Hydrogen Class 11th Notes - Free NCERT Class 11 Chemistry Chapter 9 Notes - Download PDF
Class 11 Chemistry chapter 9 notes is focused on the topic of hydrogen and is organized in such a way that students can learn everything there is to know about the element in depth. Students will learn about hydrogen's isotopes, characteristics, reactions, periodic table position, and other essential topics by studying the CBSE Class 11 Chemistry chapter 9 notes. Hydrogen Class 11 notes are also constructed with the most recent NCERT Syllabus. Notes for class 11 chemistry chapter 9 contain all relevant information in the chapter Hydrogen in a concise manner. In addition to this, students can refer to the following links, and use them as a reference tool to finish their revisions on time, prepare effectively, and get better results on final exams.
This Story also Contains
- NCERT Class 11 Chemistry Chapter 9 Notes
- Position of Hydrogen in The Periodic Table
- NCERT Class 11 Chemistry Chapter 9 Notes: Topic 2
- Dihydrogen
- NCERT Class 11 Chemistry Chapter 9 Notes: Topic 3
- Preparation of Dihydrogen
- NCERT Class 11 Chemistry Chapter 9 Notes: Topic 4
- Properties of Dihydrogen
- NCERT Class 11 Chemistry Chapter 9 Notes: Topic 5
- Hydrides
- NCERT Class 11 Chemistry Chapter 9 Notes: Topic 6
- Water
- NCERT Class 11 Chemistry Chapter 8 Notes: Topic 7
- Hardness of Water
- NCERT Class 11 Chemistry Chapter 9 Notes: Topic 8
- Hydrogen Peroxide (H2O2)
- NCERT Class 11 Chemistry Chapter 9 Notes: Topic 9
- Heavy Water (D2O)
- NCERT Class 11 Chemistry Chapter 9 Notes: Topic 10
- Dihydrogen as a Fuel
- NCERT Class 11 Notes Chapter-Wise
Also, students can refer,
NCERT Class 11 Chemistry Chapter 9 Notes
NCERT Class 11 Chemistry Chapter 9 Notes: Topic 1
Position of Hydrogen in The Periodic Table
Hydrogen is found on Earth in the form of the H2 molecule (dihydrogen). It accounts for 10% of the universe's mass. Many compounds, such as carbohydrates and hydrocarbons, release hydrogen. It is the third most abundant element on the planet.
Hydrogen has similarities with alkali metals and halogens.
Similarities with alkali metals
The number of valence electrons is 1
Electropositive character
Oxidation state =+1
Powerful reducing agent.
Similarities with halogens
The ionization energy of hydrogen is remarkably similar to that of halogens.
Hydrogen and halogens exist as diatomic.
Covalent nature exists in many hydrogen and halogen compounds.
Despite the fact that hydrogen shares some similarities with alkali metals and halogens, it is different from them too. As a result, it has an unique behaviour and placed separately in the periodic table.
NCERT Class 11 Chemistry Chapter 9 Notes: Topic 2
Dihydrogen
Occurrence
Dihydrogen is the most abundant element in the universe(70%). It can be found in a variety of forms, including water, coal, animal, and vegetable materials. Hydrogen is an important component of all organic molecules.
Isotopes
Hydrogen has three isotopes
Protium 1H1
1 electron, 1 proton and no neutrons
Deuterium 1H2
1 electron, 1 proton and 1 neutron
Tritium 1H3
1 electron, 1 proton and 2 neutrons. It is radioactive.
NCERT Class 11 Chemistry Chapter 9 Notes: Topic 3
Preparation of Dihydrogen
Laboratory preparation
It is made in laboratories by mixing powdered zinc with dilute hydrochloric acid.
Zn + 2H+ → Zn2+ + H2
Zn+2 NaOH → Na2ZnO2+H2
Electrolysis of water using platinum electrodes
2H2O Electrolysis→ 2H2+O2
Electrolysis of warm aqueous barium hydroxide solution between nickel electrodes.
Electrolysis of brine solution
At anode: 2Cl- → Cl2+2e-
At cathode: 2H2O + 2e- → H2 + 2OH-
Reaction of steam on hydrocarbons
CnH2n+1 + nH2O Ni,1270 Kn →CO+(2n+1)H2
Mixture of CO and H2 is called water gas or syngas. Production of sync gas from coal is called coal gasification.
C+H2ONi,1270 →KCO+H2
Water gas shift reaction
CO+H2O 673 K,Iron chromate→ CO2+H2
NCERT Class 11 Chemistry Chapter 9 Notes: Topic 4
Properties of Dihydrogen
Physical properties
Dihydrogen is a gas that is colourless, odourless, and tasteless.
It's a flammable gas.
It is insoluble in water.
It has a lower density than air
Chemical properties
Reaction with halogens
H2+X2 →2 HX
Reaction with dinitrogen
2H2+O2→ 2H2O
Reaction with metal
3H2+N2673 K,200 atm ,Fe →2NH3
Reactions with metal ions and metal oxides
H2+Pd2+ → Pd+2H+
yH2+MxOy →xM+y H2O
Reactions with organic compounds
H2+CO+RCH=CH2 → RCH2CH2CHO
H2+ RCH2CH2CHO → RCH2CH2CH2OH
NCERT Class 11 Chemistry Chapter 9 Notes: Topic 5
Hydrides
Dihydrogen reacts with a variety of elements to form binary compounds known as hydrides.
Ionic hydrides
They are formed when high-reactivity metals of group 1 and group 2 combine with hydrogen. Lithium, Beryllium, and Magnesium hydrides have the maximum covalent nature of all. They are crystalline in structure and have a high melting and boiling point. They have a higher density than metals. They conduct electricity and release H2 gas when melted.
Covalent hydrides
They're mostly made up of p-block elements and a few s-block elements, which have a smaller electronegativity difference than hydrogen. They are binary compounds with covalent bond.They are of 3 types
Electron deficient
They have fewer electrons than the octet. Eg:B2H6
Electron precise
They have a sufficient number of electrons. Eg:CH4
Electron rich
They have additional electrons than the octet. Eg: NH3
Molecular /non-stochiometric/interstitial hydrides
Molecular hydrides are produced when hydrogen reacts with the d and f elements in this reaction. Groups 6,7,8,9 and 3,4,5,10,11,12 do not produce hydrides, whereas group 3,4,5,10,11,12 can. This is referred to as the Hydride Gap (the inability of these groups to form hydrides is called Hydride gap). They don't have enough hydrogen. Non-stochiometric hydrides are another name for them. Interstitial spaces between these atoms are occupied by hydrogen.
NCERT Class 11 Chemistry Chapter 9 Notes: Topic 6
Water
Water makes up roughly 65 percent of the human body, while it makes up nearly 95 percent of some plants.
Physical properties
Water makes up roughly 65 percent of the human body, while it makes up nearly 95 percent of some plants.
At 4°C, the maximum density of water is 1 gm cm-3
It is a tasteless, colourless liquid.
Even covalent substances like alcohol and carbohydrates dissolve in water due to hydrogen bonding with polar molecules.
Structure of water
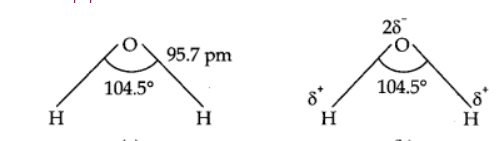
It is a bent molecule in gas phase, with a HOH bond angle of 104.5° and an O—H bond length of 95.7 pm. It has polar nature.
Structure of ice
The crystalline form of water is ice. Ice crystallizes hexagonally at atmospheric pressure. It condenses to cubic shape at low temperatures. Ice is lighter than water and has a lower density. As a result, ice cubes float in water.
Chemical properties
Amphoteric nature
H2O+NH3 ↔ OH- + NH4+
H2O+H2S ↔ H3O+ + HS-
Oxidizing and reducing nature
2H2O+2Na → 2NaOH+H2
6CO2+12H2O → C6H12O6+6H2O+6O2
2F2+2H2O → 4H++ 4F-+O2
Hydrating tendency
P4H10+6H2O → 4H3PO4
SiCl4+2H2O → SiO2+4HCl
N3-+3H2O → NH3+3OH-
Hydrates formation
Coordinated water: [Cr(H2O)6]3+ 3Cl-
Interstitial water: BaCl2.2H2O
Hydrogen bonded water:[Cu(H2O)2+ SO42-.H2O
NCERT Class 11 Chemistry Chapter 8 Notes: Topic 7
Hardness of Water
The presence of calcium and magnesium (Ca2+ and Mg2+) salts in water causes this. The water containing these salts is called hard water. Magnesium bicarbonates, sulphates, chlorides, calcium bicarbonates, sulphates, and chlorides are the most common salts Calcium and magnesium salts in hard water react with soap to generate an insoluble precipitate known as scum. It sticks to the fabric.
2C17H35COONa+M2+ → (C17H35COO)2M ↓+2 Na
Temporary hardness
It is caused by the presence of calcium and magnesium bicarbonates in the water. It's called temporary since it's easy to get rid of by simply boiling hard water. It can be removed by
Boiling
Mg(HCO3)2 ∆→ Mg(OH)2↓+2CO2
Ca(HCO3)2 ∆→ Ca(CO)3↓+H2O+CO2
Clark’s method
Ca(HCO3)2+Ca(OH)2 → 2 Ca(CO)3↓+2H2O
Mg(HCO3)2+2Ca(OH)2 → 2 Ca(CO)3↓+Mg(OH)2↓+2H2O
Permanent hardness
It is caused by the presence of calcium and magnesium chlorides and sulphates.Chemical treatments can be used to remove permanent hardness from water.
Treatment with washing soda
MCl2+Na2CO3 MCO3↓+2NaCl
MSO4+Na2CO3 MCO3↓+2Na2 SO4
Calgon’s method
Na2P6O18 → 2Na++Na4P6O182-
M2++Na4P6O182- → [Na2MP6O18]2-+2Na+
Ion exchange method(Zeolite/permutit process)
2NaZ+M2+ MZ2+ 2Na+
MZ2+2NaCl → 2NaZ+MCl2
NaZ-NaAlSiO4(Zeolite)
Synthetic resins method
Synthetic resin methods outperform ion exchange methods because they remove all forms of cations and anions, leaving only distilled water.
Cation exchange resin: It is made up of a massive hydrocarbon structure connected to fundamental groups. They are represented by the R-COOH or R-SO3H general formula. This R stands for large hydrocarbon. These resins have the ability to exchange H+ions with cations found in hard water.
Anion exchange resin: An ion exchange resin is made comprised of a large hydrocarbon matrix that is connected to basic groups such as OH-ions, commonly in the form of substituted ammonium hydroxides. They are denoted by the letters R-NH3OH, where R stands for large hydrocarbon framework. These resins may exchange hydroxide ions with anions found in hard water, such as chloride ions and sulphate ions.
NCERT Class 11 Chemistry Chapter 9 Notes: Topic 8
Hydrogen Peroxide (H2O2)
Preparation
BaO2.8H2O+H2SO4 → BaSO4+H2O2+8H2O
2HSO4- Electrolysis→ HO3SOOSO3 Hydrolysis→ 2HSO4-+2H++H2O2
2-ethylanthraquinol O2→ H2O2
Physical properties
It is a clear liquid with no colour.
It is miscible in all quantities with water and produces the hydrate H2O2. H2O
Structure
H2O2 has a nonpolar structure.
Chemical properties
Oxidation reactions in acidic medium
2Fe2++2H++H2O2 → 2Fe3++2H2O
PbS+4H2O2 → PbSO4+4H2O
Oxidation reactions in basic medium
2Fe2++H2O2 → 2Fe3++2OH-
Mn2++H2O2 → Mn3++2OH-
Reduction reactions in acidic medium
2MnO4-+6H++5H2O2 → 2Mn2++8H2O+5O2
HOCl+H2O2 → H3O++Cl-+O2
Reduction reactions in basic medium
I2+H2O2+2OH- → 2I-+2H2O+O2
2MnO4-+3H2O2 2MnO2+3O2+2H2O+2OH-
NCERT Class 11 Chemistry Chapter 9 Notes: Topic 9
Heavy Water (D2O)
It is produced via electrolysis of water over a long period of time. It is employed in nuclear reactors as a moderator. It's used to investigate reaction mechanisms through exchange reactions. Heavy water is used for the preparation of other dueterium compounds.
CaC2+2D2O C2 →D2+Ca(OD)2
SO3+D2O → D2SO4
Al2C3+12D2O → 3CD4+4Al(OD)3
NCERT Class 11 Chemistry Chapter 9 Notes: Topic 10
Dihydrogen as a Fuel
The conveyance and storage of energy in the form of liquid or gaseous dihydrogen is the core premise of the hydrogen economy. The fact that energy is transmitted in the form of dihydrogen rather than electric power is an advantage. As a fuel, it has the following advantages: - It can be used in fuel cells to generate electricity. One of the key benefits of hydrogen combustion is that it produces very little pollution and does not emit any unburnt carbon particles in the form of smoke. The study shows that dihydrogen, both in gaseous and liquefied form, releases more energy when burned than other regularly used fuels. CNG is blended with 5% dihydrogen for use in four-wheeler vehicles.
Significance of NCERT Class 11 Chemistry Chapter 9 Notes
In Chemistry, hydrogen is a significant topic. It is appropriate for real-time applications and uses in our everyday life. Students can improve their final test scores by studying this Hydrogen Class 11 notes and learning about its real-world uses and applications, as well as how to employ it in real-time situations. Students can use the review Hydrogen Class 11 notes to gain a better understanding of the material and to answer the questions in the board exam as well as other competitive exams including NEET and JEE MAIN. Students can use Class 11 Chemistry chapter 9 notes pdf download for learning in offline mode.
NCERT Class 11 Notes Chapter-Wise
NCERT Class 11 Chemistry Chapter 9 Notes |
NCERT Class 11 Chemistry Chapter 11 Notes |
NCERT Class 11 Chemistry Chapter 12 Notes |
Subject Wise NCERT Exemplar Solutions
- NCERT Exemplar Class 11 Solutions
- NCERT Exemplar Class 11 Maths
- NCERT Exemplar Class 11 Physics
- NCERT Exemplar Class 11 Chemistry
- NCERT Exemplar Class 11 Biology
Subject Wise NCERT Solutions
Frequently Asked Questions (FAQs)
Ans: Class 11 Chemistry chapter 9 notes mention physical properties of hydrogen. Hydrogen is a colourless, odourless, and tasteless gas. It’s vapour density is one, making it lighter than air. In addition, it is non-poisonous. Hydrogen has relatively poor solubility in water.
Ans-. Due to its high calorific value, it's employed as a rocket fuel. Welding with an oxygen-hydrogen flame is possible. It's also used to make nitric acid, ammonia, hydrochloric acid and other compounds. It is also used as a reducing agent.
Ans- Hard water contain many minerals and when this water is combined with soap, no lather is produced. It contains Calcium and magnesium ions. But when soap is mixed with soft water, lather forms. Soft water contains sodium ions.
Ans- Water molecules are extremely polar in nature. A considerable amount of hydration energy is released when ionic compounds are combined in water. This energy is higher than the energy required to break the water molecules. As a result of the high hydration energy, ionic compounds solvate in water.
Ans- Hydrogen compounds of metals and nonmetals are known as hydrides.
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
