NCERT Solutions for Class 11 Chemistry Chapter 5 States of Matter
NCERT solutions for class 11 chemistry chapter 5 States of Matter- The matter is existing in 3 physical states which are solid, liquid and gas. In NCERT solutions for class 11 chemistry chapter 5 States of matter, you will deal with the two states of matter namely liquid state and gaseous state, the laws governing the behaviour of ideal gases and properties associated with liquids. In NCERT syllabus class 11 chemistry chapter 5 States of Matter, there are 23 questions in the exercise.
Also Read :
- States Of Matter Class 11 NCERT Exemplar Solutions
- States Of Matter Class 11 Chemistry Chapter 5 Notes
The NCERT solutions for class 11 chemistry chapter 5 States of Matter are prepared and solved by subject experts. These NCERT solutions will help you in class 11 exam preparation as well as in the preparation of various competitive exams like NEET, JEE, BITSAT etc. By referring to the NCERT solutions for class 11, students can understand all the important concepts and practice questions well enough before their examination.
Some common characteristics of three forms of matter solid liquid and gas are summarised below-
S.No. | Gases | Liquids | Solids |
1 | No definite shape | No definite shape | Definite shape |
2 | Indefinite volume | Definite volume | Definite volume |
3 | Compressible | Slightly compressible | Nearly compressible |
4 | Low density | Intermediate density | High density |
NCERT Solutions for Class 11 Chemistry Chapter 5 States of Matter- Exercise Questions
Answer :
Given the volume of air to be compressed is $500dm^3$ at $1 bar$ pressure to $200dm^3$ at $30^{\circ}C$ .
So, as the temperature remains constant at $30^{\circ}C$ .
We have Boyle's law where, $P_{1}V_{1} = P_{2}V_{2}$
To calculate the final pressure $P_{2}$ .
We have,
$P_{1}V_{1} = P_{2}V_{2}$
$P_{2} = \frac{P_{1}V_{1}}{V_{2}}$
$= \frac{1\times 500}{200} bar = 2.5\ bar$
Therefore, the minimum pressure required is $2.5\ bar$ .
Answer :
Given volume of air to be transferred from the capacity of $120 \; mL$ vessel at $1.2\ bar$ pressure to $180 \; mL$ vessel at 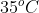
So, as the temperature remains constant at 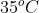 .
.
We have Boyle's law where, $P_{1}V_{1} = P_{2}V_{2}$
To calculate the final pressure $P_{2}$ .
We have,
$P_{1}V_{1} = P_{2}V_{2}$
$P_{2} = \frac{P_{1}V_{1}}{V_{2}}$
$= \frac{1.2\times 120}{180} bar = 0.8\ bar$
Therefore, the pressure required is $0.8\ bar$ .
Answer :
Given ideal gas equation ;
$PV = nRT$
So, at given fixed temperature T. we have the
$Density\ of\ gas (\rho ) = \frac{mass(m)}{Volume(V)}$
And we know $n = \frac{Mass\ of\ gas}{Molar\ mass\ of\ gas}$
so, we get $P = \frac{mRT}{MV}$
$P = \frac{\rho RT}{M}$
But at constant Temperature, we have $P \propto \rho$ .
Answer :
Given the condition :
Temperature $= 0^{\circ}C$ ,
$Pressure = 2\ bar$ for oxide gas and $Pressure = 5\ bar$ for dinitrogen.
As Density of gas is represented by, $d = \frac{PM}{RT}$
And given that density is the same for both the gases at the same temperature condition.
So, we have $M_{1}P_{1} = M_{2}P_{2}$ (as R is constant}
or, $M_{1}\times 2 =28\times 5$ $(\because Molecular\ mass\ of\ N_2 = 28\ u)$
or, $M_{1} = 70\ u$
Answer :
Given Pressure of 1g of an ideal gas A at $27^{\circ}C$ is $P_{A} = 2\ bar$ .
When 2g of another ideal gas B is introduced in the same flask at the same temperature,
The pressure becomes $P_{A}+P_{B}= 3\ bar$ .
$\implies P_{B }= 1\ bar$ .
We can assume the molecular masses of A and B be $M_{A}\ and\ M_{B}$ respectively.
The ideal gas equation, $PV=nRT$
So, we have $P_{A}V=n_{A}RT$ and $P_{B}V=n_{B}RT$
Therefore, $\frac{P_{A}}{P_{B}} = \frac{n_{A}}{n_{B}} = \frac{1M_{B}}{2M_{A}} = \frac{M_{B}}{2M_{A}}$
or, $\frac{M_{B}}{M_{A}} = 2\times\frac{P_{A}}{P_{B}} = 2\times \frac{2}{1} =4$
Hence the relation between the two gases is $M_{B} =4M_{A}$ .
Answer :
We have the chemical reaction:
$2 Al +2NaOH +H_2O \rightarrow 2NaAlO_2+3H_{2}$
(Where dihydrogen is being produced.)
So, at $20^{\circ}C$ and $1\ bar$ pressure, the volume of gas that will be released when $0.15g$ of aluminium reacts.
As we can see from the reaction equation that 2 moles of aluminium produce 3 moles of dihydrogen.
i.e., $2\times 27g$ reacts to give $3\times 22.4Litres$
At STP condition
$273.15K$ and $1atm$ ,
$54g(2\times 27g)$ of Al gives $3\times 22400mL$ of $H_{2}$ .
Therefore $0.15g$ of Al will give $= \frac{3\times22400\times0.15}{54}mL$ of $H_{2}$
i.e., $186.67mL$ of $H_{2}$ .
At STP condition:
$273.15K$ and $1atm$ ,
Now, $P_{1} =1atm$ , $V_{1} = 186.67mL$ , $T_{1} = 273.15K$
Then we assume the volume of dihydrogen be $V_{2}$ at the pressure $P_{2} = 0.987atm$
(Since 1 bar = 0.987atm) and temperature $T_{2} = 20^{\circ}C$
$\implies (273.15+20)K = 293.15K$
$\frac{P_{1}V_{1}}{T_{1}} = \frac{P_{2}V_{2}}{T_{2}}$
$V_{2}= \frac{P_{1}V_{1}T_{2}}{P_{2}T_{1}}$
$= \frac{1\times186.67\times293.15}{0.987\times273.15}$
$= 202.98mL\approx 203mL$
Therefore, 203mL of dihydrogen will be released.
Answer :
Given a mixture of 3.2g of methane and 4.4g of carbon dioxide contained a $9dm^3$ flask at $27 ^{o}C$ .
So, the pressure exerted by the mixture will be
$P =\frac{n}{V}RT = \frac{m}{M}\frac{RT}{V}$
The pressure exerted by the Methane gas, $P_{CH_{4}} =(\frac{3.2mol}{16}) \frac{0.0821 dm^3 atm K^{-1}mol^{-1}\times300K}{9 dm^3} = 0.55\ atm$
The pressure exerted by the Carbon dioxide gas,
$P_{CO_{2}} =(\frac{4.4mol}{44}) \frac{0.0821 dm^3 atm K^{-1}mol^{-1}\times300K}{9 dm^3} = 0.27\ atm$
So, the total pressure exerted $= 0.55+0.27 = 0.82\ atm$
And in terms of SI units, we get,
$R = 8.314p\ m^3\ K^{-1}mol^{-1},\ V= 9\times 10^{-3}\ m^3$
$P = 5.543\times10^4 Pa +2.771\times 10^4Pa = 8.314\times10^4 Pa.$
Answer :
Pressure of the gas mixture will be the sum of partial pressure of $H_{2}$ gas and partial pressure of $O_{2}$ gas.
So, calculating the partial pressures of each gas.
Partial pressure of $H_{2}$ in a 1L volume vessel.
$P_{1} = 0.8\ bar$ , $P_{2},\ V_{1} = 0.5L,\ V_{2} =1L$
As the temperature remains constant, $P_{1}V_{1} =P_{2}V_{2}$
$\implies (0.8\ bar)(0.5L) = P_{2}(1L)$
or $P_{2} = 0.4\ bar$ or $P_{H_{2}} = 0.4\ bar$
Now, calculating the partial pressure of $O_{2}$ gas in 1L vessel.
$P'_{1}V_{1} = P'_{2} V'_{2}$
$\implies (0.7\ bar)(2L) = P_{2}(1L)$
$\implies P'_{2} = 1.4\ bar$ or $P_{O_{2}} = 1.4\ bar$
Therefore the total pressure $= P_{H_{2}} +P_{O_{2}} = 0.4\ bar+1.4\ bar = 1.8\ bar$
Question 5.9 Density of a gas is found to be $5.46 \: g/dm^{3}$ at $27^{o}C$ at 2 bar pressure. What will be its density at STP ?
Answer :
Given the density of a gas is equal to $5.46g/dm^3$ at $27^{\circ}C$ and at 2bar pressure.
Density $d = \frac{PM}{RT}$ , for the same gas at different temperatures and pressures we can apply,
$\frac{d_{1}}{d_{2}} = \frac{P_{1}}{T_{1}}\times\frac{T_{2}}{P_{2}}.$
Here, 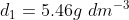 , $T_{1} =27^{\circ}C =300K$ , $P_{1} =2bar.$
, $T_{1} =27^{\circ}C =300K$ , $P_{1} =2bar.$
then at STP, we have $d_{2},\ T_{2} = 0^{\circ}C =273K,\ P_{2} =1bar.$
$\therefore \frac{5.46g\ dm^{-3}}{d_{2}} = \frac{2bar}{300K}\times\frac{273K}{1bar}$ or $d_{2} = 3g\ dm^{-3}$
Question 5.10 $34.05 \; mL$ of phosphorus vapour weighs 0.0625 g at $546 ^{o}C$ and 0.1 bar pressure. What is the molar mass of phosphorus?
Answer :
Given,
The volume of phosphorus vapour $= 34.05mL$ which weights about $0.0625g$ .
Temperature $= 546^{\circ}C = 273+546 = 819K$
Pressure $=0.1 bar$
Hence we apply the ideal gas equation,
$PV=nRT$ , where the number of moles will be $n =\frac{PV}{RT}$
$= \frac{1bar\times (34.05\times 10^{-3} dm^3)}{0.083 bar\ dm^3\ K^{-1}Mol^{-1}\times 819K}$
$= 5\times 10^{-4} mol$
$\therefore Mass\ of\ 1\ mole = \frac{0.0625}{5\times 10^{-4}}g = 125g$
Therefore the molar mass is $125g\ mol^{-1}$
Answer :
Assume the round-bottomed flask has volume $= V\ cm^3$
so, the volume of air in the flask at $27^{\circ}C$ $= V\ cm^3$ .
To find out how much air has been expelled out, we use Charle's Law:
$\frac{V_{1}}{T_{1}} = \frac{V_{2}}{T_{2}}$
Therefore $Volume\ expelled = 2.5V-V =1.5V$
Or, Fraction of air expelled $=\frac{1.5V}{2.5V} =\frac{3}{5}$
Answer :
We have the ideal gas equation,
$PV=nRT$
Pressure, p=3.32bar
Volume, V=5 $dm^3$
Number of moles, n=4 mol
Gas constant R= 0.083
Temperature, T=?
$T = \frac{PV}{nR} = \frac{3.32bar\times 5\ dm^3}{4\ mol\times 0.083 bar\ dm^3\ K^{-1}mol^{-1}} = 50K$
so, temperature, T=50K
Question 5.13 Calculate the total number of electrons present in 1.4 g of dinitrogen gas.
Answer :
Dinitrogen $N_{2}$ has a molar mass $= 28g\ mol^{-1}$ .
Given 1.4g of $N_{2}$ gas.
That means $\frac{1.4}{28} = 0.05\ mol$
$= 0.05\times6.02\times10^{23} = 3.01\times 10^{23}$ number of molecules.
And as 1 molecule of $N_{2}$ has 14 electrons.
So, $3.01\times 10^{23}$ molecules of $N_{2}$ has $14\times 3.01\times 1023 = 4.214\times 10^{23}$ electrons.
Question 5.14 How much time would it take to distribute one Avogadro number of wheat grains, if 1010 grains are distributed each second ?
Answer :
Given that $10^{10}$ grains are distributed each second.
Time taken to distribute $10^{10}$ grains = 1second.
Time taken to distribute one Avogadro number of wheat grains.
$=\frac{1s\times 6.022\times 10^{23}grains}{10^{10}grains}$
$= \frac{6.022\times10^{23}}{60\times 60\times 24\times 365} = 1.9\times 10^{6}\ years.$
Answer :
Given the mass of oxygen gas and mass of hydrogen gas.
Molar mass of $O_{2} =32g\ mol^{-1}$
Therefore $8g\ O_{2} = \frac{8}{32}mol = 0.25\ mol$
Molar mass of $H_{2} = 2g\ mol^{-1}$
Therefore $4g H_{2} =\frac{4}{2} =2\ mol$
$\Rightarrow$ Total number of moles of mixture $n = 2+0.25 =2.25$
and $V = 1\ dm^3,\ T = 27^{\circ }C = 300K,\ R=0.083\ bar\ dm^{3}K^{-1}\ mol^{-1}$
So, Ideal gas equation; $PV = nRT$
or $P = \frac{nRT}{V} = \frac{(2.25mol\times) (0.083 bar\ dm^3K^{-1}mol^{-1})(300K)}{1 dm^3}$
Therefore Total pressure is $= 56.025\ bar$
(Density of $air = 1.2 \: kg\: m^{-3}$ and $R = 0.083 \: bar \: dm^{3} K^{-1} mol^{-1}$ ).
Answer :
The payload can be defined as:
(Mass of the displaced air - Mass of the balloon)
Given the radius of the balloon, r = 10 m.
Mass of the balloon, m = 100 kg.
Therefore, the volume of the balloon will be:
$V = \frac{4}{3}\pi r^3 = \frac{4}{3}\times\frac{22}{7}\times10^3 = 4190.5\ m^3$
Now, the volume of the air displaced:
$V_{d} = 4190.5\ m^3$
The mass of the air displaced :
$m_{d} = density \times V_{d} = 1.2kgm^{-3}\times 4190.5\ m^3$
$= 5028.6kg$
Let $W$ be the mass of helium gas filled into the balloon, then
$PV=(\frac{W}{m})rt$
Or, $W = \frac{PVM}{RT}$
$= \frac{1.66\times4190.5\times10^3\times4}{0.083\times300} = 1117\ kg$ approximately.
The balloon is filled with He with total mass of $= 1117+100= 1217kg$
$\therefore$ The payload of the balloon will be:
$= 5028.6 -1217 = 3811.6kg$
Question 5.17 Calculate the volume occupied by 8.8 g of $CO_{2}$ at 31.1°C and 1 bar pressure. $R = 0.083 \: bar \: L\: K^{-1} mol^{-1}$ .
Answer :
Given the mass of carbon dioxide is 8.8grams at $31.1^{\circ}C$ and at 1 bar pressure.
So, the number of moles of
$CO_{2} (n) = \frac{Mass\ of\ CO_{2}}{Molar\ Mass}$
$= \frac{8.8g}{44g\ mol^{-1}} = 0.2\ mol$
Now, the pressure of $CO_{2} (P) = 1\ bar$
Given $R = 0.083 \: bar \: L\: K^{-1} mol^{-1}$
also, the $Temperature (T) = 273+31.1 = 304.1K$
The Ideal gas equation; $PV=nRT$
$V=\frac{nRT}{P} = \frac{0.2\times 0.083\times304.1}{1\ bar} = 5.048L$
So volume occupied by 8.8 g of carbon dioxide is 5.048L
Answer :
Let the molar mass of the gas be $M_{gas}$ ,
Then, given that $2.9g$ of a gas at $95^{\circ}C$ occupied the same volume as
$0.184g$ of $H_{2}$ at $17^{\circ }C$
So, we have the relation, $P_{1}=P_{2}$ and $V_{1} = V_{2}$
Therefore $P_{1}V_{2} = P_{2} V_{2}$
wihch gives, $n_{1}RT_{1} =n_{2}RT_{2}$
$\implies n_{1}T_{1} =n_{2}T_{2}$
Or, $\frac{2.9}{M_{gas}}\times (95+273) = \frac{0.184}{2}\times(17+273)$
Or, $M_{gas} = \frac{2.9\times368\times 2 }{0.184\times 290} = 40g\ mol^{-1}$
Answer :
A mixture of dihydrogen and dioxygen at one bar pressure contains 20% by weight of dihydrogen.
So, let us assume the weight of dihydrogen in a total mixture weight of $W$ be $20Wgrams$ and dioxygen be $80Wgrams$ .
Then, the number of moles of $H_{2}$ will be, $n_{H_{2}} = \frac{20W}{2} = 10W\ moles$ and the number of moles of $O_{2}$ , $n_{O_{2}} = \frac{80W}{32} = 2.5W\ moles$ .
And given that the total pressure of the mixture is $P_{Total} = 1\ bar$ .
Then we have a partial pressure of $H_{2}$ ,
$P_{H_{2}}=(Mol.\ fraction)_{H_{2}}\times P_{Total}$
$= \frac{n_{H_{2}}}{n_{H_{2}}+n_{O_{2}}}\times P_{Total}$
$= \frac{10W}{10W+2.5W}\times 1\ bar$
$=0.8\ bar$
Therefore, the partial pressure of $H_{2}$ is $0.8\ bar$ .
Question 5.20 What would be the $SI$ unit for the quantity $pV^{2}T^{2}/n$ ?
Answer :
Given quantity, $pV^{2}T^{2}/n$ ,
The SI units of each factors are,
For pressure p is $Nm^{-2}$ .
For Volume V is $m^3$ .
For Temperaute T is $K$ .
For number of moles, n is mol.
Therefore we have for the quantity
The SI unit is $=\frac{(Nm^{-2})(m^3)^2)(K)^2}{mol}$
$= Nm^4K^2 mol^{-1}$
Question 5.21 In terms of Charles’ law explain why –273 °C is the lowest possible temperature.
Answer :
Charles' Law states that pressure remaining constant, the volume of a fixed mass of a gas is directly proportional to its absolute temperature.
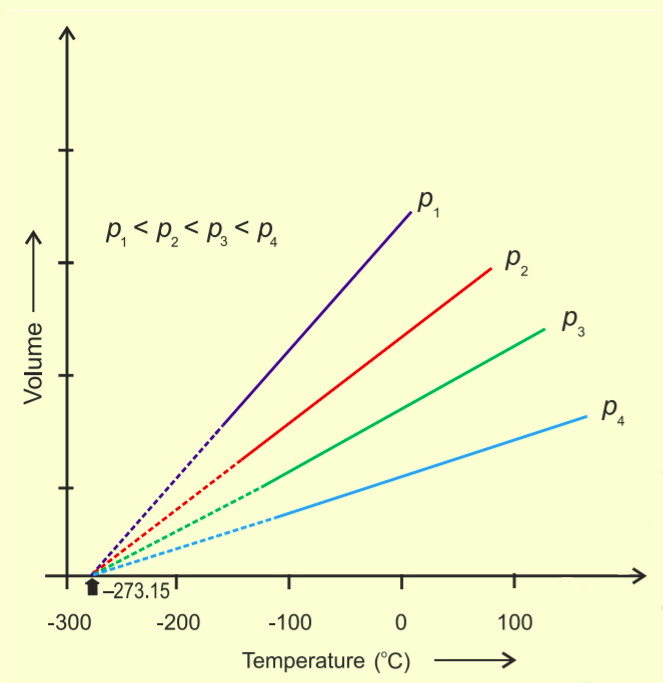
Charles found that for all gases, at any given pressure, the graph of volume vs temperature (in Celsius) is a straight line and on extending to zero volume, each line intercepts the temperature axis at – 273.15 ° C.
We can see that the volume of the gas at – 273.15 ° C will be zero. This means that gas will not exist. In fact, all the gases get liquified before this temperature is reached.
Answer :
The critical temperature we know is the highest temperature at which liquid exists above it is gas.
Given that the critical temperature of Carbon dioxide and Methane are. $31.1 ^{\circ}C\ and\ -81.9^{\circ}C$
Higher is the critical temperature of a gas, easier is its liquefaction.
So, as the critical temperature increases the gas is now easier to liquefaction.
That means the intermolecular forces of attraction between the molecules of a gas are directly proportional to its Critical temperature.
Hence, Carbon dioxide has stronger intermolecular forces than Methane.
Question 5.23 Explain the physical significance of van der Waals parameters.
Answer :
The equation of van der Waals after taking into account the corrections for pressure and volume,
$\left ( p+\frac{an^2}{V^2} \right )\left ( V-nb \right ) =nRT$
Where a and b are called van der Waals constants or parameters.
Here the significance of a and b is important:
Value of ' a' is a measure of the magnitude of intermolecular attractive forces within the gas and is independent of temperature and pressure.
And the value of 'b' is the volume occupied by the molecule and 'nb' is the total volume occupied by the molecules.
More About NCERT Solutions For States Of Matter Class 11 Chemistry
NCERT solutions for class 11 chemistry chapter 5 States of Matter is an important chapter for class 11 students because the topics of this chapter are the basics to the topics to be studied in class 12 NCERT book. The important topics of this chapter are intermolecular forces, thermal energy, the gaseous state, ideal gas equation, kinetic molecular theory of gases, liquid State and more. After completing the NCERT solutions for class 11 chemistry chapter 5 states of matter students will be able to explain the existence of different states of matter, explain the laws governing the behaviour of ideal gases; able to apply gas laws in real life situations; describe the conditions required for liquefaction of gases and also able to differentiate between vapours and gaseous state.
NCERT Solutions for Class 11 Chemistry
Chapter 1 | |
Chapter-2 | |
Chapter-3 | |
Chapter-4 | |
Chapter-5 | States of Matter |
Chapter-6 | |
Chapter-7 | |
Chapter-8 | |
Chapter-9 | |
Chapter-10 | |
Chapter-11 | |
Chapter-12 | |
Chapter-13 | |
Chapter-14 |
NCERT Solutions for Class 11 Subject Wise
Benefits of NCERT Solutions for Class 11 Chemistry Chapter 5 States of Matter
- The solutions are written in a comprehensive manner in the NCERT solutions for class 11 chemistry chapter 5 States of Matter will help you writing answers in your exam.
- Revision will be easy because the detailed solutions will help you to remember the concepts and get you good marks.
- Homework problems will be easier for you, all you need to do is check the detailed NCERT solutions for class 11 chemistry and you are ready to go.
If you have a doubt or question that is not available here or in any of the chapters, contact us. You will get all the answers that will help you score well in your exams.
Also Check NCERT Books and NCERT Syllabus here:
Frequently Asked Questions (FAQs)
This chapter holds weightage of 7 marks in JEE mains
You can find complete solutions of NCERT class 11 chemistry below. You can also find CBSE syllabus and last year papers. You can also find important questions in this app. And you can also practice test papers.
https://school.careers360.com/ncert/ncert-class-11th-chemistry-notes
https://school.careers360.com/ncert/ncert-solutions-class-11-chemistry
Most of the questions are asked directly from NCERT, hence it is must to do ncert solutions
Yes, it is helpful to a large extent
5 marks
This chapter holds weightage of 2%
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
