The P-Block Elements Class 11th Notes - Free NCERT Class 11 Chemistry Chapter 11 Notes - Download PDF
The P-Block elements are one of the important chapters of Class 11 Chemistry. It includes the elements present in the groups from 13 to 18. And is very important to learn more about the periodic table. It also includes the physical and chemical properties of elements. And also uses and properties of some of its compounds. The P-block elements Class 11 notes include the very important details of P-Block elements and it will help students to acquire basic information of the periodic table.
This Story also Contains
- NCERT Notes for Class 11 Chemistry Chapter 11
- NCERT Notes for Class 11 Chemistry Chapter 11: Topic 1
- P block Elements
- NCERT Notes for Class 11 Chemistry Chapter 11; Topic 2
- Boron Family
- NCERT Notes for Class 11 Chemistry Chapter 11; Topic 3
- Atomic radii
- NCERT Notes for Class 11 Chemistry Chapter 11; Topic 4
- Ionization Enthalpy
- NCERT Notes for Class 11 Chemistry Chapter 11; Topic 5
- Physical Properties
- NCERT Notes for Class 11 Chemistry Chapter 11; Topic 6
- Chemical Properties
- NCERT Notes for Class 11 Chemistry Chapter 11; Topic 7
- Some of the Compounds of Boron
- NCERT notes for Class 11 Chemistry Chapter 11; Topic 8
- Carbon Family
- NCERT Notes for Class 11 Chemistry Chapter 11; Topic 9
- Atomic radii
- NCERT Notes for Class 11 Chemistry Chapter 11; Topic 10
- Ionization Enthalpy
- NCERT Notes for Class 11 Chemistry Chapter 11; Topic 11
- Electronegativity
- NCERT Notes for Class 11 Chemistry Chapter 11; Topic 12
- Physical Properties
- NCERT Notes for Class 11 Chemistry Chapter 11; Topic 13
- Chemical Properties
- NCERT Notes for Class 11 Chemistry Chapter 11; Topic 14
- Allotropes of Carbon
- NCERT Notes for Class 11 Chemistry Chapter 11; Topic 15
- Uses of Carbon
- NCERT Notes for Class 11 Chemistry Chapter 11; Topic 16
- Silicon Dioxide
- NCERT Notes for Class 11 Chemistry Chapter 11; Topic 17
- Carbon Dioxide
- NCERT Class 11 Notes Chapter-Wise
The purpose of CBSE Class 11 Chemistry chapter 11 notes is to assist students to collect information and recalling the key points before the test. The structure and properties of allotropes of carbon are also covered in the Class 11 Chemistry chapter 11 notes. Students who wish to score top marks in the CBSE exams need proper study materials and guidance. NCERT Class 11 Chemistry chapter 11 notes are made to help students with that. And thereby they can succeed in examinations.
Also, students can refer,
NCERT Notes for Class 11 Chemistry Chapter 11
NCERT Notes for Class 11 Chemistry Chapter 11: Topic 1
P block Elements
The elements present in the group from 13 to 18 of the periodic table are the p block elements. Except for helium, the general outer electronic configuration of p block elements is ns2 np1-6. Based on the first element of the group can be classified as boron family carbon family nitrogen family oxygen family halogens and noble gases.
NCERT Notes for Class 11 Chemistry Chapter 11; Topic 2
Boron Family
Group 13 of the p block is the boron family with the outer electronic configuration ns2np1.
NCERT Notes for Class 11 Chemistry Chapter 11; Topic 3
Atomic radii
The atomic and ionic radii of boron family elements are comparatively smaller than that of alkali and alkaline earth metals. This is because of the effective charge increasing on moving from left to right in a period. Angel on moving down the group the atomic and ionic radii increase this is due to the addition of extra electron shell.
NCERT Notes for Class 11 Chemistry Chapter 11; Topic 4
Ionization Enthalpy
The first ionization enthalpies of boron family elements are lesser in comparison to the alkaline group 2 elements present in the same period. The reason for it is the removal of a p electron is much easier than the electron in the S orbitals. And also on moving down group 13 the first ionization enthalpy decreases this is due to the increase in atomic size.
NCERT Notes for Class 11 Chemistry Chapter 11; Topic 5
Physical Properties
Except for boron, all the other members of the boron family possess a low melting point.
Boron is a hard and black-colored non-metallic compound. While all the other members of this family are soft metals.
NCERT Notes for Class 11 Chemistry Chapter 11; Topic 6
Chemical Properties
The oxidation state of boron and Aluminium is +3. While all the other members show an oxidation state in addition to +3 also.
NCERT Notes for Class 11 Chemistry Chapter 11; Topic 7
Some of the Compounds of Boron
Boric acid: Boric acid is a white crystalline solid and causes a soft soapy touch. It has a very important application that it can be used in the manufacture of heat-resistant borosilicate glasses, and also can be used in the manufacture of enamels. The structure of boric acid is,
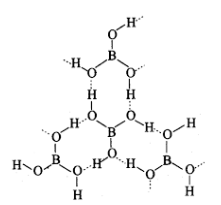
Structure of boric acid
Diborane: The compound formed between boron and hydrogen in a series is called boranes. The brain can be prepared by the reaction of boron trifluoride and Lithium aluminum hydride in the presence of diethyl ether. Diborane is a colorless compound that is highly toxic and it will spontaneously flame in the air. Borane can be easily hydrolyzed with water and it will form boric acid. The structure of diborane is,
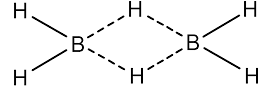
Structure of diborane
NCERT notes for Class 11 Chemistry Chapter 11; Topic 8
Carbon Family
The element present in group 14 is the carbon family and it includes, silicon, tin, and lead. And the outer electronic configuration of the carbon family is ns2np1.
Carbon: It is the 17th abundant element by weight in the earth's crust. It is present in the form of coal, graphite, and diamond and is also present in its combined state in metal carbonates hydrocarbons, and carbon dioxide gas in the air.
NCERT Notes for Class 11 Chemistry Chapter 11; Topic 9
Atomic radii
The covalent radius of group 14 elements increases in moving from carbon to silicon. And there is only a small increase in moving from silicon to lead. The reason for this is the addition of a new electronic shell in each element on moving down the group.
NCERT Notes for Class 11 Chemistry Chapter 11; Topic 10
Ionization Enthalpy
In comparison to group 13 elements, the first ionization enthalpy of group 14 is higher. The reason for this is the increased effective nuclear charge. While on moving down the group from carbon to silicon there is a decrease in first ionization enthalpy while the first ionization enthalpy of lead increases slightly than that of tin.
NCERT Notes for Class 11 Chemistry Chapter 11; Topic 11
Electronegativity
The electronegativity of group 14 elements is higher than that of group 13 elements.
NCERT Notes for Class 11 Chemistry Chapter 11; Topic 12
Physical Properties
All elements present in the carbon family are solids and their less metallic than the group 13 elements.
The melting and boiling points of group 14 elements are high.
NCERT Notes for Class 11 Chemistry Chapter 11; Topic 13
Chemical Properties
Of the element carbon and silicon show +4 oxidation state while germanium forms+4 oxidation state and fewer components also shows+2 oxidation State.
Anomalous Behavior of Carbon
In comparison to other elements of the carbon, family carbon shows some anomalous behavior. This is due to the small atomic and ionic size of carbon and also due to the higher ionization enthalpy. The higher electronegativity and also the absence of d orbitals in the valency shell is the reason behind the anomalous behavior of carbon. And there are so many allotropes of carbon too.
NCERT Notes for Class 11 Chemistry Chapter 11; Topic 14
Allotropes of Carbon
Carbon can accept any two allotropic forms that are crystalline and amorphous. The crystalline form of carbon includes graphite and fullerene.
Diamond: The hybridization of carbon in diamonds is Sp3 and it is carbon is tetrahedrally linked to 4 other carbon atoms. And the diamond is the hardest substance on earth and is so it can be used for sharpening hard tools. The structure of diamond is,
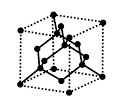
Structure of diamond
Graphite: The hybridization of carbon in graphite is Sp2 and has a two-dimensional sheet-like structure that consists of hexagonal rings fused. The layers present in graphite are held by van der Waals forces. Graphite conducts electricity and is a soft and slippery nature. It can be used as a lubricant in the machine if the oil cannot be used. The structure of graphite is,
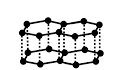
Structure of graphite
Fullerenes: it is also an allotrope of carbon and is prepared by heating graphite in an electric Arc in the presence of inert gases like helium or argon. Fullerenes cause a cage-like structure and in which C60 has a sociable shape so it is called buckminsterfullerene and is the most stable among the fullerenes. The hybridization of fullerene is Sp2. Fullerenes are soluble in organic solvent due to their covalent nature and they also form platinum complexes. The structure of fullerene is,
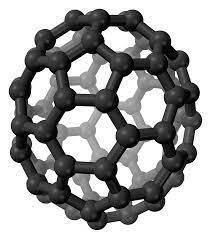
Structure of fullerene
NCERT Notes for Class 11 Chemistry Chapter 11; Topic 15
Uses of Carbon
As the black pigment in black ink carbon black is used.
As a reducing agent in metallurgy, Coke is used.
Diamond is very important and precious stone.
As Graphite is a very good conductor of electricity it can be used for making electrodes for batteries and industrial electrolysis.
The fibers of graphite are used in making sports goods.
Activated charcoal can be used for absorbing poisonous gases.
NCERT Notes for Class 11 Chemistry Chapter 11; Topic 16
Silicon Dioxide
Silicon dioxide is an industrial very important compound of silicon. Quartz, termite, and cristobalite are some of the crystalline forms of silica. Silicon dioxide is a three-dimensional network consisting of covalent bonds and each silicon atom is covalently bonded to four oxygen atoms. Quartz is an extensively used piezoelectric material and also silica Gel is used as an adsorbent in chromatography techniques. The structure of silicon dioxide is,
NCERT Notes for Class 11 Chemistry Chapter 11; Topic 17
Carbon Dioxide
It is also a very important compound of carbon and is prepared by the combustion of carbon.
C+O2→CO2
It is an odorless, colorless gas and is slightly soluble in water. When carbon dioxide is dissolved in water a compound called carbonic acid is formed and is toxic. Income bad to carbon monoxide carbon dioxide is not so poisonous. But due to the increased combustion of fossil fuels and also due to the decomposition of limestone in the cement manufacturing unit in the presence of carbon dioxide in the atmosphere goes on the increase and is the reason behind the greenhouse effect.
Significance of NCERT Notes for Class 11 Chemistry Chapter 11
The P-block elements Class 11 notes pdf download contains some important points about the elements present in the P-Block of the periodic table. This is an important topic to learn for students. The notes for Class 11 Chemistry Chapter 11 helps students in getting good marks in the examinations and also it will give proper revision for students. Class 11 Chemistry Chapter 11 notes pdf download shares some important points for NEET and JEE MAIN examinations and it will act as a revision kit for students before attempting the examination. The P-block elements Class 11 notes will also help students to process their studies even when they are offline.
NCERT Class 11 Notes Chapter-Wise
NCERT Class 11 Chemistry Chapter 11 Notes |
NCERT Class 11 Chemistry Chapter 12 Notes |
Subject Wise NCERT Exemplar Solutions
- NCERT Exemplar Class 11 Solutions
- NCERT Exemplar Class 11 Maths
- NCERT Exemplar Class 11 Physics
- NCERT Exemplar Class 11 Chemistry
- NCERT Exemplar Class 11 Biology
Subject Wise NCERT Solutions
Frequently Asked Questions (FAQs)
The p block elements properties are influenced by the atomic sizes electronegativity, electron gain enthalpy and ionization enthalpy and these topics are discussed in the notes for Class 11 Chemistry chapter 11.
Helium is the one element that is an exception to the P-block element.
Aluminum is a p block element present in group 13 of the periodic table that is the boron family. This is because the last electron enters into the outermost p orbital. About outer electronic configuration and everything is discussed in detail in the Class 11 Chemistry chapter 11 notes pdf download.
To give colored compounds there should be a transition. 4 p block elements the transitions are s-p and p-d. While these transitions require energy that is UV radiations are required so these are not possible through visible light hence they seem colorless.
As the atomic size decreases on moving from left to right along the period the effective nuclear charge also increases. Therefore p block elements possess higher ionization enthalpy than the S block elements and are also increased when we move from left to right along the period. The facts about the ionization enthalpy are explained in detail in the Class 11 the P-block elements notes.
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
