NCERT Class 11 Chemistry Chapter 8 Notes Redox Reaction - Download PDF
Do you know that 80% of electricity is generated through redox-based reactions, or the batteries used in phones, the energy needed by our body to move, think, breathe, and even the process of rusting of iron is driven by redox-based mechanisms? This chapter helps in understanding such important phenomena. Various industrial processes, workings of batteries, combustion of fuels, etc, are very well explained by redox reaction concepts. These NCERT notes provide detailed descriptions of topics like the process of oxidation and reduction, the change in oxidation state of elements during chemical reactions, and different types of oxidation reactions.
This Story also Contains
- NCERT Notes for Class 11 Chemistry Chapter 7 Redox Reactions: Download PDF
- NCERT Notes for Class 11 Chemistry Chapter 7 Redox Reactions
- Redox Reactions Previous Year Questions and Answers
- How to Master Class 11 Chemistry Chapter 7 Redox Reactions
- Advantages of Using Class 11 Chemistry Chapter 7 Redox Reactions Notes
- CBSE Class 11 Chemistry Chapter-Wise Notes
- NCERT Solutions for Class 11 Chemistry
- Subject-Wise NCERT Exemplar Solutions
- Subject-Wise NCERT Solutions
Redox Reactions are significant in our day-to-day existence. They help extract metals from ores, are used in electroplating to apply a thin layer of metal to objects, and are necessary for the manufacturing of fuel cells, which are devices that produce electricity. The NCERT Class 11 Chemistry Notes are prepared by our experts, which will work as a key guide to excel in your examinations. Students can also access NCERT Class 11 notes for understanding the concepts better. These notes cover all the topics as prescribed by the latest CBSE syllabus and selected previous year questions are also added to help enhance the clarity and problem-solving ability of students.
NCERT Notes for Class 11 Chemistry Chapter 7 Redox Reactions: Download PDF
Download ncert class 11 chemistry chapter 7 redox reaction notes pdf to access a clear explanation, important reactions of this chapter. These notes cover all the key concepts of the Redox Reactions. You can download the PDF from the button given below.
Also Read
NCERT Notes for Class 11 Chemistry Chapter 7 Redox Reactions
Redox reactions, commonly known as oxidation-reduction reactions, are fundamental chemical processes in which molecules exchange electrons. Redox reactions notes Class 11 Chemistry are given below, and explain every concept of the NCERT textbook easily and comprehensively. Whether revising for exams or studying for the first time, these notes are essential, students can also refer to the NCERT Solutions for additional practice and understanding.
Classical Idea of Redox reactions- Oxidation and Reduction reactions
The classical idea of redox reactions explains oxidation and reduction reactions in a simple way, helping students build a strong foundation in redox reactions concepts.
Oxidation Reaction
Oxidation is described as the addition of oxygen to an element or a compound or the removal of hydrogen/ electropositive element from a substance.
$\begin{aligned} & 2 \mathrm{Mg}(\mathrm{s})+\mathrm{O}_2(\mathrm{~g}) \rightarrow 2 \mathrm{MgO}(\mathrm{s}) \\ & \mathrm{Mg}(\mathrm{s})+\mathrm{Cl}_2(\mathrm{~g}) \rightarrow \mathrm{MgCl}_2(\mathrm{~s})\end{aligned}$
Reduction Reaction
It is defines as removal of oxygen/electronegative element from a substance or addition of hydrogen/ electropositive element to a substance.
$\begin{aligned} & 2 \mathrm{FeCl}_3(\mathrm{aq})+\mathrm{H}_2(\mathrm{~g}) \rightarrow 2 \mathrm{FeCl}_2(\mathrm{aq})+2 \mathrm{HCl}(\mathrm{aq}) \\ & 2 \mathrm{HgO}(\mathrm{s}) \rightarrow 2 \mathrm{Hg}(\mathrm{l})+\mathrm{O}_2(\mathrm{~g})\end{aligned}$
Redox Reactions in terms of electron transfer reactions
According to electronic concept every redox reaction consists of two steps known as half reactions
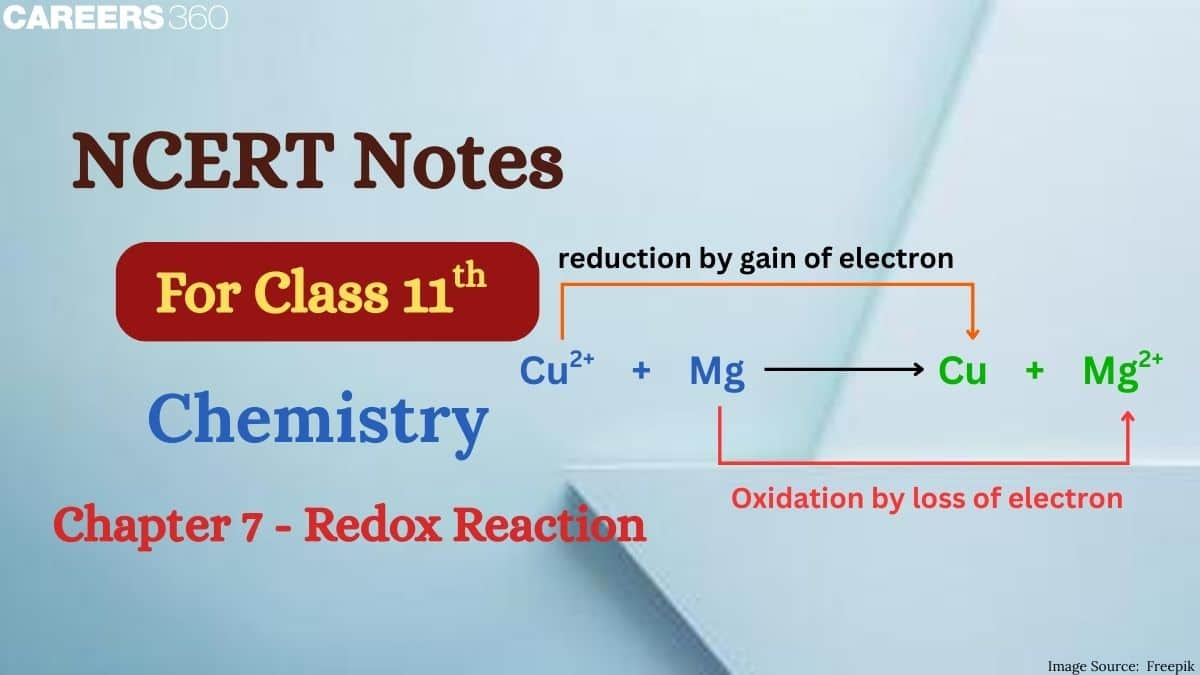
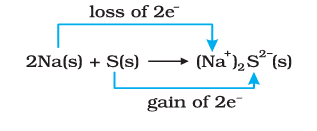
Redox reactions involve the transfer of electrons between species. Oxidation is the loss of electrons, while reduction is the gain of electrons. The substance that loses electrons is oxidised, and the one that gains electrons is reduced. These reactions always occur simultaneously and can be represented as two half-reactions one for oxidation and one for reduction. To make your preparation easier, you can also download the class 11 chemistry chapter 7 redox reaction notes pdf for quick revision and better exam practice.
Oxidising agent : Acceptor of electron(s).
Reducing agent : Donor of electron(s).
Competitive Electron Transfer Reactions
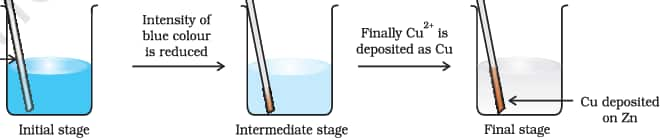
Place a strip of metallic zinc in an aqueous solution of copper nitrate for about one hour. Then you observed that, reddish copper deposits on the strip and the blue colour fades, indicating the formation of
$\mathrm{Zn}^{2+}$ ions. Passing $\mathrm{H}_2 \mathrm{~S}$ through the now colourless solution forms white ZnS upon adding ammonia, confirming the presence of $\mathrm{Zn}^{2+}$.
$\mathrm{Zn}(\mathrm{s})+\mathrm{Cu}^{2+}(\mathrm{aq}) \mathrm{Zn}^{2+}(\mathrm{aq})+\mathrm{Cu}(\mathrm{s})$
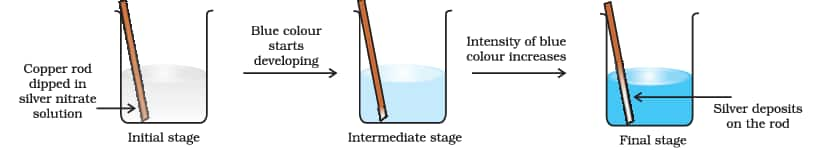
$\mathrm{Cu}(\mathrm{s})+2 \mathrm{Ag}^{+}(\mathrm{aq}) \mathrm{Cu}^{2+}(\mathrm{aq})+2 \mathrm{Ag}(\mathrm{s})$
Let us extend electron transfer reaction now to copper metal and silver nitrate solution in water and arrange a set-up as shown below. The solution develops blue colour due to the formation of $\mathrm{Cu}^{2+}$ ions on account of the reaction.
Oxidation Number
Oxidation number denotes the oxidation state of an element in a compound ascertained according to a set of rules formulated on the basis that electron pair in a covalent bond belongs entirely to more electronegative element.
Oxidation
It is a process that involves the loss of electrons by the atoms or ions.
Reduction
It is a process that involves the gain of electrons by the atoms or ions.
Any reaction, in which the electrons are exchanged between atoms or ions, represents a simultaneous process of oxidation and reduction and is called as a Redox Reaction.
In a Redox Reaction, the species that loses electron (i.e., gets oxidised) is known as reducing agent or reductant, (since it causes reduction of other species), the species which accepts electrons from reductant (i.e., gets reduced) is known as oxidising agent or oxidant (as it causes oxidation of other species).
Oxidation Number (O.N.): It refers to the total charge on all atoms of same kind in a compound.
Oxidation State (O.S.): It refers to the charge per atom of all atoms of same kind in a compound.
Oxidation state, many times, is also referred to as Oxidation Number.
This means oxidation number of an element in a compound is equal to the oxidation state of that element multiplied by total atoms of that element in particular compound.
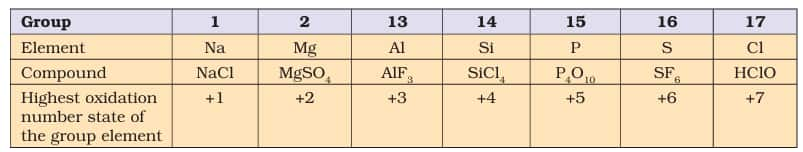
(i) In ionic compounds, it is simply the charge on corresponding cation and anion which is expressed as oxidation state of that partiular element. For example, oxidation state of potassium and chlorine in potassium chloride (KCl) is simply +1 and –1 respectively as KCl is treated as $\mathrm{K}^{+} \mathrm{Cl}^{-}$.
NOTE: (a) In $\mathrm{MgCl}_2$ and $\mathrm{AlCl}_3$, -1 is the oxidation state of Cl atom and its oxidation number is -2 and -3 respectively.
(b) In each of the cases, the sum of oxidation number of all atoms of all kinds is equal to zero since the compound is neutral.
(ii) In Covalent Compounds, it is not so easy to assign oxidation state of an atom. In order to simplify the concept, we are going to define a set of rules which would enable us to assign oxidation state to every element in any compound.
Rules for Assigning Oxidation State (O.S.) and Oxidation Number (O.N.):
- Any element in free state is assigned an oxidation state of zero. For example: O.S. of H, P, S, O in $\mathrm{H}_2, \mathrm{P}_4, \mathrm{~S}_8, \mathrm{O}_2$ respectively is zero.
- The oxidation state of any cation or anion (of form $\mathrm{A}^{+}$ or $B^{-}$) is equal to the magnitude of its charge. For example: O.S of Ca in $\mathrm{Ca}^{2+}$ = +2 and O.S of Al in $\mathrm{Al}^{3+}$ = +3.
-
The algebraic sum of the oxidation number of all atoms in a neutral compound is equal to 0. The algebraic sum of the oxidation numbers of all atoms in an ion (like $\mathrm{PO}_4{ }^{3-}$) is equal to charge on the ion.
-
The oxidation states of Alkali Metals (Group IA) is +1 in all of their compounds and that of Alkaline
Earth elements (Group IIA) is +2 in all of their compounds. -
Hydrogen in almost all of its compounds is assigned an oxidation state of +1. The exception occurs when hydrogen forms compounds with strong metals like KH, NaH, $\mathrm{MgH}_2$, $\mathrm{CaH}_2$, etc. In all of these, the oxidation state of hydrogen is -1.
-
Oxygen in almost all of its compounds is assigned an oxidation state of -2. But in certain compounds like Peroxides($\mathrm{H}_2 \mathrm{O}_2$), the oxidation state of oxygen is -1. Another exception is $\mathrm{OF}_2$, where O.S. is +2. $\mathrm{O}_2 \mathrm{~F}_2$, where O.S. is +1 and $\mathrm{KO}_2$ in which O.S. is -1/2.
-
Fluorine is most electronegative element and is assigned an O.S. of -1, in all its compounds. For other halogens, O.S. is generally -1 except when they are bonded to a more electronegative halogen or oxygen. O.S. of iodine in $\mathrm{IF}_7$ is +7, O.S. of chlorine in $\mathrm{KClO}_3$ is +5.
Types of Redox reaction:
A redox reaction can be defined as a chemical reaction in which we can see the transfer of electrons occur in between two reactants i.e. one is going to be reduced and the other is going to gain that electron.
The different types of redox reactions are:
- Combination Reaction
- Decomposition Reaction
- Displacement Reaction
- Disproportionation Reactions
1). Combination reaction:
These types of reactions are opposite to decomposition reactions. So the combination of two compounds produces a single product.
Example:
$4 \mathrm{Fe}+3 \mathrm{O}_2 \rightarrow 2 \mathrm{Fe}_2 \mathrm{O}_3$
2). Decomposition reaction:
This type of reaction involves breaking a compound into other different compounds.
Example:
-
$2 \mathrm{NaH} \rightarrow 2 \mathrm{Na}+\mathrm{H}_2$
3). Displacement reaction:
Displacement reactions, also known as replacement reactions, involve compounds and the replacing of elements. They occur as single and double replacement reactions. In other words, in these type of reactions, an atom or an ion in a compound is substituted by another element. The general representation of this reaction is as follows:
$\mathrm{X}+\mathrm{YZ} \rightarrow \mathrm{XZ}+\mathrm{Y}$
-
Metal displacement Reaction
-
Non-metal displacement Reaction
(a). Metal Displacement reaction:
A metal in a compound can be displaced by another metal in the uncombined state. Metal displacement reactions find many applications in metallurgical processes in which pure metals are obtained from their compounds in ores. A few such examples are:
$
\mathrm{CuSO}_4(\mathrm{aq})+\mathrm{Zn}(\mathrm{~s}) \rightarrow \mathrm{Cu}(\mathrm{~s})+\mathrm{ZnSO}_4(\mathrm{aq})
$
$
\mathrm{V}_2 \mathrm{O}_5(\mathrm{~s})+5 \mathrm{Ca}(\mathrm{~s}) \xrightarrow{\Delta} 2 \mathrm{~V}(\mathrm{~s})+5 \mathrm{CaO}(\mathrm{~s})
$
$
\mathrm{TiCl}_4(\mathrm{l})+2 \mathrm{Mg}(\mathrm{~s}) \xrightarrow{\Delta} \mathrm{Ti}(\mathrm{~s})+2 \mathrm{MgCl}_2(\mathrm{~s})
$
In each case, the reducing metal is a better reducing agent than the one that is being reduced which evidently shows more capability to lose electrons as compared to the one that is reduced.
(b). Non-Metal Displacement reaction:
The non-metal displacement redox reactions include hydrogen displacement and a rarely occurring reaction involving oxygen displacement. All alkali metals and some alkaline earth metals (Ca, Sr, and Ba) which are very good reductants, will displace hydrogen from cold water.
$\begin{aligned} & 2 \mathrm{Na}(\mathrm{s})+2 \mathrm{H}_2 \mathrm{O}(\mathrm{l}) \rightarrow 2 \mathrm{NaOH}(\mathrm{aq})+\mathrm{H}_2(\mathrm{~g}) \\ & \mathrm{Ca}(\mathrm{s})+2 \mathrm{H}_2 \mathrm{O}(\mathrm{l}) \rightarrow \mathrm{Ca}(\mathrm{OH})_2(\mathrm{aq})+\mathrm{H}_2(\mathrm{~g})\end{aligned}$
Less active metals such as magnesium and iron react with steam to produce dihydrogen gas:
4). Disproportionation Reactions
Disproportionation reactions are those reactions in which a single element in one oxidation state is simultaneously oxidized and reduced. Some examples include:
Balancing of redox reaction
Two methods are used to balance chemical equations for redox processes. One of these methods is based on the change in the oxidation number of reducing agent and the oxidising agent and the other method is based on splitting the redox reaction into two half reactions.
(1) Oxidation Number Method:
While balancing a given reaction by this method, following steps are to be followed :
-
Assign oxidation state to each element (atom) on both sides of the equation and identify which element has been oxidised and which reduced.
-
Write two oxidation and reduction reactions (two half-reactions) separately involving only atoms. Now balance the atoms on both sides of equation in each half-reaction.
-
Balance charge on both sides by adding electrons to whichever side is deficient in electrons. (i.e., negative charge)
-
Add two half-reactions together. In doing this we want electrons to cancel from both sides. For this, multiply the equations by appropriate coefficients so that number of electrons produced in oxidation reaction equals to that used up in reduction reaction.
-
Now compare this balanced equation with original unbalanced equation. Here, notice whether the given equation is a molecular equation or an ionic equation.
-
For molecular equation, to balance Oxygen (O) and Hydrogen (H), add required water to the side deficient in H and check for Oxygen atoms on both sides. (They will be equal on both sides).
-
For ionic equation, apart from balancing O and H atoms, charge needs to be balanced. It depends upon the medium in which the reaction is taking place: Acidic (containing $\mathrm{H}^{+}$ ions or any acid) or Alkaline (containing OH- ions or any base).
-
In Acidic medium, count total charge on both sides and balance it by adding $\mathrm{H}^{+}$ ions to the required side (i.e., to the side deficient in +ve charge). Finally, add enough water molecules to balance H and O atoms to the required side.
-
In Basic medium, balance the charge by adding $\mathrm{OH}^{-}$ ions to the side with excess of +ve charge and finally add required number of $\mathrm{H}_2 \mathrm{O}$ molecules to the appropriate side to balance O and H.
(2) Half Reaction Method:
In this method, the two half equations are balanced separately and then added together to give balanced equation. Electrons are included in the half-reactions. These are then balanced so that the number of electrons lost is equal to the number of electrons gained. Finally, the two half-reactions are added back together.
(i). Write the unbalanced ionic equation.
$\mathrm{Fe}^{2+}(a q)+\mathrm{Cr}_2 \mathrm{O}_7^{2-}(a q) \rightarrow \mathrm{Fe}^{3+}(a q)+\mathrm{Cr}^{3+}(a q)$
(ii). Write separate half-reactions for the oxidation and the reduction processes. Determine the oxidation numbers first, if necessary.
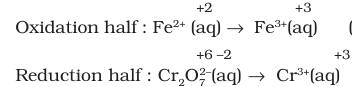
(iii). Balance the atoms in the half-reactions other than hydrogen and oxygen. In the oxidation half-reaction above, the iron atoms are already balanced. The reduction half-reaction needs to be balanced with the chromium atoms.
$\mathrm{Cr}_2 \mathrm{O}_7^{2-}(a q) \rightarrow 2 \mathrm{Cr}^{3+}(a q)$
(iv). Balance oxygen atoms by adding water molecules to the appropriate side of the equation. For the reduction half-reaction above, seven $\mathrm{H}_2 \mathrm{O}$ molecules will be added to the product side.
$\mathrm{Cr}_2 \mathrm{O}_7^{2-}(a q) \rightarrow 2 \mathrm{Cr}^{3+}(a q)+7 \mathrm{H}_2 \mathrm{O}(l)$
(v). Now the hydrogen atoms need to be balanced. In an acidic medium, add hydrogen ions to balance. In this example, fourteen H+ ions will be added to the reactant side.
$\mathrm{Cr}_2 \mathrm{O}_7^{2-}(\mathrm{aq})+14 \mathrm{H}^{+}(\mathrm{aq}) \rightarrow 2 \mathrm{Cr}^{3+}(\mathrm{aq})+7 \mathrm{H}_2 \mathrm{O}(\mathrm{l})$
(vi). Balance the charges by adding electrons to each half-reaction. For the oxidation half-reaction, the electrons will need to be added to the product side. For the reduction half-reaction, the electrons will be added to the reactant side. By adding one electron to the product side of the oxidation half-reaction, there is a 2+ total charge on both sides.
$\mathrm{Fe}^{2+}(a q) \rightarrow \mathrm{Fe}^{3+}(a q)+\mathrm{e}^{-}$
There is a total charge of 12+ on the reactant side of the reduction half-reaction (14−2). The product side has a total charge of 6+ due to the two chromium ions (2×3). To balance the charge, six electrons need to be added to the reactant side.
$6 \mathrm{e}^{-}+14 \mathrm{H}^{+}(a q)+\mathrm{Cr}_2 \mathrm{O}_7^{2-}(a q) \rightarrow 2 \mathrm{Cr}^{3+}(a q)+7 \mathrm{H}_2 \mathrm{O}(l)$
Now equalize the electrons by multiplying everything in one or both equations by a coefficient. In this example, the oxidation half-reaction will be multiplied by six.
$6 \mathrm{Fe}^{2+}(a q) \rightarrow 6 \mathrm{Fe}^{3+}(a q)+6 \mathrm{e}^{-}$
(vii). We add the two half reactions to achieve the overall reaction and cancel the electrons on each side. This gives the net ionic equation as :
$6 \mathrm{Fe}^{2+}(a q) \rightarrow 6 \mathrm{Fe}^{3+}(a q)+6 \mathrm{e}^{-}$
(viii). Verify that the equation contains the same type and number of atoms and the same charges on both sides of the equation.
$14 \mathrm{H}^{+}(a q)+6 \mathrm{Fe}^{2+}(a q)+\mathrm{Cr}_2 \mathrm{O}_7^{2-}(a q) \rightarrow 6 \mathrm{Fe}^{3+}(a q)+2 \mathrm{Cr}^{3+}(a q)+7 \mathrm{H}_2 \mathrm{O}(l)$
Redox Titrations as the basis of titrations:
In the process of titration, oxidation-reduction reactions occur and are known as redox titration. During the chemical reaction in such a technique transfer of electrons can be seen in the aqueous solution. Students can understand these concepts better by using redox reaction class 11 chemistry chapter 7 CBSE notes.
The Redox titration can further be classified on the basis of the reagent used in the redox titration.
Sub-Divisions of Redox Titration
(i) Permanganate Titrations- In some redox titrations, the reagent itself, like permanganate ion ($\mathrm{MnO}_4^{-}$) acts as a self-indicator. The endpoint is marked by a faint pink colour, appearing after complete oxidation of the reductant.
(ii). Dichromate Titrations- When no visible auto-colour change occurs, redox indicators are used. For example, dichromate $\mathrm{Cr}_2 \mathrm{O}_7^{2-}$ is not a self-indicator but oxidises diphenylamine after the equivalence point, producing an intense blue colour to mark the endpoint.
(iii). Iodometric and Iodometric Titrations- Another common redox titration method involves reagents that oxidise iodide I- to iodine $\mathrm{I}_2$, such as $\mathrm{Cu}^{2+}$. The liberated $\mathrm{I}_2$ forms a blue complex with starch, serving as the indicator. $\mathrm{I}_2$ then reacts with thiosulphate $\mathrm{S}_2 \mathrm{O}_3{ }^{2-}$
, and the blue colour disappears once all $\mathrm{I}_2$ is consumed.
Limitation of The Concept of Oxidation Number
It is not practically possible to apply the classical approach where the oxidation number concept is verified; the reaction is the so-called Redox reaction.
Redox reactions and electrode processes:
A redox couple is defined as when a substance is taking part in the oxidation and reduction process- half-reaction the combination of both oxidized and reduced parts of the substance is present in that reaction.
The electrode potential of a Galvanic cell can be defined as the tendency of losing or gaining the electrodes.
Dependency of electrode potential:
The electrode potential can be dependent upon:
-
Nature of the metal,
-
The concentration of the ions and
-
The temperature of an electrolyte
Electrode potential: The potential difference set up between the metal and its own ions in the solution is called the electrode potential. In general, it is the tendency of an electrode to gain or lose electrons.
Standard electrode potential (E°): If the concentration of each species taking part in the electrode reaction is unity and further the reaction is carried out at 298 K, then the potential of each electrode is called standard electrode potential. Standard electrode potential of hydrogen is taken as 0.00 volts by convention.
Electrochemical series is a series in which a list of oxidising agents are arranged in decreasing order of their strength. It is also called activity or electromotive series.
- A negative E° means that the redox couple is a stronger reducing agent than the $\mathrm{H}^{+} / \mathrm{H}_2$ couple.
- A positive E° means that the redox couple is a weaker reducing agent than the $\mathrm{H}^{+} / \mathrm{H}_2$
couple.
Redox Reactions Previous Year Questions and Answers
Previous year questions and answers of redox reactions will help students understand important exam questions and frequently asked concepts. These questions with solutions are useful for chemistry exam preparation and quick revision. The concepts used to solve these questions are explained in class 11 chemistry chapter 7 redox reaction notes.
Question 1: Which of the following electrodes will act as anodes, when connected to Standard Hydrogen Electrode?
(i) $A l / A l^{3+}, E^{\Theta}=-1.66$
(ii)$\mathrm{Fe} / \mathrm{Fe}^{2+}, E^{\Theta}=-0.44$
(iii)$\mathrm{Cu} / \mathrm{Cu}^{2+}, E^{\Theta}=+0.34$
(iv)$F_2(g) / 2 F^{-}(a q), E^{\Theta}=+2.87$
(1) (ii) and (iii)
(2) (i) and (ii)
(3) (ii) and (iv)
(4) (i) and (iv)
Answer:
The answer is the option (i) and (ii)
$A l / A l^{3+}, E^{\Theta}=-1.66$
They will act as anodes because both of the electrodes has a negative value of standard reduction potential.
Hence, the answer is the option (2).
Questions 2: Identify the correct statements with reference to the given reaction
$\mathrm{P}_4+3 \mathrm{OH}^{-}+3 \mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{PH}_3+3 \mathrm{H}_2 \mathrm{PO}_2^{-}$
(i) Phosphorus is undergoing reduction only.
(ii) Phosphorus is undergoing oxidation only.
(iii) Phosphorus is undergoing oxidation as well as reduction.
(iv) Hydrogen is undergoing neither oxidation nor reduction.
(1) (i) and (ii)
(2) (ii) and (iv)
(3) (ii) and (iii)
(4) (iii) and (iv)
Answer:
The answer is the option (iii) and (iv)
Phosphorus is undergoing oxidation as well as reduction.
Hydrogen is undergoing neither oxidation nor reduction.
This is a kind of disproportionation reaction in which phosphorus is being reduced as well as oxidized, as given in opt (iii); whereas hydrogen remains the same in the +1 oxidation state as in opt (iv). Therefore, options (iii) and (iv) are correct.
Hence, the answer is the option (4)
Question 3: The exhibition of various oxidation states by an element is also related to the outer orbital electronic configuration of its atom. Atom(s) having which of the following outermost electronic configurations will exhibit more than one oxidation state in its compounds.
(i) $3 s^1$
(ii) $3 d^1 4 s^2$
(iii) $3 d^2 4 s^2$
(iv) $3 s^2 3 p^3$
(i) +2 (ii) +3
(iii) +4 (iv) +6
(1) (i) and (ii)
(2) (ii) and (iv)
(3) (ii) and (iii)
(4) (iii) and (iv)
Answer:
Atoms having electronic configurations such as in Option (iii) and (iv) will exhibit more than one oxidation state in the compound because in Option (iii) electrons can be removed from 4s as well as 3d. Similarly, in option (iv) electron can be removed from 3p and 3s.
Hence, the answer is the option (1).
Question 4: Which of the following oxidation reactions are carried out by both $\mathrm{K}_2 \mathrm{Cr}_2 \mathrm{O}_7$ and $\mathrm{KMnO}_4$ in acidic medium?
A. $\mathrm{I}^- \rightarrow \mathrm{I}_2$
B. $\mathrm{S}^{2-} \rightarrow \mathrm{~S}$
C. $\mathrm{Fe}^{2+} \rightarrow \mathrm{Fe}^{3+}$
D. ${I }^{-} \rightarrow \mathrm{IO}_3^{-}$
E. $\mathrm{S}_2 \mathrm{O}_3{ }^{2-} \rightarrow \mathrm{SO}_4{ }^{2-}$
Choose the correct answer from the options given below :
(1) B, C and D only
(2) A, D and E only
(3) A, B and C only
(4) C, D and E only
Answer:
$\begin{array}{ll}\mathrm{I}^{-} \xrightarrow{\mathrm{H}^{+}} \mathrm{I}_2 & \mathrm{I}^{-} \xrightarrow{\mathrm{OH}^{-}} \mathrm{IO}_3^{-} \\ \mathrm{S}^{-2} \xrightarrow{\mathrm{H}^{+}} \mathrm{S} & \mathrm{S}_2 \mathrm{O}_3^{2-} \xrightarrow{\mathrm{OH^-}} \mathrm{SO}_4^{2-} \\ \mathrm{Fe}^{+2} \longrightarrow \mathrm{Fe}^{+3} \\ \mathrm{~S}_2 \mathrm{O}_3^{2-} \xrightarrow{\mathrm{H}^{+}} \mathrm{S} \downarrow+\mathrm{SO}_4^{2-}\end{array}$
Oxidation reactions are carried out by both $\mathrm{K}_2 \mathrm{Cr}_2 \mathrm{O}_7$ and $\mathrm{KMnO}_4$ in acidic medium by: A, B and C
Hence, the correct answer is option (3).
Question 5: Iron (III) catalyses the reaction between iodide and persulphate ions, in which
A. $\mathrm{Fe}^{3+}$ oxidises the iodide ion
B. $\mathrm{Fe}^{3+}$ oxidises the persulphate ion
C. $\mathrm{Fe}^{2+}$ reduces the iodide ion
D. $\mathrm{Fe}^{2+}$ reduces the persulphate ion
Choose the most appropriate answer from the options given below:
(1) B and C only
(2) B only
(3) A only
(4) A and D only
Answer:
$\begin{aligned} & 2 \mathrm{Fe}^{3+}+2 \mathrm{I}^{-} \longrightarrow 2 \mathrm{Fe}^{2+}+\mathrm{I}_2 \\ & 2 \mathrm{Fe}^{2+}+\mathrm{S}_2 \mathrm{O}_8{ }^{2-} \longrightarrow 2 \mathrm{Fe}^{3+}+2 \mathrm{SO}_4{ }^{2-}\end{aligned}$
$\mathrm{Fe}^{+3}$ oxidises $\mathrm{I}^{-}$to $\mathrm{I}_2$ and convert itself into $\mathrm{Fe}^{+2}$. This $\mathrm{Fe}^{+2}$ reduces $\mathrm{S}_2 \mathrm{O}_8{ }^{2-}$ to $\mathrm{SO}_4{ }^{2-}$ and converts itself into $\mathrm{Fe}^{+3}$
Hence, the answer is the option (4).
How to Master Class 11 Chemistry Chapter 7 Redox Reactions
These redox reaction class 11 chemistry chapter 7 CBSE notes form the foundation of many chemical processes, including energy production and industrial reactions. Given below some points on how to master this chapter.
- Students must understand the basic concepts like oxidation, reduction, and redox reactions.
- Then students must learn about oxidation numbers and rules for assigning them in compounds.
- Methods of balancing redox reactions, including oxidation-reduction method and ion-electron method in acidic and basic media are well explained in redox reaction class 11 chemistry notes.
- Questions related to electrochemical concepts, electrolysis, galvanic cells, and standard electrode potentials are often asked in exams.
- At last students must solve numerical questions on balancing redox reactions and calculating electrode potentials.
Advantages of Using Class 11 Chemistry Chapter 7 Redox Reactions Notes
NCERT Class 11 Chemistry Chapter 7 Notes Redox Reaction covers all important concepts from the NCERT book in a simple and organised manner. The advantages of using these notes are given below:
- Students can use these notes to understand topics like oxidation, reduction, oxidising and reducing agents, and redox reactions.
- These notes cover all topics from the NCERT book in a very clear and comprehensive manner.
- These notes are prepared by subject experts to make sure that the content provided is accurate, covers the whole syllabus, and easy to understand.
- The ncert class 11 chemistry chapter 7 redox reaction notes provide explanation of all important concepts, helping students in board and competitive exams.
CBSE Class 11 Chemistry Chapter-Wise Notes
In addition to redox reaction class 11 chemistry chapter 7 CBSE notes, students can refer to the NCERT notes of other Class 11 chapters provided below.
| NCERT notes for class 11 Chemistry Chapter 1 Some Basic Concepts of Chemistry |
|
NCERT notes for class 11 Chemistry Chapter 2 Structure of Atom |
|
NCERT notes for class 11 Chemistry Chapter 4 Chemical Bonding and Molecular Structure |
|
NCERT notes for class 11 Chemistry Chapter 7 Redox Reactions |
NCERT Solutions for Class 11 Chemistry
Along with redox reaction class 11 chemistry notes, follow the links below to get chapter-wise solutions of NCERT and make your learning better.
Subject-Wise NCERT Exemplar Solutions
Students can refer to the links given below for NCERT Exemplar Solutions:
Subject-Wise NCERT Solutions
Students can refer to the links given below for NCERT Solutions:
Frequently Asked Questions (FAQs)
The oxidizing agent is the species that gets reduced and thus causes the oxidation of another substance. Conversely, the reducing agent is the species that gets oxidized and causes the reduction of another substance.
NCERT Class 11 Chemistry Chapter 7 Notes Redox Reaction have numerous applications, including in batteries, metallurgy, and biological processes such as cellular respiration and photosynthesis.
Studying NCERT Class 11 Chemistry Chapter 7 Notes Redox Reaction is crucial because they form the foundation for many advanced concepts in chemistry and related fields. Understanding these reactions helps students grasp important topics such as electrochemistry, organic reactions, and even biological processes.
Redox reactions are fundamental to many everyday processes. For example, they drive the combustion of fuels in cars, the processes of digestion and metabolism in our bodies, and the rusting of metals.
Balancing a redox reaction can be done using the half-reaction method. First, separate the reaction into two half-reactions one for oxidation and one for reduction. Next, balance the atoms and charges in each half-reaction.
Redox Reactions notes are provided in a PDF for easy access, portability, and convenient revision. PDFs allow students to study anytime, highlight important points, and quickly refer to formulas, definitions, and solved examples without carrying physical books.
These redox reaction class 11 chemistry notes are concise study materials that summarise key concepts of oxidation, reduction, and electron transfer reactions. They include rules for assigning oxidation numbers, methods to balance redox reactions, electrochemical concepts, and solved examples, helping students revise quickly and prepare effectively for exams.
Students need a ncert class 11 chemistry chapter 7 redox reaction notes for quick revision, easy access to formulas and key concepts, and practice of solved examples. It helps in understanding complex reactions, preparing for exams efficiently, and strengthening problem-solving skills without going through the entire textbook repeatedly.
Redox reactions are crucial in biological systems because they are involved in cellular respiration and photosynthesis. These reactions help transfer energy through electron carriers, allowing organisms to produce ATP, the energy currency of cells.
Oxidation states indicate the degree of oxidation of an atom in a compound. They help chemists keep track of electron transfer in reactions.
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
