NCERT Solutions for Class 11 Chemistry Chapter 11 The p-Block Elements
NCERT Solutions for Class 11 Chemistry Chapter 11 The p-Block Elements- In this chapter, students will get to know the general introduction of p-block elements. P-block elements are arranged in six groups of the periodic table which are 13, 14, 15, 16, 17, and 18. This NCERT book class 11 chapter covers only two groups 13 and 14, the rest groups will be covered in the NCERT syllabus class 12th chapter 7 The p-Block elements. In this chapter, there are 38 questions in the exercise. If you are looking for the answers of any other class from 6-12 then NCERT solutions are there for you as it's the easiest way to get all the solutions of NCERT.
Also Read :
- The p Block Elements Class 11 Chemistry NCERT Exemplar Solutions
- The p Block Elements Class 11 Chemistry Chapter Notes
The NCERT solutions for Class 11 Chemistry Chapter 11 The p-Block Elements are designed and explained by our subject experts. These NCERT solutions for class 11 help students in their preparation of both CBSE class 11 final examination and in the competitive exams like JEE Main, NEET etc. It is recommended to students to go through NCERT solutions for Class 11 Chemistry Chapter 11 The p-Block Elements thoroughly to maximise their marks in examination.
NCERT Solutions for Class 11 Chemistry Chapter 11 The P-Block Elements- Exercise Questions
Question 11.1(i) Discuss the pattern of variation in the oxidation states of
$(i)\; B\; to\; Tl$
Answer :
These elements belong to group 13 of the general electronic configuration $ns^2,\ np^1$ . These elements have three valence electrons, therefore, they can exhibit a maximum only +3 oxidation state. Boron shows only +3 oxidation state but other elements also show +1 oxidation state. On moving down the group from $B\; to\; Tl$ , its stability increases due to inert pair effect. After removal of the last electron from p- orbital, the remaining two electrons behave like inert gases and don't take part in bonding. They are strongly attracted by the nucleus. This reluctance of the s- electron pair to take part in bond formation is called inert pair effect.
Question 11.1(ii) Discuss the pattern of variation in the oxidation states of
$(ii)\; C \; to \; Pb$
Answer :
These elements belong to group 14, of the general electronic configuration $ns^2,\ np^2$ . These elements have four valence electrons, therefore, they can exhibit commonly +4 oxidation state. On moving down the group from $B\; to\; Tl$ +2 oxidation state becomes more and more common. Carbon and silicon mostly show +4 oxidation state, its stability decreases on moving down the group. It is due to the inert pair effect. Though $Ge,\; Sn,\;Pb$ shows both +2 and +4 oxidation states. The stability of the lower oxidation state decreases down the group.
Question 11.2 How can you explain higher stability of $BCl_{3}$ as compared to $TlCl_{3}$ ?
Answer :
Both boron and Thallium belong to the 13th group of the periodic table. We know that the stability of lower oxidation state (+1) is increases down the group. Thus $BCl_{3}$ is more stable than $TlCl_{3}$ . It is due to the +3 of Boron is more stable than +3 of Thallium. $Tl^{3+}$ is highly oxidising in nature it reverts back to its +1 oxidation state by gaining an electron.
Question 11.3 Why does boron triflouride behave as a Lewis acid ?
Answer :
The electronic configuration of boron is $ns^2, np^1$ . It has three valance shell electrons. It can form only three covalent bonds. It means Boron has only 6 electrons and its octet is not complete, boron needs 2 more electrons to complete its octet. In $BF_{3}$ , its octet is incomplete. Hence it is an electron deficient molecules so it acts as a Lewis acid.
Question 11.4 Consider the compounds, $BCl_{3}$ and $CCl_{4}$ . How will they behave with water ? Justify.
Answer :
$BCl_{3}$ is a Lewis acid, it is an electron deficient molecules. So, it readily undergoes to hydrolysis. On hydrolysis gives boric acid.
$BCl_3+H_{2}O\rightarrow 3HCl+B(OH)_3$
$CCl_{4}$ does not undergo hydrolysis because carbon has no vacant orbital, so it cannot accept an electron from the water molecule to form intermediate.
$CCl_{4}$ + water $\rightarrow$ no reaction
Question 11.5 Is boric acid a protic acid ? Explain.
Answer :
Boric acid is not a protic acid. It is a weak monobasic acid, behaves as a Lewis acid by accepting a pair of an electron from the water molecules( $OH^-$ )
$B(OH)_{3}+H_{2}O\rightarrow [B(OH)_4]^-+H_{3}O^+$
Question 11.6 Explain what happens when boric acid is heated.
Answer :
On heating boric acid around 370 K, it converts to metaboric acid( $HBO_{2}$ ). If we continue the heating process, it becomes boric oxide.
$H_3BO_{3}\xrightarrow[370K]{\Delta }HBO_{2}\xrightarrow[red\; hot]{\Delta }B_2O_3$
Question 11.7 Describe the shapes of $BF_{3}$ and $BH_{4}^{-}$ . Assign the hybridisation of boron in these species.
Answer :
The hybridisation of Boron in $BF_{3}$ and $BH_{4}^{-}$ is $sp^2$ and $sp^3$ respectively.
Question 11.8 Write reactions to justify amphoteric nature of aluminium.
Answer :
amphoteric nature of aluminium means it shows properties of acid as well as the base. Aluminium dissolves in acid and bases.
Reaction with acids-
$2Al+6HCl\rightarrow Al^{3+}+6Cl^-+3H_{2}$
Reaction with the base-
$2Al+2NaOH+6H_{2}O\rightarrow 2Na^{+}[Al(OH)_{4}]^-+6Cl^-+3H_{2}$
Question 11.9 What are electron deficient compounds ? Are $BCl_{3}$ and $SiCl_{4}$ electron deficient species ? Explain.
Answer :
Electron-deficient compounds are those in which the octet of the central atom is not completed. Therefore, the metal wants to complete its octet by accepting an electron from a donor molecule.
$BCl_{3}$ is an electron-deficient compound because of the central atom, Boron has only 6 electrons, two electrons are needed to complete its octet.
$SiCl_{4}$ silicon has 4 valence electrons and by covalent bonding, it gets 4 electrons from four chlorine atom. Hence, $SiCl_{4}$ is not an electron-deficient molecule.
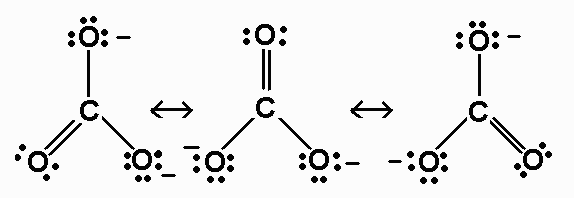
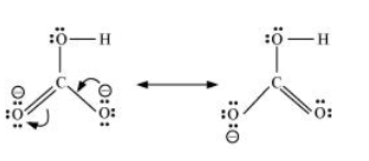
Question 11.10 Write the resonance structures of $CO_{3}^{2-}$ and $HCO_{3}^{-}$ .
Answer :
The resonance structure of the $CO_{3}^{2-}$ and $HCO_{3}^{-}$ -
![]()
Question 11.11(a) What is the state of hybridisation of carbon in
Answer :
The state of hybridization of Carbon in $CO_3^{2-}$ is $sp^2$ and it is bonded with three oxygen in a same plane.
Question 11.11(b) What is the state of hybridisation of carbon in
diamond ?
Answer :
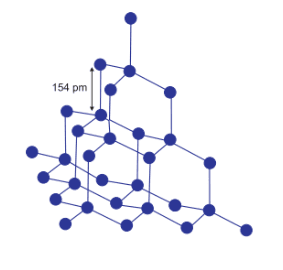
Each carbon in diamond is $sp^3$ hybridised and four carbon is attached to one central carbon.
Question 11.11(c) What is the state of hybridisation of carbon in
graphite?
Answer :
The hybridisation of each carbon in graphite is $sp^2$ . Each carbon is attached with three neighbour carbon atoms. 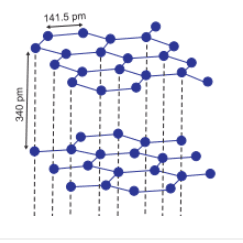
![]()
Question 11.12 Explain the difference in properties of diamond and graphite on the basis of their structures.
Answer :
The difference in the properties of carbon and graphite-
Diamond | Graphite |
It has a crystalline structure | Layered structure |
Each carbon atom is $sp^3$ hybridised | Carbon has $sp^2$ hybridisation |
Made up of tetrahedral units | Planner in geometry |
|
|
Question 11.13(a) Rationalise the given statements and give chemical reactions :
Lead (II) chloride reacts with $Cl_{2}$ to give $PbCl_{4}$ .
Answer :
$Pb$ is belong to the group 14th and it has two oxidation states +2 and +4. On moving down the group, the stability of the +2 oxidation state is more than the +4 oxidation state, it is due to the inert pair effect. This $PbCl_{4}$ is less stable than $PbCl_{2}$ . However, the formation of $PbCl_{4}$ takes places when the chlorine gas is bubbled by a saturated solution of $PbCl_{2}$ .
$PbCl_{2}+Cl_{2}\rightarrow PbCl_4$
Question 11.13(b) Rationalise the given statements and give chemical reactions :
Lead(IV) chloride is highly unstable towards heat.
Answer :
On moving down the group the +4 oxidation of lead has less stable than +2 oxidation due to the inert pair effect. Thus on heating, the lead(IV) will be reduced to lead(II).
$PbCl_4(l)\overset{\Delta }{\rightarrow}PbCl_2(s)+Cl_2(g)$
Question 11.13(c) Rationalise the given statements and give chemical reactions
Lead is known not to form an iodide, $PbI_{4}.$
Answer :
Lead is not form $PbI_{4}.$ Pb(+4) is oxidising in nature and $I^-$ is reducing in nature. So the $PbI_{4}.$ is not stable in this state. $Pb(+4)$ oxidises $I^-$ to $I_2$ and itself get reduced to $Pb(+2)$ .
$PbI_{4}\rightarrow PbI_{2}+I_{2}$
Question 11.14. Suggest reasons why the B–F bond lengths in $BF_{3}$ (130 pm) and $BF_{4}^{-}$ (143 pm) differ.
Answer :
The B-F bond length in $BF_{3}$ is shorter than the B-F bond length in $BF_{4}^{-}$ . $BF_{3}$ is an electron deficient species, in which $p$ orbital is vacant in Boron. To overcome this problem the fluorine and the boron atom undergoes $p\pi-p\pi$ back bonding. Due to this a partial double bond character is imparts on them. Because of this double bond character, the B-F bond in $BF_{3}$ molecule is shortened.
On the other hand, In the case of $BF_{4}^{-}$ , the hybridization changes from $sp^2\rightarrow sp^3$ . Now boron has 4- sigma bonds and it lose its partial double bond character. Due to this reason, the B-F bond length in $BF_{4}^{-}$ is 143 pm.
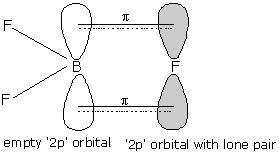
Question 11.15. If B–Cl bond has a dipole moment, explain why $BCl_{3}$ molecule has zero dipole moment.
Answer :
Due to differences in electronegativities of Boron and chlorine, the partial charges develop in B-Cl bond. $BCl_{3}$ is a non-polar compound. This molecule is trigonal planner shape (symmetrical) and we know that dipole moment is a vector quantity. In fig. we can see that, they can cancel out each other. 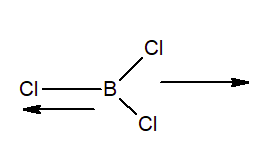
Question 11.17. Suggest a reason as to why $CO$ is poisonous.
Answer :
carbon monoxide is more poisonous than carbon dioxide, (non-toxic in nature). $CO$ has the ability to block delivery of oxygen to the organ and tissue. Also, it binds to haemoglobin to form a complex carboxyhaemoglobin, which is 300 times more stable than the oxygen-haemoglobin complex. If the concentration of these complex reaches to 3-4% then the capacity of blood to carry oxygen is reduced. On the other hand, $CO_2$ is not poisonous, it is harmful only at high concentration.
Question 11.18. How is excessive content of $CO_{2}$ responsible for global warming ?
Answer :
Carbon dioxide can ability to trap the heat emitted by the sun radiation. Higher the amount of $CO_{2}$ , higher the amount of heat trapped. Therefore, this results in an increase in temperature of the atmosphere causing global warming. Hence, excessive content of carbon dioxide is a threat to us.
Question 11.19. Explain structures of diborane and boric acid.
Answer :
Diborane-
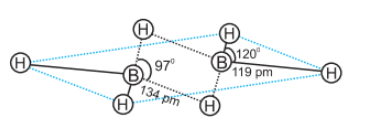
The four terminal H atom and Boron lie in the same plane but two bridge H atom is below the plane. The four terminal B-H bonds are regular two centre-two electron bonds. T he two (B-H-B) bonds are known as banana bonds(3-centre-2-electron bonds).
Boric acid-
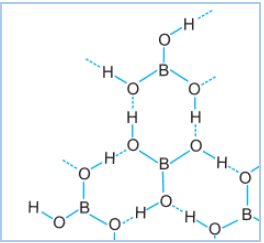
It is a weak monobasic acid. It is Lewis acid, not protic acid. It has a layered structure, the $BO_{3}$ molecule is linked by hydrogen-bonding with another $BO_{3}$ molecule. The dotted line represents H-bonding.
Question 11.20(a) What happens when
Borax is heated strongly?
Answer :
The molecular formula of borax is $Na_{2}B_{4}O_{7}.10H_{2}O$ . On heating, it loses water molecules and converts into sodium metaborate( $Na_{2}B_{4}O_{7}$ ) and if we continue the heating process it becomes anhydride, called as boric anhydride $B_2O_{3}$ .
$Na_2B_4O_7.10H_2O\rightarrow Na_2B_{4}O_{7}\rightarrow 2NaBO_{2}+B_{2}O_{3}$
Question 11.20(b) What happens when
(b) Boric acid is added to water?
Answer :
When boric acid is added to water it accepts electrons from the water molecule( $OH^-$ )and forms( $[B(OH)_4]^-$ )
$B(OH)_{3}+H_{2}O\rightarrow [B(OH)_4]^-+H_{3}O^{+}$
Question 11.20(c) What happens when
Aluminium is treated with dilute $N\! a\, \! O\! H$ ?
Answer :
Aluminium is treated with dilute $N\! a\, \! O\! H$ hydrogen gas is liberated and form sodium tetrahydroxy aluminate(III)
$2Al+2NaOH+6H_{2}O\rightarrow 2Na^{+}[Al(OH)_{4}]^-+3H_{2}$
Question 11.20(d) What happens when
$B\! F_{3}$ is reacted with ammonia ?
Answer :
When $B\! F_{3}$ is reacted with ammonia they form an borane adduct and due to that, the boron atom completes its octet.
$F_{3}B+:NH_{3}\rightarrow F_3B\leftarrow :NH_{3}$
Question 11.21(a) Explain the following reactions
Silicon is heated with methyl chloride at high temperature in the presence of copper;
Answer :
Silicon is heated with methyl chloride at high temperature in the presence of copper; as a catalyst at a temperature around 573K different types of methyl substituted chlorosilane are formed. for example- $MeSiCl_3,Me_2SiCl_2,Me_3SiCl$ and a small amount of $Me_4Si$ $2CH_{3}Cl+Si\xrightarrow[Cu\ powder]{573K}(CH_{3})Si(Cl)_{2}\overset{hydrolysis}{\rightarrow}(CH_{3})_{2}Si(OH)_{2}$
Question 11.21(b) Explain the following reactions
Silicon dioxide is treated with hydrogen fluoride;
Answer :
When Silicon dioxide is treated with hydrogen fluoride, it forms silicon tetrafluoride
$SiO_{2}+4HF\rightarrow SiF_4+2H_{2}O$
and $SiF_4$ further reacts with the HF to form hydrofluorosilic acid.
$SiF_4+2HF\rightarrow H_{2}SiF_{6}$
Question 11.21(c) Explain the following reactions
$CO$ is heated with $Z\! nO$ ;
Answer :
When CO reacts with $Z\! nO$ , it acts as a reducing agent and reduces the $Z\! nO$ to zinc.
$ZnO+CO\rightarrow Zn+CO_{2}$
Question 11.21(d). Explain the following reactions
Hydrated alumina is treated with aqueous $N\! aO\! \! \, H$ solution;
Answer :
Hydrated alumina is treated with an aqueous $N\! aO\! \! \, H$ solution leads to the formation of sodium meta aluminate
$Al_2O_{3}.2H_2O+2NaOH\rightarrow 2NaAlO_{2}+3H_{2}O$
Question 11.22(i) Give reasons
Conc. $H\! N\! O_{3}$ can be transported in aluminium container.
Answer :
Conc. $H\! N\! O_{3}$ can be transported in an aluminium container because when it reacts with the aluminium it forms a thin layer of oxide on the surface. This layer renders aluminium passive.
Question 11.22(ii) Give reasons :
A mixture of dilute $N\! aO\! H$ and aluminium pieces is used to open drain.
Answer :
When the mixture of dilute $N\! aO\! H$ and aluminium pieces reacts, they formed sodium tetrahydroxy aluminate (III)and dihydrogen gas. To open the drains, we use the pressure produced by the hydrogen gas.
$2Al+NaOH+6H_{2}O\rightarrow 2Na+[Al(OH)_{4}]^{-}+3H_{2}$
Question 11.22(iii) Give reasons :
Graphite is used as lubricant.
Answer :
Graphite has a layered structure and each layer is bonded by the weak van der Waals forces. These layers can slide over each other that's why graphite can be used as a lubricant.
Question 11.22(iv) Give reasons :
Diamond is used as an abrasive.
Answer :
The carbons of the diamond are in $sp^3$ hybridisation. Each carbon is attached with the four neighbour carbon atom by a strong covalent bond. This covalent bond gives the diamond a very rigid structure and it is very difficult to break this covalent bond and due to this reason it is the hardest substance and used as an abrasive
Question 11.22(v) Give reasons :
Aluminium alloys are used to make aircraft body.
Answer :
Aluminium has very good tensile strength and is very light in weight also. It can be alloyed with the various metals such as copper, manganese and Silicon etc. Al is malleable and ductile. Because of these properties of Aluminium, it can be used in making aircrafts bodies.
Question 11.22(vi) Give reasons :
Aluminium utensils should not be kept in water overnight.
Answer :
Aluminium utensils should not be kept in water overnight because it reacts with water with the help of atmospheric oxygen and forms an oxide layer on the surface of utensils. When we kept water on this utensils for a long period of time, some amount of oxide dissolved in water which is harmful(water become basic in nature).
Question 11.22(vii) Give reasons :
Aluminium wire is used to make transmission cables.
Answer :
Aluminium wires are good conductors of electricity and also it is cheap metal easily available, light in weight and also very good ductility. All these properties of aluminium make it better to make transmission cables.
Question 11.23 Explain why is there a phenomenal decrease in ionization enthalpy from carbon to silicon ?
Answer :
Ionisation enthalpy of carbon is quite high because it is small in size. However, on going down the group to silicon, there is a sudden decrease in the enthalpy of the silicon. This is due to the large increase in the atomic size of the silicon element.
Question 11.24 How would you explain the lower atomic radius of $Ga$ as compared to $Al$ ?
Answer :
The atomic radius of the gallium is less than the atomic radius of aluminium. This is because of the weak shielding effect of the 3d electron of gallium. The shielding effect of d-electrons are feeble so that the effective nuclear charge experienced by the valence electron in $Ga$ is quite higher than the $Al$
Answer :
The various forms of the same element are known as allotropes. They have the same chemical properties but different physical properties.
Allotropes of carbon -
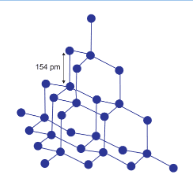
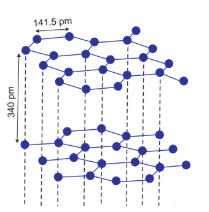
In diamond, all the carbon is $sp^3$ hybridised and each of the carbon is attached with the four neighbour carbon atom. by a strong covalent bond. This provides it with a very rigid structure and makes it the hardest substance.
In Graphite, carbon is $sp^2$ hybridised and attached with three neighbours carbon. It has a layered structure, which is attached with the weak van der Waal forces. These layers can slide over one another so that's why they are soft and used as a lubricant.
Question 11.26 Classify the given oxide as neutral, acidic, basic or amphoteric. Write suitable chemical equations to show their nature.
$CO,B_{2}O_{3},SiO_{2},CO_{2},Al_{2}O_{3},PbO_{2},Tl_2O_{3}$
Answer :
$CO$ is a neutral
Acidic oxides-
Being acidic, the oxides react with bases to form a salt. So take base = sodium hydroxide ( $NaOH$ )
$B_{2}O_{3},\ SiO_{2},\ CO_{2}$
these compounds react with a base to form salts. ex.- sodium metaborate, sodium silicate and sodium carbonate respectively.
$\\B_{2}O_{3}+2NaOH\rightarrow 2NaBO_{2}+H_{2}O\\ SiO_{2}+2NaOH\rightarrow 2Na_{2}SiO_{3}+H_{2}O\\ CO_{2}+2NaOH\rightarrow 2Na_{2}CO_{3}+H_{2}O$
Basic Oxides-
It reacts with an acid to form salts. let acid = hydrochloric acid ( $HCl$ ). for example thallium chloride.
$Tl_{2}O_{3}+6HCl\rightarrow 2TlCl_{3}+3H_{2}O$
Amphoteric Oxides-
These oxides have a tendency to react with acid as well as with the base.
$PbO_{2},\ Al_2O_{3}$
The reaction of lead oxide with acid and base-
$\\PbO_{2}+2NaOH\rightarrow Na_{2}PbO_{3}+H_{2}O\\ PbO_{2}+2H_{2}SO_{4}\rightarrow 2PbSO_{4}+2H_{2}O+O_{2}$
The reaction of aluminium oxide with base and acid-
$\\Al_2O_{3}+2NaOH\rightarrow NaAlO_{2}\\ Al_2O_{3}+3H_{2}SO_{4}\rightarrow Al_{2}(SO_{4})_3+3H_{2}O$
Answer :
Thallium belongs to group 13 of the periodic table. It shows +3 and +1 oxidation state. Due to the inert pair effect, the stability of the +1 oxidation state is more, on moving down the group. We know that aluminium and alkali metals +3 and +1 oxidation states. Thallium displays both oxidation states. Therefore it resembles both aluminium and alkali metals. Like $Al$ , it also forms compounds such as $TlCl_3$ and $Tl_{2}O_3$ also $Tl_{2}O$ and $TlCl$
Answer :
In given information, when X is treated with the sodium hydroxide it gives A(white ppt) and it is soluble in excess of $NaOH$ . Thus X must be aluminium . So, the white ppt of A is aluminium hydroxide.
The complex B is soluble in excess of sodium hydroxide So it means it is a sodium tetrahydroxy aluminate(II) complex.
$\\2Al+3NaOH\rightarrow Al(OH)_3+3Na^+\\ Al(OH)_{3}+NaOH\rightarrow Na[Al(OH)_{3}]^-$
Now, when we add dilute HCl to aluminium hydroxide it gives the compound C (Aluminum chloride).
$Al(OH)_{3}+3HCl\rightarrow AlCl_{3}+3H_{2}O$
(A) (C)
And when compound (A) is heated strongly, it gives D and this compound used to extract metal X
$2Al(OH)_{3}\rightarrow Al_2O_{3}+3H_{2}O$
(A) (D)
Question 11.29(a) What do you understand by
inert pair effect
Answer :
Inert pair effect-
On moving down the group in the periodic table, the tendency of $s$ -orbital electron to participate in bonding is decreased. This effect is known as the inert pair effect. For example, in group 13 element ( $ns^2,np^1$ )the stability of +1 oxidation is more than the +2 oxidation state due to the poor shielding of the $ns^2$ electrons by the $d$ and $f$ electron, as a result, $ns^2$ electrons are strongly held by the nucleus.
Question 11.29(b) What do you understand by
allotropy
Answer :
Allotropy-
The existence of an element in more than one form. The various forms of the same element are known as allotropes. They have the same chemical properties but different physical properties. For example- Carbon exists in three allotropic forms: diamond, fullerene and the graphite.
Question 11.29(c) What do you understand by
catenation
Answer :
Catenation-
The same atoms of element link with another same atom via a strong covalent bond to form long chains or branches. This type of property is known as catenation. Carbon and silicon show this property.
Answer :
As per the given information, the compound X is a salt of a strong base and a weak acid because salt is alkaline to litmus. And when X is heated it swells to become Y. Thus, this must be borax because borax on heating, loses water molecule and swells to form sodium metaborate if we continue to heat it becomes glassy material Y. Hence Y should be a mixture of two compound boric anhydrides and metaborate.
The reactions are shown here-
$Na_{2}B_{4}O_{7}+7H_{2}O\rightarrow 2NaOH+4H_{3}BO_{3}$ (orthoboric acid)
$Na_{2}B_{4}O_{7}.10H_{2}O\overset{\Delta }{\rightarrow} Na_{2}B_{4}O_7\overset{\Delta }{\rightarrow}B_{2}O_{3}+2NaBO_{2}$
(Borax X) (sodium metaborate) (boric anhydride)
|_______Y_________|
When a concentrated acid is added to borax, white crystals are formed
$Na_{2}B_{4}O_{7}.10H_{2}O+H_{2}SO_{4}\overset{\Delta }{\rightarrow} Na_{2}SO_{4}+4H_{3}BO_{3}+5H_{2}O$
(orthoboric acid Z)
Question 11.31 (i) Write balanced equations for:
Answer :
When boron trifluoride reacts with lithium hydride it gives diborane and lithium fluoride
$BF_{3}+LiH\rightarrow B_{2}H_{6}+6LiF$
Question 11.31(ii) Write balanced equations for:
$(ii)\; B_{2}H_{6}+H_{2}O\rightarrow$
Answer :
On treating diborane with water it gives orthoboric as the main product and liberates hydrogen gas.
$B_{2}H_{6}+H_{2}O\rightarrow2H_{3}BO_{3}+6H_{2}$
Question 11.31(iii) Write balanced equations for:
$(iii)\; NaH+B_{2}H_{6}\rightarrow$
Answer :
When diborane reacts with the sodium hydride in the presence of ether, it gives sodium borohydride.
$NaH+B_{2}H_{6}\rightarrow2NaBH_{4}$
Question 11.31(v) Write balanced equations for:
$(v)\; Al+NaOH\rightarrow$
Answer :
Aluminium on reacting with a strong alkali like sodium hydroxide with the presence of moisture gives sodium tetrahydroxoaluminate complex and liberates hydrogen gas.
$Al+NaOH+6H_{2}O\rightarrow2Na^+[Al(OH)_{4}]^-(aq)+3H_{2}$
Question 11.31(vi) Write balanced equations for:
$(vi)\; B_{2}H_{6}+NH_{3}\rightarrow$
Answer :
When diborane reacts with the ammonia it forms borazine on strong heating, which is also called inorganic benzene.
$B_{2}H_{6}+6NH_{3}\rightarrow3[BH_{2}(NH_{3})_2]^+[BH_{4}]^-\overset{\Delta }{\rightarrow} 2B_{3}N_{3}H_{6}+12H_{2}$
Question 11.32. Give one method for industrial preparation and one for laboratory preparation of $C\! \, O$ and $C\! \, O_{2}$ each.
Answer :
Laboratory method of preparation of $C\! \, O$ and $C\! \, O_{2}$ -
- Carbon dioxide can be prepared by treating calcium carbonate with dil. hydrochloric acid.
$CaCO_{3}+HCl(aq)\rightarrow CaCl_{2}(aq)+CO_2(g)+H_{2}O(l)$
- Carbon monoxide can be prepared by the dehydration of methanoic acid(formic acid) with conc. sulphuric acid at 373K.
$HCOOH\xrightarrow[H_{2}SO_{4}]{373K}H_2O+CO$
The commercial method of preparation of $C\! \, O$ and $C\! \, O_{2}$ -
- $C\! \, O_{2}$ can be prepared by heating limestone.
$CaCO_{3}\overset{\Delta }{\rightarrow}CaO+CO_{2}$
- $C\! \, O$ can be prepared by passing steam over hot coke.
$C(s)+\;steam(H_{2}O) \rightarrow CO(g)+H_{2}(g)$
|_____________|
water gas
Question 11.33. An aqueous solution of borax is
(a) neutral (b) amphoteric
(c) basic (d) acidic
Answer :
An aqueous solution of borax is basic in nature. It is salt of a strong base ( $NaOH$ ) and weak acid ( $H_{3}BO_{3}$ )
Option C is the correct answer.
Question 11.34. Boric acid is polymeric due to
(a) its acidic nature (b) the presence of hydrogen bonds
(c) its monobasic nature (d) its geometry
Answer :
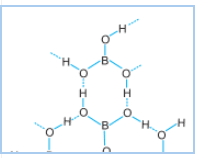
Option B is the correct answer,
Because of the inter-molecular hydrogen bonding in the boric acid, it forms the polymeric chain.
Question 11.35. The type of hybridisation of boron in diborane is
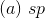 $(b) \, sp^{2}$ $(c)\, sp^{3}$ $(d)\, dsp^{2}$
$(b) \, sp^{2}$ $(c)\, sp^{3}$ $(d)\, dsp^{2}$
Answer :
In diborane, boron is $sp^2$ hybridised.
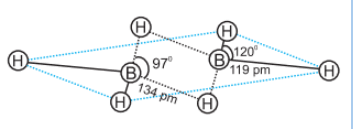
So, the correct option is C
Question 11.36. Thermodynamically the most stable form of carbon is
(a) diamond (b) graphite
(c) fullerenes (d) coal
Answer :
Graphite is a thermodynamically most stable form of carbon.
So, the correct option is B
Question 11.37. Elements of group 14
(a) exhibit oxidation state of +4 only
(b) exhibit oxidation state of +2 and +4
(c) form $M^{2-}$ and $M^{4+}$ ions
(d) form $M^{2+}$ and $M^{4+}$ ion s
Answer :
Elements of group 14 have 4 valance electrons. so the oxidation state of the group is +4 and +2. Due to the inert pair effect, the stability of +2 is more on moving down the group.
So, the correct option is B
Question 11.38. If the starting material for the manufacture of silicones is $RSiCl_{3}$ , write the structure of the product formed.
Answer :
$RSiCl_{3}+3H_{2}O\rightarrow RSi(OH)_{3}+3HCl$
$RSi(OH)_{3}$ undergoes condensation polymerisation yields straight chain polymer and water molecule as a by-product. The final product is called silicone.
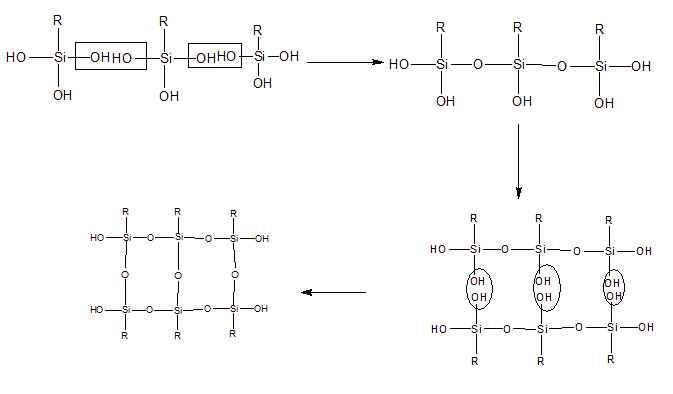
More About The p Block Elements Class 11 NCERT Chemistry Chapter
Since this is a theoritical chapter, hence most of the concepts will be learnt directly from NCERT solutions for for Class 11 Chemistry Chapter 11 The p-Block Elements. This is perhaps the longest chapter of inorganic chemistry with lot of things to mug up. Boron family and carbon family are very important topics of this chapter. Hence It is highly recommended to go through these two topics very thoroughly.
The NCERT Class 11 chemistry chapter 11 p-Block Elements is an important chapter for the class 11 students because various questions are asked from this topic in class 11 final examination. After completing the theory as well as NCERT solutions for Class 11 Chemistry Chapter 11 The p-Block Elements students will be able to explain the properties and electronic configuration of p-block elements; general trends in the p-block elements like atomic radius, electronegativity, ionisation enthalpy and electron gain enthalpy; able to describe the trends in the chemical and physical properties of group 13 and group 14 elements; explain anomalous behaviour of boron and carbon; describe allotropic forms of carbon and explain explain properties of important compounds of boron, carbon and silicon.
In p-block elements, the last electron enters the p-orbital of the outermost energy shell. The electronic configuration of p-block elements is $ns^2np^{1-6}$ (except for helium He). The number of p-orbitals is three and the maximum number of electrons that can be filled in a set of p-orbitals is six. There are a total of six groups from 13 to 18 in the p-block elements and boron, carbon, nitrogen, oxygen, fluorine and helium head the groups. The general electronic configuration and the important oxidation states of p-block elements are shown in the table given below-
Group | 13 | 14 | 15 | 16 | 17 | 18 |
General electronic configuration | $ns^2np^1$ | $ns^2np^2$ | $ns^2np^3$ | $ns^2np^4$ | $ns^2np^5$ | $\\ns^2np^6\\(1s^2\:for\:He)$ |
The first member of the group | B | C | N | O | F | He |
Group Oxidation state | +3 | +4 | +5 | +6 | +7 | +8 |
Other oxidation states | +1 | +2, -4 | +3, -3 | +4, +2, -2 | +5, +3, +1, -1 | +6, +4, +2 |
Topics of NCERT Class 11 Chemistry Chapter 11 The p-Block Elements
11.1 Group 13 Elements: The Boron Family
11.2 Important Trends and Anomalous Properties of Boron
11.3 Some Important Compounds of Boron
11.4 Uses of Boron and Aluminium and their Compounds
11.5 Group 14 Elements: The Carbon Family
11.6 Important Trends and Anomalous Behaviour of Carbon
11.7 Allotropes of Carbon
11.8 Some Important Compounds of Carbon and Silicon
NCERT Solutions for Class 11 Chemistry
Chapter 1 | |
Chapter-2 | |
Chapter-3 | |
Chapter-4 | |
Chapter-5 | |
Chapter-6 | |
Chapter-7 | |
Chapter-8 | |
Chapter-9 | |
Chapter-10 | |
Chapter-11 | The P-Block Elements |
Chapter-12 | |
Chapter-13 | |
Chapter-14 |
NCERT Solutions for Class 11 Subject Wise
- NCERT Solutions for Class 11 Biology
- NCERT Solutions for Class 11 Maths
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 11 Physics
Benefits of NCERT Solutions for Class 11 Chemistry Chapter 11 The p-Block Elements
- The solutions are written in a comprehensive manner in the NCERT solutions for class 11 chemistry chapter 11 The p-Block Elements which will help you writing answers in your exam.
- Revision will be easy because the detailed solutions will help you to remember the concepts and get you good marks.
- Homework problems will be easier for you, all you need to do is check the detailed NCERT solutions for class 11 chemistry and you are ready to go.
If you have a doubt or question that is not available here or in any of the chapters, contact us. You will get all the answers that will help you score well in your exams.
Also Check NCERT Books and NCERT Syllabus here:
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
