NCERT Solutions for Class 11 Chemistry Chapter 10 The s-Block Elements
NCERT Solutions for Class 11 Chemistry Chapter 10 The s-Block Elements- In this chapter, we discuss the s-block elements of the periodic table, which include Group 1 (alkali metals) and Group 2 (alkaline earth metals). These elements are characterized by the presence of their outermost electron(s) in the s-subshell (or s-orbital). The ns energy subshell can accommodate a maximum of two electrons, influencing the chemical reactivity and properties of these elements.
This Story also Contains
- More About The s Block Elements Class 11 NCERT Chapter
- NCERT Solutions for Class 11 Chemistry Chapter Wise
- NCERT Solutions for Class 11 Subject Wise
To strengthen conceptual understanding, this NCERT chapter includes 32 exercise questions, allowing students to practice and apply their knowledge effectively. Mastering these concepts is essential for both board exams and competitive exams like JEE, NEET, and other entrance tests.
Also Read :
- The s Block Class 11 Chemistry Element NCERT Exemplar Solutions
- The s Block Class 11 Chemistry Chapter Notes
You will get all the NCERT solutions from class 6 to 12 by clicking here. By referring to the NCERT solutions for class 11 , students can understand all the important concepts and practice questions well enough before their examination
NCERT Solutions for Class 11 Chemistry Chapter 10 The s-Block Elements - Exercise Questions
Question 10.1 What are the common physical and chemical features of alkali metals ?
Answer :
Physical and chemical features of alkali metals-
Physical features-
- All the alkali metals are silvery white, soft and light metals. Because of the large size, these elements have a low density which increases down the group from Li to Cs. However, potassium is lighter than sodium .
- The melting and boiling points of the alkali metals are low indicating weak metallic bonding due to the presence of only a single valence electron in them.
- The alkali metals and their salts impart characteristic colour to an oxidizing flame.
Chemical features-
- Due to low ionisation enthalpy, they are highly reactive. As we move down the group reactive nature increases.
- The alkali metals tarnish in dry air due to the formation of their oxides which in turn react with moisture to form hydroxides. They burn vigorously in oxygen forming oxides. Lithium forms monoxide, sodium forms peroxide, the other metals form superoxides. The superoxide $O^{2-}$ ion is stable only in the presence of large cations such as K, Rb, Cs.
- The alkali metals react with water to form hydroxide and dihydrogen.
Question 10.2 Discuss the general characteristics and gradation in properties of alkaline earth metals
Answer :
The general characteristics and gradation in properties of alkaline earth metals-
- General electronic configuration is [noble gas] $ns^{2}$ .
- Have two electrons to lose and attain their nearest noble gas configuration.
- have smaller atomic and ionic radii than corresponding alkali metals in the same periods.
- First IE is higher than those elements of group 1
- electropositive nature increase down the group from Be to Ba.
- they give a positive flame test by imparting different colours.(except Be and Mg)
Chemical properties-
- Be and Mg are inert to oxygen and water. this is because of the formation of a thin layer of oxide on their surface.(powdered Be give BeO and $Be_{3}N_{2}$ .
- React with halogens at high temperature to form halogen halides. $[M+X_{2}\rightarrow MX_{2}]$ here M = alkaline metals and X = halogen elements
- All alkaline metals react with hydrogen and form hydrides except for Beryllium
- React with acids to form salts and produce dihydrogen gas. $[M+2HCl\rightarrow MCl_{2}+H_{2}(g)]$
- Act as a reducing agent but not as powerful as alkali metals. Down the group reducing power decreases.
- Also, dissolve in liquid ammonia and give the deep blue coloured solution. $M + (x+y)NH_{3}\rightarrow [M(NH_{3})_{x}]^{2+}+2[e(NH_{3})_{y}]^{-}$
Question 10.3 Why are alkali metals not found in nature ?
Answer :
Electronic Configuration All the alkali metals have one valence electron, $ns^{1}$ outside the noble gas core. The loosely held s electron in the outermost valence shell of these elements makes them the most electropositive metals. They readily lose an electron to give monovalent M+ ions. Hence they are never found in the free or native state in nature.
Question 10.4 Find out the oxidation state of sodium in $Na_{2}O_{2}$
Answer :
Let suppose the oxidation state of Na is to be x.
In the case of peroixide, the oxygen has -1 oxidation state.
So, now
$\\2x+2(-1)=0\\ 2x = 2\\ x=1$
Question 10.5 Explain why is sodium less reactive than potassium
Answer :
As we know that in alkali metals, as we go down the group the atomic size increases and also the effective nuclear charge decreases. So, therefore, the outermost electron of potassium is easily removed. Electronic configuration of $(K\rightarrow 1s^{2},2s^{2},2p^{6},3s^{2},3p^{6},4s^{1})$ and electronic configuration of sodium (Na= $( 1s^{2},2s^{2},2p^{1}$ ). Hence the reactivity of sodium is less than of potassium.
Answer :
(i) Ionisation enthalpy-
Alkali metals have low first IE as compare to alkaline earth metals because of having a large atomic size also after losing one electron they attain noble gas configuration. In alkaline earth metals, their first IE is higher then alkali metals because they have high effective nuclear charge and small in size as compare to group 1st element. However, they have low second IE as compare to alkali metals.
(ii) Basicity of oxides-
Alkali metal oxides are more basic than alkaline earth metal oxides. this is because alkali metals are highly electropositive which makes them highly ionic, so they readily dissociate into hydroxide ions in water than alkaline metals.
(iii) The solubility of Hydroxide-
Alkali metal hydroxide is more soluble in water than alkaline earth metals. This is because of the high lattice energy of alkaline earth metals.
Question 10.7 In what ways lithium shows similarities to magnesium in its chemical behaviour?
Answer :
Chemical similarities between lithium and magnesium-
- $Li$ and $Mg$ slowly react with water and their hydroxide and oxides are also less soluble in water. On heating, their hydroxide decomposes. Both the element form nitride $Mg_{3}N_{2}$ and $Li_{3}N$ by direct reaction with nitrogen.
- $Li_{2}O$ and $MgO$ do not give superoxide on reacting with an excess of oxygen.
- Lithium and Magnesium carbonates easily decompose into their oxides and carbon dioxide.
- Chlorides of both element soluble in ethanol.
- Both chlorides can crystallise from their aqueous solution as hydrates, $LiCl.2H_{2}O$ and $MgCl_{2}.8H_{2}O$
Question 10.8 Explain why can alkali and alkaline earth metals not be obtained by chemical reduction methods?
Answer :
Alkali and alkaline earth metals not be obtained by chemical reduction methods because to reduce their oxides we need to use a strong reducing agent, but there is no other reducing agent which is stronger than them.
Question 10.9 Why are potassium and cesium, rather than lithium used in photoelectric cells?
Answer :
It is because of the low ionisation energy of K and $Cs$ . They lose electron easily. On the other hand, Lithium is smaller in size as compared to caesium and lithium, it requires high energy to lose an electron. Therefore, lithium is not used in a photoelectric cell.
Answer :
The alkali metals dissolve in liquid ammonia giving deep blue solutions which are conducting in nature.
$M +(x+y)NH_{3}\rightarrow [M(NH_{3})_{x}]^{+}+[e(NH_{3})_y]^{-}$
The blue colour of the solution is due to the ammoniated electron which absorbs energy in the visible region of light and thus imparts blue colour to the solution. The solutions are paramagnetic.
In concentrated solution, the blue colour changes to bronze colour and becomes diamagnetic
Question 10.11 Beryllium and magnesium do not give colour to flame whereas other alkaline earth metals do so. Why ?
Answer :
When the alkaline earth metals are heated the electrons are excited to higher energy levels and when they drop back to the ground state, energy is emitted in the form of visible light. Hence the colour is observed. The electrons in beryllium and magnesium are too strongly bound to get excited by flame. Hence, these elements do not impart any colour.
Question 10.12 Discuss the various reactions that occur in the Solvay process.
Answer :
Solvay process is used to prepare sodium carbonate. Reactions occurs in solvay process are -
- $2NH_{3}+H_{2}O\rightarrow (NH_{4})_{2}CO_{3}$
- $(NH_{4})_{2}CO_{3}+H_{2}O+CO_{2}\rightarrow 2NH_{4}HCO_{3}$
- $NH_{4}HCO_{3}+NaCl\rightarrow NaHCO_{3}+NH_{4}Cl$
- $2NaHCO_{3}\rightarrow Na_{2}CO_{3}+CO_{2}+H_{2}O$
Question 10.13 Potassium carbonate cannot be prepared by Solvay process. Why ?
Answer :
Solvay process cannot be used to prepare potassium carbonate because unlike sodium bicarbonate, $KHCO_{3}$ is fairly soluble in water and does not precipitate out.
Question 10.14 Why is $Li_{2}CO_{3}$ decomposed at a lower temperature whereas $Na_{2}CO_{3}$ at higher temperature?
Answer :
As we go down the group the electropositive nature of alkali metals increases. And because of that, there is an increase of stability of carbonates. But carbonates of lithium is not stable because lithium carbonate is covalent. Lithium-ion is smaller in size so it can polarise the negative carbonate ion, leading to the formation of more stable lithium oxide. therefore, Lithium decomposes at the lower temperature while sodium carbonate decomposes at a higher temperature.
$Li_{2}CO_{3}\rightarrow LiO_{2}+CO_{2}$
Answer :
(a) Nitrates-
Alkali and alkaline earth metal nitrates are soluble in water. Down the group thermal stability of nitrates increases. Nitrates of alkaline and alkali metals give corresponding nitrites except for lithium nitrate, it gives lithium oxides.
$4LiNO_{3}\rightarrow 2Li_{2}O+4NO_{2}+O_{2}$
$NaNO_{3}\rightarrow 2NaNO_{2}+O_{2}$
$2M(NO_{3})_{2}\overset{\Delta }{\rightarrow}2MO+4NO_{2}+O_{2}$ [ M = Be, Mg, Ca, Sr, Ba]
(b) Carbonates-
Alkaline earth metal carbonates decompose on heating gives carbon dioxide and oxide. On the other hand carbonates of alkali metals are stable towards heat. The solubility of carbonates increases down the group in alkali metals (except $Li_{2}CO_{3}$ ) . But carbonates of alkaline earth metals are insoluble in water.
(c)Sulphate-
thermal stability is good for both alkali and alkaline earth metals. Alkali metal sulphates are more soluble in water than alkaline earth metals. Solubility decrease down the group from $CaSO_{4}$ to $BaSO_{4}$ . sulphate of Be and Mg are readily soluble in water.
Answer :
(i) Sodium metal can be extracted from sodium chloride.In this process electrolysis of fused $NaCl$ (40%) and $CaCl_{2}$ (60%) at a temp. of 1123K in Downs cell.
steel cathode and graphite block is act as anode. sodium and calcium deposit at cathode .
$NaCl\rightarrow Na^{+}+Cl^{-}$ (Molten state)
At Cathode- $Na^{+}+e^{-}\rightarrow Na$
At Anode- $\\Cl^{-}\rightarrow Cl+e^{-}\\ Cl+Cl\rightarrow Cl_{2}$
(ii) $NaOH$ can be prepared by the electrolysis of sodium chloride. By Castner- kellner process. A brine solution is electrolysed using a mercury cathode and a carbon anode. Sodium metal discharged at the cathode combines with mercury to form sodium amalgam. Chlorine gas is evolved at the anode.
At Cathode $Na^{+}+e^{-}\rightarrow Na-amalgam$
At anode- $Cl^{-}\rightarrow 1/2Cl_{2}+e^{-}$
(iii) NaCl is electrolysed for the formation of Na metal (in downs process). this sodium metal is then heated on aluminium trays in air to form sodium peroxide.
$2Na+O_{2}\rightarrow Na_{2}O_{2}$
(iv) Sodium carbonate is preapred by solvay process. $NaHCO_{3}$ is precipitated in a reaction of sodium chloride and ammonium hydrogen carbonate.
$\\2NH_{3} +H_{2}O+CO_{2}\rightarrow (NH_{4})_{2}CO_{3}\\ (NH_{4})_{2}CO_{3}+H_{2}O+CO_{2}\rightarrow NaHCO_{3}\\ (NH_{4})_{2}CO_{3} +NaCl\rightarrow NH_{4}Cl+NaHCO_{3}$
this $NaHCO_{3}$ crystals are heatedto give sodium carbonate.
$2NaHCO_{3}\rightarrow Na_{2}CO_{3}+CO_{2}+H_{2}O$
Answer :
(i) Magnesium is more electropositive and burns with dazzling brilliance in the air to give MgO and Mg3N.
$2Mg+O_{2}\rightarrow MgO\\ 3Mg+N_{2}\rightarrow Mg_{3}N_{2}$
(ii) Quick lime combined with silica to form calcium silicate which is used as slag.
$CaO+SiO_{2}\rightarrow CaSiO_{3}$
(iii) when chlorine reacts with slaked lime it produces bleaching powder.
$2Ca(OH)_{2}+2Cl_{2}\rightarrow CaCl_{2}+Ca(OCl)_{2}+H_{2}O$
(iv) when calcium nitrate is heated is decomposed to give calcium
$2Ca_{3}(NO_{3})_{2}\rightarrow 2CaO + 4NO_{2} +O_{2}$
Question 10.18 Describe two important uses of each of the following : (i) caustic soda (ii) sodium carbonate (iii) quicklime.
Answer :
(i) Caustic Soda The chemical name is sodium hydroxide ( $NaOH$ ). Sodium hydroxide is generally prepared commercially by the electrolysis of sodium chloride in Castner-Kellner cell.
Uses-
- the manufacture of soap, paper, artificial silk and a number of chemicals
- in the purification of bauxite,
- in the textile industries for mercerising cotton fabrics
- for the preparation of pure fats and oils, and
- as a laboratory reagent.
(ii) Sodium Carbonate-
The common name is washing soda ( $Na_{2}CO_{3}$ ).Sodium carbonate is generally prepared by Solvay Process.
Uses-
- It is used in water softening, laundering and cleaning.
- It is used in the manufacture of glass, soap, borax and caustic soda.
- It is used in paper, paints and textile industries.
- It is an important laboratory reagent both in qualitative and quantitative analysis
(iii) Quick lime-
The chemical name is calcium oxide and the formula is $CaO$ . It is prepared on a commercial scale by heating limestone (CaCO3) in a rotary kiln at 1070-1270 K ( $CaCO_{3}\rightleftharpoons CaO+CO_{2}$ )
Uses-
- It is an important primary material for manufacturing cement and is the cheapest form of alkali.
- It is used in the manufacture of sodium carbonate from caustic soda.
- It is employed in the purification of sugar and in the manufacture of dye stuffs.
Question 10.19 Draw the structure of (i) $BeCl_{2}$ (vapour) (ii) $BeCl_{2}$ (solid).
Answer :
Answer-
structure of $BeCl_{2}$ in solid phase-
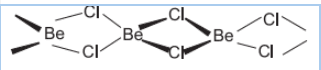
![]()
In Gaseous phase-
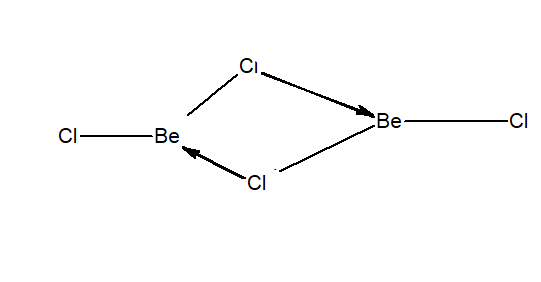
Answer :
The atomic size of sodium and potassium is lager than the magnesium and calcium. So, the lattic energy of carbonates and oxides formed by sodium and potassium are less than that of calcium and magnesium. Therefore the carbonates and oxides of $Na$ and $K$ are dissolve readily in water and Mg and $Ca$ are sparingly soluble in water
Question 10.21 Describe the importance of the following : (i) limestone (ii) cement (iii) plaster of paris.
Answer :
(i) Limestone-
The chemical formula is $CaCO_{3}$ .
Importance of limstone is-
- It is used as a building material in the form of marble and in the manufacture of quick lime
- Calcium carbonate along with magnesium carbonate is used as a flux in the extraction of metals such as iron.
- It is also used as an antacid, mild abrasive in tooth paste, a constituent of chewing gum, and a filler in cosmetics.
(ii) Cement-
Cement is an important building material. It is amixture of triclcium silicate( $Ca_{3}SiO_{5}$ ) and tricalium aluminate( $Ca_{3}Al_{2}O_{6}$ ).
Importance of cement-
It is used in concrete and reinforced concrete, in plastering and in the construction of bridges, dams and buildings
(iii) Plaster of Paris-
It is a hemihydrate of calcium sulphate. It is obtained when gypsum, $CaSO_{4}.2H_{2}O$ , is heated to 393 K.
Importance of POP-
It is used in medicine as surgical bandages and also it is used for making casts and moulds.
Question 10.22 Why are lithium salts commonly hydrated and those of the other alkali ions usually anhydrous?
Answer :
Lithium-ion is the smallest among the other alkali metal ions. Hence it has higher polarising power than others and so it can polarise water molecule more easily than other alkali metals. Hence the water molecules are more attracted towards Li salts as the water of crystallisation. Li+ has a maximum degree of hydration and for this reason, lithium salts are mostly hydrated, e.g., LiCl· 2H2O
As the down the group size of ions increase, their polarising power decreases. Hence other alkali metal ions usually form anhydrous salts.
Question 10.23 Why is LiF almost insoluble in water whereas LiCl soluble not only in water but also in acetone ?
Answer :
Both $Li^{+}$ ion and $F^{-}$ ion are smaller in size. So their size compatibility is very high. Hence lattice energy during formation is very high and it is not overcome by the hydration energy. Therefore LiF is almost insoluble in water. On the other hand, $Li^{+}$ cation and $Cl^{-}$ anion have comparable size differences, therefore they have low lattice energy and because of small in size, lithium ion has high polarising power as a result is distort the electron cloud of chloride anion. As a result, there is some covalent character in $LiCl$ . So, it soluble in water but also in acetone.
Question 10.24 Explain the significance of sodium, potassium, magnesium and calcium in biological fluids
Answer :
Significance of sodium potassium, magnesium and calcium in biological fluids-
Sodium-
- It is mainly found in blood plasma and also in the interstitial fluid which surrounds the cell.
- $Na^{+}$ ions help in the transmission of nerve signals also for the regulating water in the plasma membrane.
- Also for the transport of sugars and amino acids into the cells.
Potassium-
- These ions are highly present within cell fluids.
- Helps in activating many enzymes
- To produce ATP it oxidises the glucose molecule.
- Also helps in the transmission of nerve signals.
Magnesium and calcium-
- Plays an important role in neuromuscular function (by magnesium), interneuronal transmission, cell membrane integrity and blood coagulation(by calcium)
- Mg helps in maintains normal blood circulation in our body.
Answer :
(i) When sodium metal dropped into water it reacts rapidly and forms sodium hydroxide and hydrogen gas.
the chemical reaction is - $2Na+2H_{2}O\rightarrow 2NaOH +H_{2}$
(ii) sodium metal heated in the free supply of water-
Sodium reacts vigorously with oxygen and forming sodium peroxides.
Reaction- $2Na+O_{2}\rightarrow Na_{2}O_{2}$
(iii) when sodium peroxide dissolved in water it hydrolysed and produce sodium hydroxide and hydrogen peroxide.
Reaction- $Na_{2}O_{2}+2H_{2}O\rightarrow 2NaOH+H_{2}O_{2}$
Answer :
(a) As we know that down the group ionic size increases. Lithium-ion is the smallest in size and we know smaller the size of ion, the more hydrated it is. So lithium ion is most hydrated and $Cs^{+}$ ion is bigger in size so it is least hydrated.
Greater the size of hydrated ion less is mobility. So that the order of mobility of ions $Li^{+}<Na^{+}<K^{+}<Rb^{+}<Cs^{+}$
(b) Lithium unlike other alkali metals direct react with nitrogen and forms lithium nitride ( $Li_{3}N$ ). This is because lithium-ion is the smallest in size and therefore it has very high size compatibility with $N^{3-}$ . Hence the lattice energy released is very high.
(c) Electrode potential ( $E^{0}$ ) for $M^{2+}$ is depend on mainly three-factor- (i)ionisation enthalpy (ii)hydration enthalpy (iii)enthalpy of vaporisation. But we consider the overall effect of these three factors that should be the same for all $Ca,Sr,Ba$ . Hence their electrode potential is constant.
Answer :
(a) when sodium bicarbonate is added to water it gives sodium hydroxide (strong base). As a result, the solution becomes alkaline in nature.
$Na_{2}CO_{3}+H_{2}O\rightarrow NaOH+ H_{2}CO_{3}$
(b) Because their oxides are themselves are a very strong reducing agent in nature. So, by chemical reduction, we cannot obtain alkali metals. Also, we cannot use electrolysis of the aqueous solution method because after liberating the metals they again react with water.(
$(2M+H_{2}O\rightarrow 2M^{+}+2OH^{-}+H_{2})$ [M = alkali metals]
Question 10.28 Write balanced equations for reactions between (a) Na2O2 and water (b) KO2 and water (c) Na2O and CO2 .
Answer :
(a)The balanced reaction between $Na_{2}O_{2}$ and water-
$Na_{2}O_{2} +2H_{2}O\rightarrow 2NaOH +H_{2}O_{2}$
(b) the reaction between water and $KO_{2}$ -
$KO_{2}+H_{2}O\rightarrow KOH+H_{2}O_{2}+O_{2}$
(c) reaction between $Na_{2}O\ and\ CO_{2}$ -
$Na_{2}O+CO_{2}\rightarrow Na_{2}CO_{3}$
Answer :
(i) $Be^{2+}$ is small in size so it has high polarising power and $O^{2-}$ is also small in size. Compatibility of both the cation and anion are very high. So their lattice energy is also very high. When BeO is dissolved in water it's hydration energy is not sufficient to overcome its lattice energy. So, therefore, it is insoluble in water.
On the other hand, $SO_{4}^{2-}$ ions are large in size. Hence $Be^{+}$ ion can easily polarise $SO_{4}^{2-}$ ions and making it unstable and because of that lattice energy of $BeSO_{4}$ is not very high and so it is soluble in water.
(ii) $BaO$ is soluble because $Ba^{2+}$ cation is large in size as compare to $O^{2-}$ anion. Size compatibility between them is not good. Therefore $BaO$ is unstable. Hence lattice energy during the formation of their lattice is not high So it can be easily overcome by hydration energy. Therefore $BaO$ is soluble in water. In case of $BaSO_{4}$ , we know that down the group hydration enthalpy decreases and both the anion and cation have very good size compatibility. So, lattice energy cannot be overcome by hydration energy. As a result, $BaSO_{4}$ is not soluble in water.
(iii) The $Li^{+}$ ion has high polarising power. It is very small in size as compare to $K^{+}$ ion. So, it has a high tendency to distort the electron cloud around the negative iodide ( $I^{-}$ ) ion. As a result of high polarizability, it has high covalent character than. $KI$ Hence it is more soluble in methanol.
Question 10.30 Which of the alkali metal is having least melting point ? (a) Na (b) K (c) Rb (d) Cs
Answer :
The strength of metallic bond decreases down the group in the periodic table because as the size of cation increases the binding energies of their atoms in the crystal lattice decreases.
order of melting point $Na>k>Rb>Cs$
So, ans is (a) Na
Question 10.31 Which one of the following alkali metals gives hydrated salts ? (a) Li (b) Na (c) K (d) Cs
Answer :
The smaller the size of ion higher is the charge density and high polarising power. And among the alkali metals, lithium-ion is the smallest in size and has the power to attract the water molecules.
Ans is (a) Li
Question 10.32 Which one of the alkaline earth metal carbonates is thermally the most stable ? (a) MgCO3 (b) CaCO3 (c) SrCO3 (d) BaCO3
Answer :
Thermal stability $\propto$ size of the cation in carbonates
increasing order of cationic size in alkaline earth metal is $Mg<Ca<Sr<Ba$
Therefore the most thermal stable carbonates are of Barium
Ans. (d) $BaCO_{3}$
More About The s Block Elements Class 11 NCERT Chapter
The NCERT Solutions for Class 11 Chemistry Chapter 10 The s-Block Elements are designed and solved by our chemistry experts. These are the detailed explanation of NCERT textbook questions. These NCERT solutions for class 11 chemistry chapter 10 The s-Block Elements help students in their preparation of class 11 final examination as well as in the various competitive exams like JEE, NEET, BITSAT etc.
In s-block, there is a total of two groups (1&2). In NCERT solutions for Class 11 Chemistry Chapter 10 The s-Block Elements we will discuss important concepts of s-block elements like properties of group 1 and group 2 elements, their electronic configuration, their occurrence, general characteristics of the compounds of alkali metals and alkaline earth metal, anomalous behaviour of lithium and beryllium, important compounds of sodium and calcium and at the end NCERT syllabus discuss the biological importance of sodium, potassium, magnesium and calcium.
We have already discussed that s-block elements are divided into two groups. The elements of Group 1 are lithium(Li), sodium(Na), potassium(K), rubidium(Rb), caesium(Cs) and francium(Fr). They are collectively known as the alkali metals . The elements of Group 2 beryllium(Be), Magnesium(Mg), Calcium(Ca), Strontium(Sr), Barium(Ba) and Radium(Ra). They are collectively known as alkaline earth metals . In NCERT solutions for Class 11 Chemistry Chapter 10 The s-Block Elements, we will also discuss some important trends like atomic radius, diagonal relationship etc.
The s-Block Elements Class 11 Chemistry Chapter 10 ; Some Important Points
1. General electronic configuration of Group 1 elements is $ns^1$ .
2. General electronic configuration of Group 2 elements is $ns^2$ .
3. Generally, densities of alkali metals increase down the group from Li to Cs except for density of K < Na.
4. Alkali metals have low boiling and melting point and these decreases down the group.
NCERT The s-Block Elements Class 11 Chemistry Chapter 10 Topics
10.1 Group 1 Elements: Alkali Metals
10.2 General Characteristics of the Compounds of the Alkali Metals
10.3 Anomalous Properties of Lithium
10.4 Some Important Compounds of Sodium
10.5 Biological Importance of Sodium and Potassium
10.6 Group 2 Elements: Alkaline Earth Metals
10.7 General Characteristics of Compounds of the Alkaline Earth Metals
10.8 Anomalous Behaviour of Beryllium
10.9 Some Important Compounds of Calcium
10.10 Biological Importance of Magnesium and Calcium
NCERT Solutions for Class 11 Chemistry Chapter Wise
Chapter 1 | |
Chapter-2 | |
Chapter-3 | |
Chapter-4 | |
Chapter-5 | |
Chapter-6 | |
Chapter-7 | |
Chapter-8 | |
Chapter-9 | |
Chapter-10 | The S-Block Elements |
Chapter-11 | |
Chapter-12 | |
Chapter-13 | |
Chapter-14 |
NCERT Solutions for Class 11 Subject Wise
- NCERT Solutions for Class 11 Biology
- NCERT Solutions for Class 11 Maths
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 11 Physics
Benefits of NCERT Solutions for Class 11 Chemistry Chapter 10 The s-Block Elements
- The solutions are written in a comprehensive manner in the NCERT Solutions for Class 11 Chemistry Chapter 10 The s-Block Elements will help you writing answers in your exam.
- Revision will be easy because the detailed solutions will help you to remember the concepts and get you good marks.
- Homework problems will be easier for you, all you need to do is check the detailed NCERT solutions for class 11 chemistry and you are ready to go.
If you have a doubt or question that is not available here or in any of the chapters, contact us. You will get all the answers that will help you score well in your exams.
Also Check NCERT Books and NCERT Syllabus here:
Frequently Asked Questions (FAQs)
Important topics-
- Electronic configuration
- Occurrence
- Compounds: Sodium Carbonate, Sodium Chloride, Sodium Hydroxide and Sodium Hydrogen Carbonate
- Anomalous properties of the first element of each group -
- Diagonal relationship
- Trends in chemical reactivity with oxygen, water, hydrogen and halogens, uses
- Preparation and Properties of Some Important
For complete solutions of NCERT, students can refer to this link: https://school.careers360.com/ncert/ncert-solutions-class-11-chemistry
The weightage of NCERT class 11 Chemistry chapter 10 in NEET is 2% of total marks
Atleast 1 question from this chapter is asked in JEE mains every year
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
