NCERT Solutions for Class 11 Chemistry Chapter 9 Hydrogen
NCERT Solutions for Class 11 Chemistry Chapter 9 Hydrogen- Hydrogen is the simplest and most abundant element in our universe. In the NCERT solutions for class 11 chemistry chapter 9 hydrogen, we will learn about hydrogen element, its occurrence, its position in the periodic table, dihydrogen, preparation and properties of dihydrogen, hydrides etc. And also discuss the isotopes of hydrogen, how they are prepared and its properties. NCERT book class 11 chemistry chapter 9 is an important chapter for class 11 students because many questions asked in CBSE class 11 final examination and competitive exams.
Also Read :
- NCERT Exemplar Solutions For Class 11 Chemistry Chapter 9 Hydrogen
- Hydrogen Class 11 Chemistry Chapter Notes
In this chapter, there are 36 questions in the exercise. The NCERT Solutions for Class 11 Chemistry Chapter 9 Hydrogen are designed by our chemistry experts. These NCERT solutions help students in the preparation of their class 11 final examination as well as in the various competitive exams like JEE Mains, NEET, etc. If you are looking for the answers of any other class from 6-12 then NCERT solutions are there for you as it's the easiest way to get all the solutions of NCERT. By referring to the NCERT solutions for class 11 , students can understand all the important concepts and practice questions well enough before their examination
NCERT Solutions for Class 11 Chemistry Chapter 9 Hydrogen- Exercise Questions
Question 9.1 Justify the position of hydrogen in the periodic table on the basis of its electronic configuration
Answer :
Answer-The electronic configuration of the H atom is $1s^{1}$ . Due to the presence of only one electron, it exhibits dual behaviour,i.e., it resembles both alkali metals of group 1 and also along with the halogens of group 17.
It can lose one electron to form a unipositive ion. Both H and alkali metals form monovalent cations by losing one electron from its outer shell. Both alkali and hydrogen shows +1 oxidation numbers.both have an affinity towards electronegative elements and readily combine with them and forms oxide, halides, and sulphides.
It has a very high ionization enthalpy and does not possess metallic characteristics under normal conditions. In fact, in terms of ionization enthalpy, hydrogen resembles more with halogens, ionization enthalpy of Li is 520 kJ mol–1, F is 1680 kJ mol–1 and that of H is 1312 kJ mol–1. Like halogens, it forms a diatomic molecule, combines with elements to form hydrides and a large number of covalent compounds.
Question 9.2 Write the names of isotopes of hydrogen. What is the mass ratio of these isotopes ?
Answer :
Isotopes of Hydrogen are-
- Protium $(H^{1}_{1})$
- Deuterium $(H^{2}_{1}/D)$
- Tritium $(H^{3}_{1}/T)$
The mass ratio is 1:2:3
Question 9.3 Why does hydrogen occur in a diatomic form rather than in a monoatomic form under normal conditions ?
Answer :
Ionisation enthalpy of hydrogen is quite high(1312 kJ/mol). Hence it is very difficult to remove its only one electron. As a result, the tendency to exist in the monoatomic form is very low.
Question 9.4 How can the production of dihydrogen, obtained from ‘coal gasification’, be increased?
Answer :
The process of producing syngas from coal is known as 'coal gasification'
$C(s)+H_{2}(g)\rightarrow CO(g)+H_{2}(g)$
The production of dihydrogen can be increased by reacting carbon monoxide of syngas mixture with the steam in the presence of iron chromate as a catalyst. This is called 'water gas shift reaction '. carbon dioxide can be removed by scrubbing with a sodium solution.
$CO(g)+H_{2}O(g)\rightarrow CO_{2}(g)+H_{2}(g)$
Answer :
The dihydrogen is prepared by electrolysis of acidified water using platinum electrodes. generally acid is sulphuric acid and base is sodium hydroxide is used.
$2H_{2}O\rightarrow 2H_{2}(g)+O_{2}(g)$
Role of electrolyte-
The electrical conductivity of pure water is very low due to the absence of ions in it. Hence electrolysis of water takes place at slow rate. If we add an electrolyte as an acid or base it will increase the rate of electrolysis. Electrolytes provide ions in this process for the better conduction of electricity.
Question 9.6 Complete the following reactions
Answer :
Answer- Complete reactions are-
$\\(i)H_{2}(g)+MmOo(s)\rightarrow mM(s)+H_{2}O(l)\\ (ii)CO(g)+H_{2}(g)\rightarrow CH_{3}OH(l)\\ (iii)C_{3}H_{8}(g)+3H_{2}O(g)\rightarrow 3CO(g)+7H_{2}(g)\\ (iv)Zn(s)+NaOH(aq)\rightarrow Na_{2}ZnO_{2}(aq)+H_{2}(g)$
Question 9.7 Discuss the consequences of high enthalpy of H–H bond in terms of chemical reactivity of dihydrogen?
Answer :
The ionization enthalpy of the H-H bond is very high (1312kJ/mol). It means hydrogen has less tendency to form $H^{+}$ ions. Its ionization enthalpy is comparable to halogens. Hence it forms a diatomic molecule, hydrides with the elements, and a large number of covalent bonds. It does not possess any metallic character like metals
Answer :
(i)Electron-deficient hydrides-
Those compounds having fewer electrons to writing its conventional Lewis structure. examples $B_{2}H_{6}$ (all the elements of group 13 form electron deficient hydrides)
(ii)Electron-precise hydrides-
Those compounds have the required number of electrons to write their conventional Lewis structure. ex- $CH_{4}$ (all elements of group 14 form such compounds)
(iii)Electron-rich hydrides-
Electron-rich hydrides have excess electrons which are present as lone pairs. elements of group 15-17 form such compounds like $NH_{3},H_{2}O$ has one lone pair and two lone pair respectively.
Answer :
Electron-deficient hydrides do not have required electrons to form a regular bond in which two electrons are shared by two atoms. e.g. $B_{2}H_{6},Al_{2}H_{6}$ etc
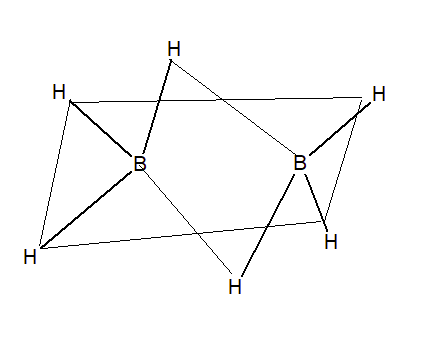
Because of electron deficiency, they have the tendency to accept electrons. hence they act as Lewis acid.
$\\B_{2}H_{6}+2NMe\rightarrow 2BH_{3}.NMe_{3}\\B_{2}H_{6}+2CO\rightarrow 2BH_{3}.CO$
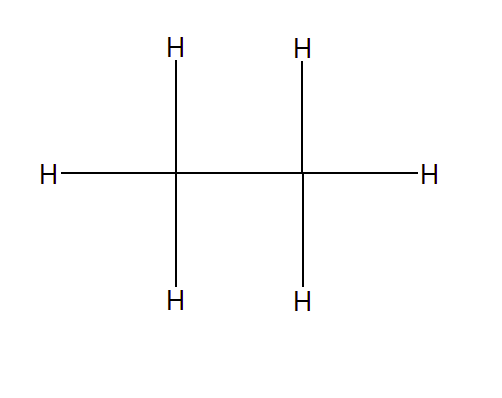
Question 9.10 Do you expect the carbon hydrides of the type (CnH2n + 2) to act as ‘Lewis’ acid or base? Justify your answer.
Answer :
the general term $C_{n}H_{2n+2}$
for n = 1, 2, 3.. we get $CH_{4},C_{2}H_{6},C_{3}H_{8}$ ...
Lewis acids are an electron acceptor, So the above compound should be electron deficient species.
$C_{2}H_{6}$ taking an example
Here we can directly count that both the Carbon atom has perfect 8 electrons in sharing. they follow the octet rule. It is electron precise hydride and it neither donate or accept electrons to act Lewis acid or base
Answer :
Non-stoichiometric hydrides are hydrogen deficient compounds. they are formed by d & f block elements. Such hydrides do not follow the law of constant composition.
Examples- $LaH_{2.87},YbH_{2.55},ZrH_{1.3-1.75}$ etc.
Alkali metals do not form these types of hydrides. Alkali metals form stoichiometric hydrides. These hydrides are ionic in nature.
Question 9.12 How do you expect the metallic hydrides to be useful for hydrogen storage? Explain
Answer :
Metallic hydrides are hydrogen deficient and they don't hold the law of constant composition.it is established that hydrides of nickel, palladium, and Ce have lattice different where hydrogen occupies the interstitial position in the lattices allowing further absorption of hydrogen on these metals. Some of the metals like Pd and Pt accommodate a very large volume of hydrogen and therefore, can be used for hydrogen storage.
Question 9.13 How does the atomic hydrogen or oxy-hydrogen torch function for cutting and welding purposes? Explain.
Answer :
Atomic hydrogen can be prepared by dissociation of a bond of dihydrogen with the help of an electric arc. This release a huge amount of energy around 135 kJ/mol and this energy can be used to generate a high temperature of 4000K, which is desirable for welding and cutting of metals. Therefore, atomic hydrogen or oxy-hydrogen torch are used for welding purpose.
Question 9.14 Among NH3, H2O and HF, which would you expect to have highest magnitude of hydrogen bonding and why?
Answer :
The strength of hydrogen bonding depends on the electronegativity of an atom
electronegativity order $N<O<F$
The expected order should be $NH_{3}<H_{2}O<HF$
But the actual order is $NH_{3}<HF<H_{2}O$
This is because of the extent of H bonding in a water molecule is more due to the presence of one extra hydrogen atom. Also, Oxygen atom forms a huge ring like structure through its high ability of hydrogen bonding
Answer :
Saline hydrides are known to react with water violently and produce fire.
$MH(s)+H_{2}O\rightarrow MOH(aq)+H_{2}(g)$ this is the general reaction formula. We see that the product is a base and hydrogen gas.
Carbon dioxide is heavier than oxygen. It is used in fire extinguisher because it cuts the supply of oxygen by acting as a blanket on fire.
Carbon dioxide can be used in nowadays and it is heavier than dihydrogen also so that it will isolate the burning surface from $H_{2}$ and $O_{2}$
Question 9.16(i) Arrange the following
CaH2, BeH2 and TiH2 in order of increasing electrical conductance
Answer :
increasing order of electrical conductance-
$BeH_{2}<CaH_{2}<TiH_{2}$
electrical conductivity depends on its ionic nature . more is the ionic more is the conductivity.
Berrium hydride is covalent in nature so it has least conductivity. Titanium hydride is metallic in nature so it will also conduct.
we also know that greater is the size of cation more is the ionic in nature.
Question 9.16(ii) Arrange the following
LiH, NaH and CsH in order of increasing ionic character
Answer :
the increasing order of ionic character -
$LiH<NaH<CsH$
The ionic character can be measured by the electronegativity difference between the atom. we know that down the group electronegativity decreases. Therefore, Cs has most electronegative character then Na then Li.
Question 9.16(iii) arrange the following
H–H, D–D and F–F in order of increasing bond dissociation enthalpy
Answer :
$F-F<H-H<D-D$ increasing order of bond dissociation enthalpy
In F-F high repulsion force is acting so breaking should be easy.
while in case of H-H and D-D, bond pair of D-D is more strongly attracted by the nucleus because of the higher nucleus mass in D2. we know that higher the attraction high is the bond strength.
Question 9.16(iv) Arrange the following $NaH, MgH_2,$ and $H_2O$ in order of increasing reducing property
Answer :
increasing order of reducing property-
$H_{2}O<MgH_{2}<NaH$
Na can easily donate electrons so it has a higher reducing property. Both $H_{2}OandMgH_{2}$ are covalent hydrides. water has less reducing property because it has high bond dissociation energy than $MgH_{2}$
Question 9.17 Compare the structures of H2O and H2O2.
Answer :
In the gaseous phase water is a bent molecule with a bond angle of 104.5 degree, and O-H bond length of 95.7 pm. It is a highly polar molecule.
Hydrogen peroxide has a non-planner structure both in the gas phase and solid phase. The dihedral angle in the gas phase is 115.5 degree and in the solid phase is 90.2 degree.
Question 9.18 What do you understand by the term ’auto-protolysis’ of water? What is its significance?
Answer :
It means water molecules can react with each other and form hydronium ion and hydroxide ion. (self-ionization)
$H_{2}O(l)+H_{2}O(l)\rightarrow H_{3}O^{+}(aq)+OH^{-}(aq)$
it indicates its amphoteric nature; means behave as acid as well as the base.
the Hydronium Ion - conjugate acid
the hydroxide ion - conjugate base
Question 9.19 Consider the reaction of water with F2 and suggest, in terms of oxidation and reduction, which species are oxidized/reduced.
Answer :
The reaction of fluorine with water-
$2F_{2}+2H_{2}O\rightarrow 4HF+O_{2}$
$F_{2}(0)\rightarrow H^{+1}F^{-1}$ -----------reduction (O.N of F changes from 0 to -1)
$H^{+1}_{2}O^{-2}\rightarrow O_{2}^{0}$ ------------- Oxidation( O.N of O changes from -2 to 0)
Fluorine reduced by gaining an electron and water oxidized by losing an electron.
Question 9.20(i) Complete the following chemical reactions.
$(i)PbS(s)+H_{2}O_{2}(aq)\rightarrow$
Classify the above into (a) hydrolysis, (b) redox and (c) hydration reactions
Answer :
$(i)PbS(s)+H_{2}O_{2}(aq)\rightarrow PbSO_{4}(s)+H_{2}O(l)$
Here hydrogen peroxide oxidizes the lead sulfide, act as an oxidising agent. Hence it is a redox reaction.
Question 9.20(ii) Complete the following chemical reactions.
$MnO_{4}^{-}(aq)+H_{2}O_{2}(aq)\rightarrow$
Classify the above into (a) hydrolysis, (b) redox and (c) hydration reactions.
Answer :
Reaction is- $MnO_{4}^{-}(aq)+H_{2}O_{2}(aq)\rightarrow 6H^{+}(aq)+Mn^{2+}(aq)+8H_{2}O(l)+5O_{2}(g)$
Here hydrogen peroxide reduces the $MnO_{4}^{-}$ into $Mn^{2+}$ act as reducing agent. Hence it's a redox reaction.
Question 9.20(iii) Complete the following chemical reactions.
$CaO(s)+H_{2}O(g)\rightarrow$
Classify the above into (a) hydrolysis, (b) redox and (c) hydration reactions
Answer :
The reaction is - $CaO(s)+H_{2}O(g)\rightarrow Ca(OH)_{2}(aq)$
it is a hydrolysis reaction because we know that the reaction in which water reacts with water to produce another compound is hydrolysis.
Question 9.20 (iv) Complete the following chemical reactions
$AlCl_{3}(g)+H_{2}O(l)\rightarrow$
Classify the above into (a) hydrolysis, (b) redox and (c) hydration reactions.
Answer :
The complete reaction is -
$AlCl_{3}(g)+H_{2}O(l)\rightarrow Al_{2}O_{3}(s)+6HCl(aq)$
The given reaction is hydrolysis reaction.
Question 9.20(v) Complete the following chemical reactions
$Ca_{3}N_{2}(s)+H_{2}O(l)\rightarrow$
Classify the above into (a) hydrolysis, (b) redox and (c) hydration reactions
Answer :
the complete reaction is -
$Ca_{3}N_{2}(s)+H_{2}O(l)\rightarrow 3Ca(OH)_{2}(aq)+2NH_{3}(g)$
The reaction in which water reacts with water and produce other compound are called hydrolysisreaction.
Question 9.21 Describe the structure of the common form of ice.
Answer :
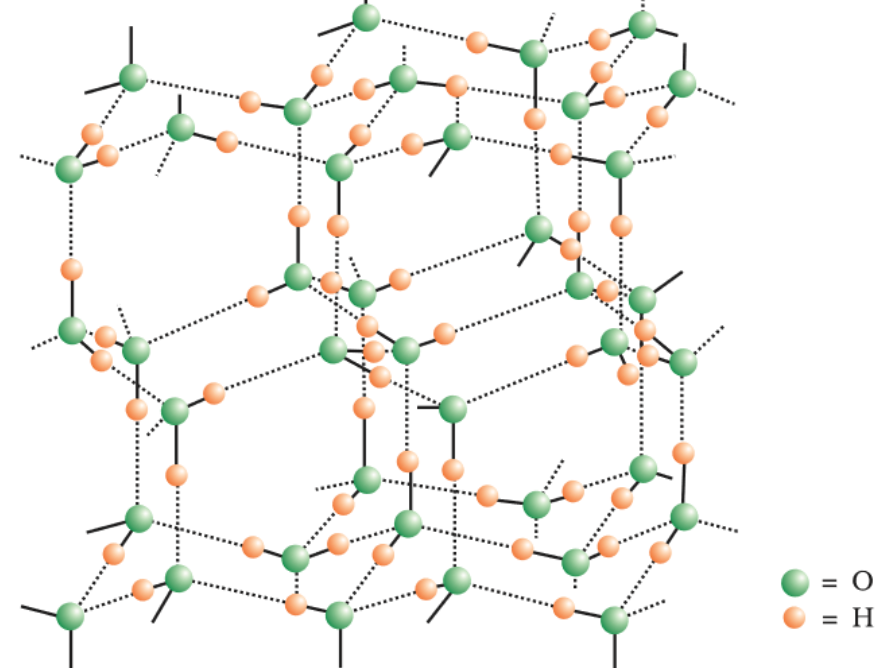
Ice is a crystalline form of water. At atmospheric pressure, ice crystallizes in the hexagonal form, but at low temperature, it condenses to cubic form. The density of ice is less than water; therefore it floats on the water surface. Ice has a highly ordered three-dimensional hydrogen-bonded structure. (shown above) Each Oxygen atom is surrounded by four H atom tetrahedrally.
Question 9.22 What causes the temporary and permanent hardness of water ?
Answer :
Answer- Temporary hardness of water is because of the presence of salts of magnesium and calcium hydrogen carbonates. $(MHCO_{3})$ [ M = Mg,Ca]. Permanent hardness is due to the presence of soluble salts of magnesium and calcium in the form of chlorids and sulphates in water. e.g, $MCl_{2}/MSO_{4}$
[M = Mg and Ca]
Question 9.23 Discuss the principle and method of softening of hard water by synthetic ionexchange resins.
Answer :
The treatment process of permanent hard water using synthetic resin is based on cations exchange $(Ca^{2+},Mg^{2+},Na^{+})$ and anion exchange $((Cl^{-},SO_{4}^{2-},HCO_{3}^{-})$ ;present in water by $H^{+}$ ions and $OH^{-}$ ions.
There are two types-
(i) Cation exchange resins
(ii)anion exchange resins
Cation exchange resin is large organic molecules contain $-SO_{3}H$ group and are water-insoluble. Ion exchange resin (RSO3H) is changed to RNa by treating it with NaCl. The resin exchanges $Na^{+}$ ions with $Ca^{2+}$ and $Mg^{2+}$ ions present in hard water to make the water soft.
$2RNa(s)+M^{2+}(aq)\rightarrow R_{2}M(s)+2Na^{+}(aq)$
There are cation exchange resins in H + form. The resins exchange H + ions for Na + , Ca 2+ , and Mg 2+ ions.
$2RH+M^{2+}\rightleftharpoons MR_{2}+2H^{+}$
Anion exchange resins exchanges $OH^{-}$ ions for anions like $((Cl^{-},SO_{4}^{2-},HCO_{3}^{-})$
$\\RNH_{2}+H_{2}O\rightleftharpoons RNH_{3}^{+}.OH(s)\\RNH_{3}^{+}.OH^{-}+X^{-}\rightleftharpoons RNH_{3}^{+}.X^{-}+OH^{-}$
Water first passes through the cation exchange process. The water obtained is acidic in nature and this acidic water then passed through anion exchange process where $OH^{-}$ ions neutralize the H + ions
Question 9.24 Write chemical reactions to show the amphoteric nature of water.
Answer :
It has the ability to act as an acid as well as a base. In the Bronsted sense, it acts as an acid with ammonia and a base with hydrogen sulphide.
Reactions to show the amphoteric nature of water -
$H_{2}O(l)+NH_{3}(aq)\rightleftharpoons OH^{-}(aq)+NH_{4+}(aq)$
$\\H_{2}O(l)+NH_{3}(aq)\rightleftharpoons OH^{-}(aq)+NH_{4}^{+}(aq)\\ H_{2}O(l)+H_{2}S(aq)\rightleftharpoons H_{3}O^{+}(aq)+HS^{-}(aq)$
Question 9.26 What is meant by ‘demineralised’ water and how can it be obtained?
Answer :
Demineralized water means free from all types of cations and anions and also soluble mineral salts.
It can be obtained by passing water successively through a cation exchange (in the $H^{+}$ ion form) and anion exchange (in the form of $OH^{-}$ ion) resins:
Cation exchange process-
$2RH(s)+M^{2+}\rightleftharpoons MR_{2}(s)+2H^{+}(aq)$ { exchange for $Na^{+}, Ca^{2+},Mg{2+}$ and other cation present in water.}
Anion exchange process-
$OH^{-}$ exchanges for anions like $Cl^{-},HCO_{3}^{-},SO_{4}^{2-}$ etc present in the water $\\RNH_{2}(s)+H_{2}O(l)\rightleftharpoons RNH_{3}^{+}.OH^{-}(s)\\RNH_{3}^{+}.OH^{-}(s)+X^{-}(aq)\rightleftharpoons RNH_{3}^{+}X^{-}(s)+OH^{-}(aq)$
$OH^{-}$ ions liberated in anion exchange neutralizes the $H^{+}$ ions liberated in cation exchange, thereby forming water
Question 9.27 Is demineralized or distilled water useful for drinking purposes? If not, how can it be made useful?
Answer :
Water contains several minerals that are necessary or required by human beings, plants, animals for survival. And we know that demineralized water is free from all these minerals. So it is not useful for drinking purpose.
It can be made useful by adding desired minerals in a certain amount which are favourable for growth.
Question 9.28 Describe the usefulness of water in biosphere and biological systems.
Answer :
Water is essential for all living beings. It plays an important role in the biosphere. its has high specific heat, thermal conductivity, surface tension, dipole moment, and dielectric constant.
The high heat of vaporisation and heat capacity are responsible for the moderation of the climate and body temperature of living beings. It is an excellent solvent for transportation of ions and molecules required for plant and animal metabolism.
Question 9.29 What properties of water make it useful as a solvent? What types of a compound can it (i) dissolve, and (ii) hydrolyze?
Answer :
Water has high dielectric constant ( $78.4 C^{2}/Nm^{2}$ ) and dipole moment which makes it universal solvent. It dissolves the most ionic as well as covalent compounds. [ionic compound because of ion-dipole interaction and covalent due to their tendency to form a hydrogen bond with water.] Water is also able to hydrolyze the metallic and non-metallic oxides, hydrides and nitrides, etc.
Question 9.30 Knowing the properties of H2O and D2O, do you think that D2O can be used for drinking purposes?
Answer :
$D_{2}O$ is heavy water, it acts as a moderator which slow down the rate of the chemical reaction. Due to this property, we cannot use it for drinking purpose. if we use, it will slow down our metabolic reaction happens in the body.
Question 9.31 What is the difference between the terms ‘hydrolysis’ and ‘hydration’ ?
Answer :
Hydrolysis - it means the reaction of the water molecule $(H^{+}/OH^{-})$ with another compound and forming a new product. for example.
$KH +H_{2}O\rightarrow KOH+H_{2}$
Hydration - It means the addition of one or more than one water molecule to form a hydrated compound.
ex. $CuSO_{4}+5H_{2}O\rightarrow CuSO_{4}.5H_{2}O$
Question 9.32 How can saline hydrides remove traces of water from organic compounds?
Answer :
The saline hydrides react with water to form metal hydroxide and liberate hydrogen gas.
reaction- $MH+H_{2}O\rightarrow MOH+H_{2}$ [M = Na, K, Ca...]
when added to the organic compounds they form metal hydroxide along with the liberation of hydrogen gas (escape into the atmosphere) and leave behind only hydroxide part.
Answer :
Atomic Number 15 19 23 and 44 are phosphorus, potassium, vanadium, and ruthenium respectively.
hydrides of these elements are
(i) $PH_{3}$ - its an electron rich species also a covalent molecule.
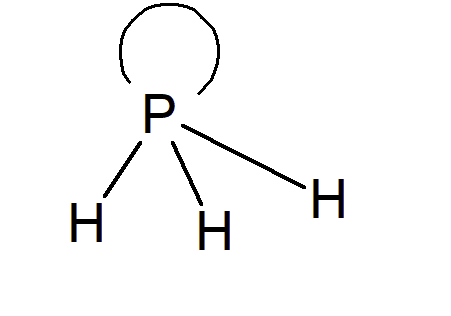 a lone pair of electron.
a lone pair of electron.
(ii)Hydrides of potassium- dihydrogen forms an ionic hydride. It is crystalline and non-volatile in nature
(iii) hydrides of vanadium and ruthenium- Both elements belong to d- block. They form metallic and non-stoichiometric hydrides. They are metallic in nature.
Behavior towards water-
- D block element does not react with water
- phosphorus reacts with water (behave as Lewis base and accept $H^{+}$ and form $PH_{4}^{+}$ )
- potassium react with water and form KOH and liberate hydrogen gas
Answer :
- KCl is salt of KOH(strong base) and HCl(strong acid). So it does not hydrolyze in normal water because it is neutral in nature. It just simply ionizes in water.
$KCl\rightarrow K^{+}+Cl^{-}$ In acidified and alkaline water they do not dissociate into ions and remains the same.
- $AlCl_{3}$ is the salt of a weak base $Al(OH)_{3}$ and HCl( strong acid).So it hydrolyzes in normal water.
$AlCl_{3}+H_{2}O\rightarrow Al(OH)_{3}+HCl$
In acidic water, there are $H^{+}$ ions which react with $Al(OH)_{3}$ and form water molecule and give $Al^{3+}$ ions. In alkaline water, $OH^{-}$ ions react with $Al(OH)_{3}$ and form $[Al(OH)_{4}]^{-}$ and water molecules.
$Al(OH)_{3}+OH^{-}\rightarrow [Al(OH)_{4}]^{-}+H_{2}O$
Question 9.35 How does H2O2 behave as a bleaching agent?
Answer :
Hydrogen peroxide $(H_{2}O_{2})$ act as an oxidizing agent in basic as well as the acidic medium. In the exposure of light, it decomposes and gives nascent oxygen which combines with coloring matter which in turn gets oxidized. So the bleaching action of $(H_{2}O_{2})$ is mainly due to the oxidation of coloring matter.
Answer :
(i) Hydrogen Economy - Technique of using dihydrogen in an efficient way. The basic principle of the hydrogen economy is the transportation and storage of energy in the form of liquid or gaseous dihydrogen.
(ii) Hydrogenation- The addition of dihydrogen to the reactant. This process can be done with the help of a suitable catalyst like nickel and palladium.
(iii) Syngas- The mixture of $CO\&H_{2}$ . This mixture of carbon monoxide and dihydrogen is used for the synthesis of methanol and a number of hydrocarbons, its called syngas, water gas or synthesis gas.
(iv) Water gas shift reaction- To increase the production of dihydrogen, by reacting with carbon monoxide of syngas mixture with steam in the presence of iron chromate as a catalyst. $CO(g)+H_{2}O(g)\rightarrow H_{2}+CO_{2}$ This is called water gas shift reaction.
(v) Fuel-cell- These are the devices that produce electrical energy from the liquid fuel with the help of suitable electrolytes.dihdrogen can be used in these cells to produce electrical energy. It is eco-friendly in nature.
Hydrogen is the most abundant element in our universe (more than 80% of the universe) and also the 3rd most abundant element after oxygen and silicon on the surface of the globe. Therefore it is important to know all about the hydrogen element for a chemistry student. Hydrogen is being visualized as the major future source of energy. After completing NCERT solutions for Class 11 Chemistry Chapter 9 Hydrogen students will be able to explain the position of hydrogen in the periodic table; describe isotopes of hydrogen; able to explain how different elements combine with hydrogen to form, molecular, ionic and non-stoichiometric compounds; understand the structure of water, hydrogen peroxide, etc.
Hydrogen Class 11 Chemistry Chapter 9: Points To Remember-
1. Hydrogen is the 1st element in the periodic table. Its electronic configuration is $1s^1$ . Its electronic configuration is similar to the electronic configuration of two groups which are alkali metals $(ns^1)$ and halogens $(ns^2np^5)$ .
2. Hydrogen shows dual nature because it behaves as electropositive like alkali metals and electronegative like halogens.
3. Hydrogen exists in three isotopes which are $_{1}^{1}\textrm{H}$ (protium), $_{1}^{2}\textrm{H}$ or D (deuterium), and $_{1}^{3}\textrm{H}$ or T (tritium).
4. Three main sources from which dihydrogen may be prepared are (i) Water, (ii) Acids and (iii) Alkalies.
5. Dihydrogen is an odourless, colourless, and tasteless gas.
Hydrogen Class 11 Chemistry NCERT Syllabus Topics
9.1 Position of Hydrogen in the Periodic Table
9.2 Dihydrogen, $H_2$
9.3 Preparation of Dihydrogen, $H_2$
9.4 Properties of Dihydrogen
9.5 Hydrides
9.6 Water
9.7 Hydrogen Peroxide $(H_2O_2)$
9.8 Heavy Water, $D_2O$
9.9 Dihydrogen as a Fuel
NCERT Solutions for Class 11 Chemistry
Chapter 1 | |
Chapter-2 | |
Chapter-3 | |
Chapter-4 | |
Chapter-5 | |
Chapter-6 | |
Chapter-7 | |
Chapter-8 | |
Chapter-9 | Hydrogen |
Chapter-10 | |
Chapter-11 | |
Chapter-12 | |
Chapter-13 | |
Chapter-14 |
NCERT Solutions for Class 11 Subject Wise
- NCERT Solutions for Class 11 Biology
- NCERT Solutions for Class 11 Maths
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 11 Physics
Benefits of NCERT Solutions for Class 11 Chemistry Chapter 9 Hydrogen
- The solutions are written in a comprehensive manner in the NCERT solutions for class 11 chemistry chapter 9 Hydrogen will help you writing answers in your exam.
- Revision will be easy because the detailed solutions will help you to remember the concepts and get you good marks.
- Homework problems will be easier for you, all you need to do is check the detailed NCERT solutions for class 11 chemistry and you are ready to go.
If you have a doubt or question that is not available here or in any of the chapters, contact us. You will get all the answers that will help you score well in your exams.
Also Check NCERT Books and NCERT Syllabus here:
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
