NCERT Solutions for Class 11 Physics Chapter 13 Oscillations
Have you ever felt the back and forward motion of a playground swing or felt your mobile phone vibrating when it is on a call? These are the classic examples of the oscillatory motion, and the NCERT Solutions of the Class 11 Physics Chapter 13 - Oscillations enable students to understand the science of the oscillatory motion in a well-structured and clear way. This chapter is extremely significant as oscillations can be found everywhere, as in a pendulum, musical instruments, sound waves, electric circuits, and even such natural phenomena as earthquakes.
This Story also Contains
- Class 11 Physics Chapter 13 - Oscillations question answers: Download PDF
- NCERT Solutions for Class 11 Physics Chapter 13 - Oscillations: Exercise Questions
- Oscillations NCERT Solutions: Additional Questions
- Class 11 Physics Chapter 13 - Oscillations: Higher Order Thinking Skills (HOTS) Questions
- NCERT Solutions for Class 11 Physics Chapter 13 - Oscillations: Topics
- NCERT Solutions for Class 11 Physics Chapter 13 - Oscillations: Important Formulas
- How Can NCERT Solutions for Class 11 Physics Chapter 13 Oscillations Help in Exam Preparation?
- Approach to solve the NCERT Class 11 Physics Chapter 13 - Oscillations
- Importance of NCERT Solutions for Class 11 Physics Chapter 13: Oscillations
- What Students Learn from NCERT Solutions for Class 11 Physics Chapter 13: Oscillations?
- What Extra Should Students Study Beyond NCERT for JEE/NEET?
- NCERT Solutions for Class 11 Physics Chapter-Wise
.jpg)
Strictly adhering to the current CBSE and NCERT syllabus 2025-26, the NCERT Solutions for Class 11 Physics Chapter 13 - Oscillations are authored by the subject experts to ensure that even the most complicated topics seem simple and easy to understand. The access to these step-by-step NCERT solutions is possible not only online, but also the solutions to Chapter 13 Oscillations as a free PDF can be downloaded and studied at any time and from any place. Besides the exercise questions, the NCERT Solutions for Class 11 Physics Chapter 13 - Oscillations also contain questions that were asked before to improve the conceptual clarity and develop problem-solving skills, as well as advanced numericals and HOTS (Higher Order Thinking Skills) questions. The major points to be discussed are the types of oscillations, the motions of a wave, simple harmonic motion (SHM), resonance, and vibrations, which are extremely important not only in board exams but also in competitive tests, such as JEE and NEET. The students, having these well-structured and exam-oriented solutions, become more confident, more analytical, and further understand the oscillatory motion in Physics
Also Read,
Class 11 Physics Chapter 13 - Oscillations question answers: Download PDF
The Oscillations class 11 question answers offer a clear step-by-step explanation to all the textbook questions, which helps to understand complex subjects like SHM, resonance, and vibrations. These Oscillations class 11 question answers are according to the latest CBSE syllabus and can be easily downloaded in PDF to enable quick and effective preparation towards exams.
NCERT Solutions for Class 11 Physics Chapter 13 - Oscillations: Exercise Questions
The NCERT Solutions for Class 11 Physics Chapter 13 - Oscillations; Exercise Questions offer detailed, well-structured answers to all problems from the textbook. These class 11 physics chapter 13 Oscillations question answers help students strengthen concepts like periodic motion, simple harmonic motion, and energy in oscillations, making exam preparation more effective and hassle-free.
Q. 13.1 Which of the following examples represents periodic motion?
(a) A swimmer completing one (return) trip from one bank of a river to the other and back.
(b) A freely suspended bar magnet displaced from its N-S direction and released.
(c) A hydrogen molecule rotating about its centre of mass.
(d) An arrow released from a bow
Answer:
(a) The motion is not periodic, though it is to and fro.
(b) The motion is periodic.
(c) The motion is periodic.
(d) The motion is not periodic.
Q. 13.2 Which of the following examples represent (nearly) simple harmonic motion and which represent periodic but not simple harmonic motion?
(a) The rotation of the Earth about its axis.
(b) Motion of an oscillating mercury column in a U-tube.
(d) General vibrations of a polyatomic molecule about its equilibrium position.
Answer:
(a) Periodic but not S.H.M.
(b) S.H.M.
(c) S.H.M.
(d) Periodic but not S.H.M. [A polyatomic molecule has a number of natural frequencies, so its vibration is a superposition of SHM’s of a number of different frequencies. This is periodic but not SHM.
Q. 13.3 Fig. 13.18 depicts four x-t plots for the linear motion of a particle. Which of the plots represents periodic motion? What is the period of motion (in case of periodic motion)?

Fig 13.18
Answer:
The x-t plots for linear motion of a particle in Fig. 13.18 (b) and (d) represent periodic motion, with both having a period of motion of two seconds.
Q. 13.4 (a) Which of the following functions of time represent
$sin\; \omega t-cos\; \omega t$
Answer:
$
\begin{aligned}
& \sin \omega t-\cos \omega t \\
& =\sqrt{2}\left(\frac{1}{\sqrt{2}} \sin \omega t-\frac{1}{\sqrt{2}} \cos \omega t\right) \\
& =\sqrt{2}\left(\cos \frac{\pi}{4} \sin \omega t-\sin \frac{\pi}{4} \cos \omega t\right) \\
& =\sqrt{2} \sin \left(\omega t-\frac{\pi}{4}\right)
\end{aligned}
$
Since the above function is of form $Asin(\omega t+\phi )$ it represents SHM with a time period of $\frac{2\pi }{\omega }$
Answer:
$\\sin3\omega t =3sin\omega t -4sin^{3}\omega t $
$sin^{3}\omega t=\frac{1}{4}\left ( 3sin\omega t - sin3\omega t \right )\\$
The two functions individually represent SHM, but their superposition does not give rise to SHM. The motion will definitely be periodic with a period of $\frac{2\pi }{\omega }$
Answer:
The function represents SHM with a period of $\frac{\pi }{\omega }$
$cos\; \omega t+cos\; 3\omega t+cos\; 5\omega t$
Answer:
Here, each individual function is SHM. But superposition is not SHM. The function represents periodic motion but not SHM.
$period=LCM(\frac{2\pi}{\omega},\frac{2\pi}{3\omega},\frac{2\pi}{5\omega})=\frac{2\pi}{\omega}$
Answer:
The given function is exponential and, therefore, does not represent periodic motion.
Answer:
The given function does not represent periodic motion.
Q. 13.5 (a) A particle is in linear simple harmonic motion between two points, A and B, $10cm$ apart. Take the direction from A to B as the positive direction and give the signs of velocity, acceleration and force on the particle when it is at the end A,
Answer:
Velocity is zero. Force and acceleration are in the positive direction.
Q. 13.5 (b) A particle is in linear simple harmonic motion between two points, $A$ and $B$, $10cm$ apart. Take the direction from A to $B$ as the positive direction and give the signs of velocity, acceleration and force on the particle when it is at the end $B$,
Answer:
Velocity is zero. Acceleration and force are negative.
Q. 13.5 (c) A particle is in linear simple harmonic motion between two points, $A$ and $B$, $10 \; cm$ apart. Take the direction from $A$ to $B$ as the positive direction and give the signs of velocity, acceleration and force on the particle when it is at the mid-point of AB going towards $A$,
Answer:
Velocity is negative, that is, towards A, and its magnitude is maximum. Acceleration and force are zero.
Q. 13.5 (d) A particle is in linear simple harmonic motion between two points, $A$ and $B$, $10cm$ apart. Take the direction from $A$ to $B$ as the positive direction and give the signs of velocity, acceleration and force on the particle when it is at $2cm$ away from $B$ going towards $A$,
Answer:
The velocity is negative. Acceleration and force are also negative.
Q.13.5 (e) A particle is in linear simple harmonic motion between two points, $A$ and $B$, $10cm$ apart. Take the direction from $A$ to $B$ as the positive direction and give the signs of velocity, acceleration and force on the particle when it is at $3cm$ away from $A$ going towards $B$,
Answer:
Velocity is positive. Acceleration and force are also positive.
Q. 13.5 (f) A particle is in linear simple harmonic motion between two points, $A$ and $B$, $10cm$ apart. Take the direction from $A$ to $B$ as the positive direction and give the signs of velocity, acceleration and force on the particle when it is at $4cm$ away from $B$ going towards $A.$
Answer:
Velocity, acceleration and force are all negative
Answer:
Only the relation given in (c) represents simple harmonic motion as the acceleration is proportional in magnitude to the displacement from the midpoint, and its direction is opposite to that of the displacement from the mean position.
Q. 13.7 The motion of a particle executing a simple harmonic motion is described by the displacement function, $x(t)=A \; cos(\omega t+\phi ).$ If the initial $(t=0)$ position of the particle is $1\; cm$ and its initial velocity is $\omega \; cm/s,$ what are its amplitude and initial phase angle? The angular frequency of the particle is $\pi s^{-1}.$ If instead of the cosine function, we choose the sine function to describe the SHM: $x=B\; sin(\omega t+\alpha ),$ what are the amplitude and initial phase of the particle with the above initial conditions?
Answer:
$\omega =\pi\ rad\ s^{-1}$
$x(t)=Acos(\pi t+\phi )$
at t = 0
$x(0)=Acos(\pi \times 0+\phi )$
$ 1=Acos\phi ....(i)$
$v=\frac{\mathrm{d}x(t) }{\mathrm{d} t}$
$ v(t)=-A\pi sin(\pi t+ \phi )$
at t = 0
$v(0) =-A\pi sin(\pi \times 0+ \phi )$
$ \omega =-A\pi sin\phi$
$ 1 =-A sin\phi ....(ii)$
Squaring and adding equations (i) and (ii), we get
$1^{2}+1^{2}=(Acos\phi )^{2}+(-Asin\phi )^{2} $
$2=A^{2}cos^{2}\phi +A^{2}sin^{2}\phi $
$ 2=A^{2}$
$ A=\sqrt{2}$
Dividing equation (ii) by (i) we get
$\tan\phi =-1$
$\phi =\frac{3\pi }{4},\frac{7\pi }{4},\frac{11\pi }{4}......$
$x(t)=Bsin(\pi t+\alpha )$
at t = 0
$\\x(0)=Bsin(\pi \times 0+\alpha )$
$ 1=Bsin\alpha ....(iii)$
$\\v=\frac{\mathrm{d}x(t) }{\mathrm{d} t}$
$ v(t)=B\pi cos(\pi t+ \alpha )$
at t = 0
$\\v(0) =B\pi cos(\pi \times 0+ \alpha )$
$ \omega =B\pi cos\alpha$
$ 1 =B cos\alpha ....(iv)$
Squaring and adding equations (iii) and (iv), we get
$\\1^{2}+1^{2}=(Bsin\alpha )^{2}+(Bcos\alpha )^{2} $
$2=B^{2}sin^{2}\alpha +B^{2}cos^{2}\alpha$
$ 2=B^{2}$
$ B=\sqrt{2}$
Dividing equation (iii) by (iv), we get
$\tan\alpha =1$
$\alpha =\frac{\pi }{4},\frac{5\pi }{4},\frac{9\pi }{4}......$
Answer:
The spring constant of the spring is given by
$\begin{aligned}
k & =\frac{\text { Weight of Maximum mass the scale can read }}{\text { Maximum displacement of the scale }} \\
k & =\frac{50 \times 9.8}{20 \times 10^{-2}} \\
k & =2450 \mathrm{Nm}^{-1}
\end{aligned}$
The time period of a spring attached to a body of mass $m$ is given by
$\begin{aligned}
T & =2 \pi \sqrt{\frac{m}{k}} \\
m & =\frac{T^2 k}{4 \pi^2} \\
m & =\frac{(0.6)^2 \times 2450}{4 \pi^2} \\
m & =22.34 \mathrm{~kg} \\
w & =m g \\
w & =22.34 \times 9.8 \\
w & =218.95 \mathrm{~N}
\end{aligned}$
Determine the frequency of oscillations,
Answer:
The frequency of oscillation of an object of mass m attached to a spring of spring constant k is given by
$\begin{aligned}
\nu & =\frac{1}{2 \pi} \sqrt{\frac{k}{m}} \\
\nu & =\frac{1}{2 \pi} \times \sqrt{\frac{1200}{3}} \\
\nu & =3.183 \mathrm{~Hz}
\end{aligned}$
Determine the maximum acceleration of the mass,
Answer:
A body executing S.H.M experiences maximum acceleration at the extreme points
$\begin{aligned}
& a_{\max }=\frac{F_A}{m} \\
& a_{\max }=\frac{k A}{m} \\
& a_{\max }=\frac{1200 \times 0.2}{3} \\
& a_{\max }=8 \mathrm{~ms}^{-2}
\end{aligned}$
Determine the maximum speed of the mass.
Answer:
Maximum speed occurs at the mean position and is given by
$\begin{aligned}
& v_{\max }=A \omega \\
& v_{\max }=0.02 \times 2 \pi \times 3.18 \\
& v_{\max }=0.4 \mathrm{~ms}^{-1}
\end{aligned}$
Q. 13.10 (a) In Exercise 13.9, let us take the position of mass when the spring is unstretched as $x=0,$ and the direction from left to right as the positive direction of the x-axis. Give x as a function of time t for the oscillating mass if at the moment we start the stopwatch $(t=0),$ the mass is at the mean position. In what way do these functions for SHM differ from each other, in frequency, in amplitude or the initial phase?
Answer:
Amplitude is A = 0.02 m
Time period is $\\\omega$
$\begin{aligned}
\omega & =\sqrt{\frac{k}{m}} \\
\omega & =\sqrt{\frac{1200}{3}} \\
\omega & =20 \mathrm{rad} / \mathrm{s}
\end{aligned}$
(a) At t = 0 the mass is at mean position i.e. at t = 0, x = 0
$\\x(t)=0.02sin\left ( 20t \right )$
Here x is in metres and t is in seconds.
Q. 13.10 (b) In Exercise 13.9, let us take the position of mass when the spring is unstretched as $x=0,$ and the direction from left to right as the positive direction of x-axis. Give x as a function of time t for the oscillating mass if at the moment we start the stopwatch $(t=0),$ the mass is at the maximum stretched position. In what way do these functions for SHM differ from each other, in frequency, in amplitude or the initial phase?
Answer:
Amplitude is A = 0.02 m
Time period is $\\\omega$
$\begin{aligned}
&\begin{aligned}
\omega & =\sqrt{\frac{k}{m}} \\
\omega & =\sqrt{\frac{1200}{3}} \\
\omega & =20 \mathrm{rad} / \mathrm{s}
\end{aligned}\\
&\text { (b) At } \mathrm{t}=0 \text { the mass is at the maximum stretched position. }\\
&\begin{aligned}
& x(0)=\mathrm{A} \\
& \phi=\frac{\pi}{2} \\
& x(t)=0.02 \sin \left(20 t+\frac{\pi}{2}\right) \\
& x(t)=0.02 \cos (20 t)
\end{aligned}
\end{aligned}$
Here x is in metres and t is in seconds.
Q. 13.10 (c) In Exercise 13.9, let us take the position of mass when the spring is unstretched as $x=0,$ and the direction from left to right as the positive direction of x-axis. Give x as a function of time t for the oscillating mass if at the moment we start the stopwatch $(t=0),$ the mass is at the maximum compressed position. In what way do these functions for SHM differ from each other, in frequency, in amplitude or the initial phase?
Answer:
Amplitude is A = 0.02 m
Time period is $\\\omega$
$\begin{aligned}
&\begin{aligned}
\omega & =\sqrt{\frac{k}{m}} \\
\omega & =\sqrt{\frac{1200}{3}} \\
\omega & =20 \mathrm{rad} / \mathrm{s}
\end{aligned}\\
&\text { (c) At } \mathrm{t}=0 \text { the mass is at the maximum compressed position. }\\
&\begin{aligned}
& \mathrm{x}(0)=-\mathrm{A} \\
& \phi=\frac{3 \pi}{2} \\
& x(t)=0.02 \sin \left(20 t+\frac{3 \pi}{2}\right) \\
& x(t)=-0.02 \cos (20 t)
\end{aligned}
\end{aligned}$
Here x is in metres and t is in seconds.
The above functions differ only in the initial phase and not in amplitude or frequency.
Answer:
(a) Let the required function be $x(t)=a \sin ( \pm \omega t+\phi)$
Amplitude $=3 \mathrm{~cm}=0.03 \mathrm{~m}$
$\mathrm{T}=2 \mathrm{~s}$
$\begin{aligned}
\omega & =\frac{2 \pi}{T} \\
\omega & =\pi \mathrm{rad} \mathrm{~s}
\end{aligned}$
Since initial position $\mathrm{x}(\mathrm{t})=0, \phi=0$
As the sense of revolution is clockwise
$\begin{aligned}
& x(t)=0.03 \sin (-\omega t) \\
& x(t)=-0.03 \sin (\pi t)
\end{aligned}$
Here x is in metres and t is in seconds.
(b) Let the required function be $x(t)=a \sin ( \pm \omega t+\phi)$
Amplitude $=2 \mathrm{~m}$
$\mathrm{T}=4 \mathrm{~s}$
$\begin{aligned}
& \omega=\frac{2 \pi}{T} \\
& \omega=\frac{\pi}{2} \mathrm{rad} \mathrm{~s}
\end{aligned}$
Since initial position $x(t)=-A, \phi=\frac{3 \pi}{2}$
As the sense of revolution is anti-clockwise
$\begin{aligned}
& x(t)=2 \sin \left(\omega t+\frac{3 \pi}{2}\right) \\
& x(t)=-2 \cos \left(\frac{\pi}{2} t\right)
\end{aligned}$
Here x is in metres and t is in seconds.
Answer:
$\begin{aligned}
& x=-2 \sin (3 t+\pi / 3) \\
& x=2 \cos \left(3 t+\frac{\pi}{3}+\frac{\pi}{2}\right) \\
& x=2 \cos \left(3 t+\frac{5 \pi}{6}\right)
\end{aligned}$
The initial position of the particle is $x(0)$
$\begin{aligned}
& x(0)=2 \cos \left(0+\frac{5 \pi}{6}\right) \\
& x(0)=2 \cos \left(\frac{5 \pi}{6}\right) \\
& x(0)=-\sqrt{3} \mathrm{~cm}
\end{aligned}$
The radius of the circle, i.e. the amplitude, is 2 cm
The angular speed of the rotating particle is $\omega=3 \mathrm{rad} \mathrm{s}{ }^{-1}$
The initial phase is
$\begin{aligned}
\phi & =\frac{5 \pi}{6} \\
\phi & =150^{\circ}
\end{aligned}$
The reference circle for the given simple Harmonic motion is
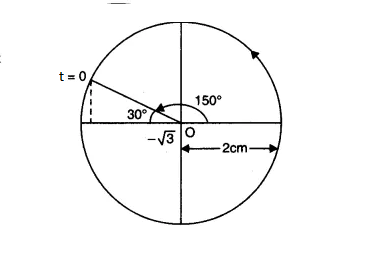
Answer:
$\\x(t)=cos(\frac{\pi }{6}-t)$
$x(t)=cos(t-\frac{\pi }{6})$
The initial position of the particle is x(0)
$\\x(0)=cos(0-\frac{\pi }{6})$
$ x(0)=cos(\frac{\pi }{6})$
$ x(0)=\frac{\sqrt{3}}{2}cm$
The radius of the circle, i.e. the amplitude, is 1 cm
The angular speed of the rotating particle is $\omega =1rad\ s^{-1}$
The initial phase is
$\\\phi =-\frac{\pi }{6}\\ \phi =-30^{o}$
The reference circle for the given simple Harmonic motion is
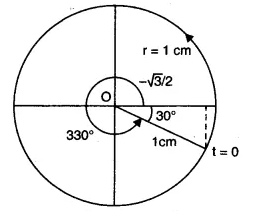
Answer:
$\begin{aligned}
x&=3 \sin (2 \pi t+\pi / 4) \\
x&=-3 \cos \left(2 \pi t+\frac{\pi}{4}+\frac{\pi}{2}\right) \\
& =3 \cos \left(2 \pi t+\pi+\frac{\pi}{4}+\frac{\pi}{2}\right) \\
& =3 \cos \left(2 \pi t+\frac{3 \pi}{2}+\frac{\pi}{4}\right) \\
& =3 \cos \left(2 \pi t+\frac{7 \pi}{4}\right)
\end{aligned}$
At t= 0
$phase=\frac{7\pi}{4}$
The reference circle is as follows

Answer:
$\\x(t)=2cos(\pi t)\\$
The initial position of the particle is x(0)
$\\x(0)=2cos(0)$
$x(0)=2cm$
The radius of the circle, i.e. the amplitude, is 2 cm
The angular speed of the rotating particle is $\omega =\pi rad\ s^{-1}$
The initial phase is
$\phi =0^{o}$
The reference circle for the given simple Harmonic motion is
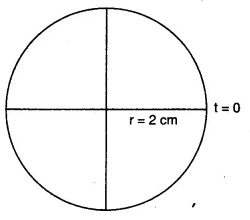
(b) is stretched by the same force F.
(a) What is the maximum extension of the spring in the two cases?
Answer:
(a) Let us assume the maximum extension produced in the spring is x.
At maximum extension
$\\F=Kx$
$x=\frac{F}{k}$
(b) Let us assume the maximum extension produced in the spring is x. That is x/2 due to the force towards the left and x/2 due to the force towards the right
$\\F=k\frac{x}{2}+k\frac{x}{2}$
$\Rightarrow x=\frac{F}{k}$
Answer:
Amplitude of SHM = 0.5 m
angular frequency is
$\begin{aligned}
& \omega=200 \mathrm{rad} / \mathrm{min} \\
& \omega=3.33 \mathrm{rad} / \mathrm{s}
\end{aligned}$
If the equation of SHM is given by
$x(t)=A \sin (\omega t+\phi)$
The velocity would be given by
$\begin{aligned}
& v(t)=\frac{\mathrm{d} x(t)}{\mathrm{d} t} \\
& v(t)=\frac{\mathrm{d}(A \sin (\omega t+\phi))}{\mathrm{d} t} \\
& v(t)=A \omega \cos (\omega t+\phi)
\end{aligned}$
The maximum speed is therefore
$\begin{aligned}
& v_{\max }=A \omega \\
& v_{\max }=0.5 \times 3.33 \\
& v_{\max }=1.67 \mathrm{~ms}^{-1}
\end{aligned}$
Answer:
The time period of a simple pendulum of length l executing S.H.M. is given by
$\begin{aligned}
& T=2 \pi \sqrt{\frac{l}{g}} \\
& \mathrm{~g}_{\mathrm{e}}=9.8 \mathrm{~m} \mathrm{~s}^{-2} \\
& \mathrm{~g}_{\mathrm{m}}=1.7 \mathrm{~m} \mathrm{~s}^{-2}
\end{aligned}$
The time period of the pendulum on the surface of Earth is $\mathrm{T}_{\mathrm{e}}=3.5 \mathrm{~s}$
The time period of the pendulum on the surface of the moon is $T_m$
$\begin{aligned}
& \frac{T_m}{T_e}=\sqrt{\frac{g_e}{g_m}} \\
& T_m=T_e \times \sqrt{\frac{g_e}{g_m}} \\
& T_m=3.5 \times \sqrt{\frac{9.8}{1.7}} \\
& T_m=8.4 \mathrm{~s}
\end{aligned}$
Answer:
Acceleration due to gravity = g (in downward direction)
Centripetal acceleration due to the circular movement of the car = a c
$a_{c}=\frac{v^{2}}{R}$ (in the horizontal direction)
Effective acceleration is
$\\g'=\sqrt{g^{2}+a_{c}^{2}}\\ g'=\sqrt{g^{2}+\frac{v^{4}}{R^{2}}}$
The time period is 'T'
$\\T'=2\pi \sqrt{\frac{l}{g'}}$
$ T'=2\pi \sqrt{\frac{l}{\sqrt{g^{2}+\frac{v^{4}}{R^{2}}}}}$
Q. 13.17 A cylindrical piece of cork of density $\rho$ of base area A and height h floats in a liquid of density $\rho _{\imath }$. The cork is depressed slightly and then released. Show that the cork oscillates up and down simply harmonically with a period $T=2\pi \sqrt{\frac{h\rho }{\rho _{ 1}g}}$ where $\rho$ is the density of the cork. (Ignore damping due to the viscosity of the liquid).
Answer:
Let the cork be displaced by a small distance x in the downward direction from its equilibrium position, where it is floating.
The extra volume of fluid displaced by the cork is Ax
Taking the downwards direction as positive, we have
$\begin{aligned}
&\begin{aligned}
& m a=-\rho_1 g A x \\
& \rho A h a=-\rho_1 g A x \\
& \frac{\mathrm{~d}^2 x}{\mathrm{~d} t^2}=-\frac{\rho_1 g}{\rho h} x
\end{aligned}\\
&\text { Comparing with } \mathrm{a}=-\mathrm{kx} \text { we have }\\
&\begin{aligned}
k & =\frac{\rho_1 g}{\rho h} \\
T & =\frac{2 \pi}{\sqrt{k}} \\
T & =2 \pi \sqrt{\frac{\rho h}{\rho_1 g}}
\end{aligned}
\end{aligned}$
Answer:
Let the height of each mercury column be h.
The total length of mercury in both columns = 2h.
Let the cross-sectional area of the mercury column be A.
Let the density of mercury be $\rho$
When either of the mercury columns dips by a distance x, the total difference between the two columns becomes 2x.
The weight of this difference is $2Ax\rho g$
This weight drives the rest of the column to the original mean position.
Let the acceleration of the column be a. Since the force is restoring
$\\2hA\rho (-a)=2xA\rho g\\ a=-\frac{g}{h}x$
$\frac{\mathrm{d}^{2}x }{\mathrm{d} t^{2}}=-\frac{g}{h}x$ which is the equation of a body executing S.H.M
The time period of the oscillation would be
$T=2\pi \sqrt{\frac{h}{g}}$
Oscillations NCERT Solutions: Additional Questions
The Class 11 Physics Chapter 13 - Oscillations; Additional Questions provide extra practice beyond the textbook, covering advanced problems and tricky concepts. These questions enhance analytical skills, deepen understanding of oscillatory motion, and prepare students for competitive exams like JEE and NEET.

Fig 1
Answer:
Let the initial volume and pressure of the chamber be V and P.
Let the ball be pressed by a distance x.
This will change the volume by an amount ax.
Let the change in pressure be $\Delta P$
Let the Bulk modulus of air be K.
$\\K=\frac{\Delta P}{\Delta V/V}$
$ \Delta P=\frac{Kax}{V}$
This pressure variation would try to restore the position of the ball.
Since force is restoring in nature, displacement and acceleration due to the force would be in different directions.
$\begin{aligned}
& F=a \Delta P \\
& -m \frac{\mathrm{~d}^2 x}{\mathrm{~d} t^2}=a \Delta p \\
& \frac{\mathrm{~d}^2 x}{\mathrm{~d} t^2}=-\frac{k a^2}{m V} x
\end{aligned}$
The above is the equation of a body executing S.H.M.
The time period of the oscillation would be
$T=\frac{2\pi }{a}\sqrt{\frac{mV}{k}}$
Q. 2 (a) You are riding in an automobile of mass $3000\; kg.$. Assuming that you are examining the oscillation characteristics of its suspension system. The suspension sags $15\; cm$ when the entire automobile is placed on it. Also, the amplitude of oscillation decreases by $50^{o}/_{o}$ during one complete oscillation. Estimate the value of the spring constant $K$
Answer:
Mass of automobile (m) = 3000 kg
There are a total of four springs.
Compression in each spring, x = 15 cm = 0.15 m
Let the spring constant of each spring be k
$\\4kx=mg$
$ k=\frac{3000\times 9.8}{4\times 0.15}$
$ k=4.9\times 10^{4}\ N$
Q. 2 (b) You are riding in an automobile of mass $3000kg\;$. Assuming that you are examining the oscillation characteristics of its suspension system. The suspension sags $15\; cm$ when the entire automobile is placed on it. Also, the amplitude of oscillation decreases by $50^{o}/_{o}$ during one complete oscillation. Estimate the values of the damping constant b for the spring and shock absorber system of one wheel, assuming that each wheel supports $750 \; kg$.
Answer:
The amplitude of oscillation decreases by 50 % in one oscillation, i.e. in one time period.
$\begin{aligned}
T & =2 \pi \sqrt{\frac{m}{k}} \\
T & =2 \pi \times \sqrt{\frac{3000}{4 \times 4.9 \times 10^4}} \\
T & =0.77 \mathrm{~s}
\end{aligned}$
For the damping factor b, we have
$\begin{aligned}
& x=x_0 e^{\left(-\frac{b t}{2 m}\right)} \\
& \mathrm{x}=\mathrm{x}_0 / 2 \\
& \mathrm{t}=0.77 \mathrm{~s} \\
& \mathrm{~m}=750 \mathrm{~kg} \\
& e^{-\frac{0.75 \%}{2 \times 750}}=0.5 \\
& \ln \left(e^{-\frac{0.775}{2 \times 780}}\right)=\ln 0.5 \\
& \frac{0.77 b}{1500}=\ln 2 \\
& b=\frac{0.693 \times 1500}{0.77} \\
& b=1350.2287 \mathrm{~kg} \mathrm{~s}^{-1}
\end{aligned}$
Answer:
Let the equation of oscillation be given by $x=Asin(\omega t)$
Velocity would be given as
$\\v=\frac{dx}{dt}$
$ v=A\omega cost(\omega t)$
Kinetic energy at an instant is given by
$
\begin{aligned}
K(t) & =\frac{1}{2} m(v(t))^2 \\
K(t) & =\frac{1}{2} m(A \omega \cos (\omega t))^2 \\
K(t) & =\frac{1}{2} m A^2 \omega^2 \cos ^2 \omega t
\end{aligned}
$
The time period is given by
$T=\frac{2\pi }{\omega }$
The Average Kinetic Energy would be given as follows
$
\begin{aligned}
& K_{a v}=\frac{\int_0^T K(t) d t}{\int_0^T d t} \\
& K_{a v}=\frac{1}{T} \int_0^T K(t) d t \\
& K_{a v}=\frac{1}{T} \int_0^T \frac{1}{2} m A^2 \omega^2 \cos ^2 \omega t d t \\
& K_{a v}=\frac{m A^2 \omega^2}{2 T} \int_0^T \cos ^2 \omega t d t \\
& K_{a v}=\frac{m A^2 \omega^2}{2 T} \int_0^T\left(\frac{1+\cos 2 \omega t}{2}\right) d t \\
& K_{a v}=\frac{m A^2 \omega^2}{2 T}\left[\frac{t}{2}+\frac{\sin 2 \omega t}{4 \omega}\right]_0^T \\
& K_{a v}=\frac{m A^2 \omega^2}{2 T}\left[\left(\frac{T}{2}+\frac{\sin 2 \omega T}{4 \omega}\right)-(0+\sin (0))\right] \\
& K_{a v}=\frac{m A^2 \omega^2}{2 T} \times \frac{T}{2} \\
& K_{a v}=\frac{m A^2 \omega^2}{4}
\end{aligned}
$
The potential energy at an instant T is given by
$
\begin{aligned}
& U(t)=\frac{1}{2} k x^2 \\
& U(t)=\frac{1}{2} m \omega^2(A \sin (\omega t))^2 \\
& U(t)=\frac{1}{2} m \omega^2 A^2 \sin ^2 \omega t
\end{aligned}
$
The Average Potential Energy would be given by
$
\begin{aligned}
& U_{a v}=\frac{\int_0^T U(t) d t}{\int_0^T d t} \\
& U_{a v}=\frac{1}{T} \int_0^T \frac{1}{2} m \omega^2 A^2 \sin ^2 \omega t d t \\
& U_{a v}=\frac{m \omega^2 A^2}{2 T} \int_0^T \sin ^2 \omega t d t \\
& U_{a v}=\frac{m \omega^2 A^2}{2 T} \int_0^T \frac{(1-\cos 2 \omega t)}{2} d t \\
& U_{a v}=\frac{m \omega^2 A^2}{2 T}\left[\frac{t}{2}-\frac{\sin 2 \omega t}{4 \omega}\right]_0^T \\
& U_{a v}=\frac{m \omega^2 A^2}{2 T}\left[\left(\frac{T}{2}-\frac{\sin 2 \omega T}{4 \omega}\right)-(0-\sin 0)\right] \\
& U_{a v}=\frac{m \omega^2 A^2}{2 T} \times \frac{T}{2} \\
& U_{a v}=\frac{m \omega^2 A^2}{4}
\end{aligned}
$
We can see K av = U av
Answer:
$J=-a\; \theta$
The moment of Inertia of the disc about the axis passing through its centre and perpendicular to it is
$
\begin{aligned}
& I=\frac{M R^2}{2} \\
& J=I \frac{\mathrm{~d}^2 \theta}{\mathrm{~d} t^2} \\
& -a \theta=\frac{M R^2}{2} \frac{\mathrm{~d}^2 \theta}{\mathrm{~d} t^2} \\
& \frac{\mathrm{~d}^2 \theta}{\mathrm{~d} t^2}=-\frac{2 a}{M R^2} \theta
\end{aligned}
$
The period of Torsional oscillations would be
$
\begin{aligned}
& T=2 \pi \sqrt{\frac{M R^2}{2 a}} \\
& a=\frac{2 \pi^2 M R^2}{T^2} \\
& a=\frac{2 \pi^2 \times 10 \times(0.15)^2}{(1.5)^2} \\
& a=1.97 \mathrm{~N} \mathrm{~m} \mathrm{rad}{ }^{-1}
\end{aligned}
$
Q. 5 (a) A body describes simple harmonic motion with an amplitude of $5\; cm$ and a period of $0.2\; s$. Find the acceleration and velocity of the body when the displacement is $5\; cm$
Answer:
$\begin{aligned}
& \mathrm{A}=5 \mathrm{~cm}=0.05 \mathrm{~m} \\
& \mathrm{~T}=0.2 \mathrm{~s} \\
& \omega=\frac{2 \pi}{T} \\
& \omega=\frac{2 \pi}{0.2} \\
& \omega=10 \pi \mathrm{rad} \mathrm{~s}^{-1}
\end{aligned}$
At displacement x acceleration is $a=-\omega ^{2}x$
At displacement x velocity is $v=\omega \sqrt{A^{2}-x^{2}}$
(a)At displacement 5 cm
$
\begin{aligned}
& v=10 \pi \sqrt{(0.05)^2-(0.05)^2} \\
& v=0 \\ \\
& a=-(10 \pi)^2 \times 0.05 \\
& a=-49.35 m s^{-2}
\end{aligned}
$
Q. 5 (b) A body describes simple harmonic motion with an amplitude of $5\; cm$ and a period of $0.2 \; s$. Find the acceleration and velocity of the body when the displacement is $3\; cm$
Answer:
$
\begin{aligned}
& \mathrm{A}=5 \mathrm{~cm}=0.05 \mathrm{~m} \\
& \mathrm{~T}=0.2 \mathrm{~s} \\
& \omega=\frac{2 \pi}{T} \\
& \omega=\frac{2 \pi}{0.2} \\
& \omega=10 \pi \mathrm{rad} \mathrm{~s}^{-1}
\end{aligned}
$
At displacement x acceleration is $a=-\omega ^{2}x$
At displacement x velocity is $v=\omega \sqrt{A^{2}-x^{2}}$
(a)At displacement 3 cm
$
\begin{aligned}
& v=10 \pi \sqrt{(0.05)^2-(0.03)^2} \\
& v=10 \pi \sqrt{0.0016} \\
& v=10 \pi \times 0.04 \\
& v=1.257 \mathrm{~ms}^{-1} \\ \\
& a=-(10 \pi)^2 \times 0.03 \\
& a=-29.61 \mathrm{~ms}^{-2}
\end{aligned}
$
Q. 5 (c) A body describes simple harmonic motion with an amplitude of $5\; cm$ and a period of $0.2\; s$. Find the acceleration and velocity of the body when the displacement is $0 \; cm$
Answer:
$
\begin{aligned}
& \mathrm{A}=5 \mathrm{~cm}=0.05 \mathrm{~m} \\
& \mathrm{~T}=0.2 \mathrm{~s} \\
& \omega=\frac{2 \pi}{T} \\
& \omega=\frac{2 \pi}{0.2} \\
& \omega=10 \pi \mathrm{rad} \mathrm{~s}^{-1}
\end{aligned}
$
At displacement x acceleration is $a=-\omega ^{2}x$
At displacement x velocity is $v=\omega \sqrt{A^{2}-x^{2}}$
(a)At displacement 0 cm
$
\begin{aligned}
& v=10 \pi \sqrt{(0.05)^2-(0)^2} \\
& v=10 \pi \times 0.05 \\
& v=1.57 \mathrm{~ms}^{-1} \\ \\
& a=-(10 \pi)^2 \times 0 \\
& a=0
\end{aligned}
$
Answer:
At the maximum extension of the spring, the entire energy of the system would be stored as the potential energy of the spring.
Let the amplitude be A
$\begin{aligned}
& \frac{1}{2} k A^2=\frac{1}{2} m v_0^2+\frac{1}{2} k x_0^2 \\
& A=\sqrt{x_0^2+\frac{m}{k} v_0^2}
\end{aligned}$
The angular frequency of a spring-mass system is always equal to $\sqrt{\frac{k}{m}}$
Therefore
$A=\sqrt{x_0^2+\frac{v_0^2}{\omega^2}}$
Class 11 Physics Chapter 13 - Oscillations: Higher Order Thinking Skills (HOTS) Questions
The Class 11 Physics Chapter 13 - Oscillations HOTS Questions are designed to challenge students with advanced problem-solving and application-based scenarios. These questions go beyond the basics, sharpening logical reasoning and preparing students for competitive exams like JEE and NEET.
Q1. A particle executes simple harmonic motion with a time period of 2 seconds and an amplitude of 1 cm. If D and d are the total distance and displacement covered by the particle in 12.5 s, then $\frac{\mathrm{D}}{\mathrm{d}}$ is:-
Answer:
$\mathrm{A}=1 \mathrm{~cm}$
$\mathrm{T} = 2 \mathrm{~sec}$
Now, 0.5 sec = T/4
$\therefore d = 1 \mathrm{~cm} \sin(\frac{2\pi}{T}\frac{T}{4}) = 1 \mathrm{~cm}$
Number of complete cycles in 12.5 sec = 12.5 / 2 = 6.25 cycles.
Distance covered in one cycle = 4A = 4cm
$\therefore D = 6.25 \times 4 = 25 \mathrm{~cm}$
$\frac{\mathrm{D}}{\mathrm{d}}=25$
Hence, the answer is 25.
Q2. With a period of 5s, a particle moves in simple harmonic motion. The particle takes 1/a time to cover a displacement from the mean location equal to half of its amplitude. To the closest integer, the value of "a" is:
Answer:
Time period, T=5sec
Time taken by the particle, $\mathrm{t}=1 / \mathrm{a}$
$x=A \sin (\omega t+\phi)$ [ Where, phase constant $\phi=0$ at mean position, $\omega$ is angular velocity, A is amplitude.]
$ \frac{A}{2}=A \sin \left(\frac{2 \pi}{T}\right) t $
$ \frac{2 \pi}{T} t=\sin ^{-1}\left(\frac{1}{2}\right) $
$\frac{2 \pi}{T} t=\frac{\pi}{6} $
$ t=\frac{1}{6}$
So, $a=6$
Hence, the answer is 6.
NCERT Solutions for Class 11 Physics Chapter 13 - Oscillations: Topics
The NCERT Class 11 Physics Chapter Topics of Chapter 13 Oscillations include the periodic motion, simple harmonic motion (SHM), velocity and acceleration in SHM, simple pendulum. These concepts form the bridge to the concept of vibrations, sound waves and real-life multiple oscillatory systems.
13.1 Introduction
13.2 Periodic And Oscillatory Motions
13.3 Simple harmonic motion
13.4 Simple harmonic motion and uniform circular motion
13.5 Velocity and acceleration in simple harmonic motion
13.6 Force law for simple harmonic motion
13.7 Energy In Simple Harmonic Motion
13.8 The simple pendulum
NCERT Solutions for Class 11 Physics Chapter 13 - Oscillations: Important Formulas
The key equations of Class 11 Physics Chapter 13 - Oscillations serve as the foundation for resolving numerical and conceptual problems. These equations include displacement, velocity, acceleration, energy and the time period of oscillatory motion, and therefore, these equations are necessary both to appear in board exams as well as in competitive exams like NEET or JEE. Having them all together will enable students to revise within a shorter time and become more effective in solving problems.
1. Displacement in SHM:
$
x(t)=A \sin (\omega t+\phi) \text { or } x(t)=A \cos (\omega t+\phi)
$
where $A=$ amplitude, $\omega=$ angular frequency, $\phi=$ phase constant
2. Velocity in SHM:
$v(t)=\frac{d x}{d t}=\omega A \cos (\omega t+\phi)$ or $v(t)=-\omega A \sin (\omega t+\phi)$
3. Acceleration in SHM:
$a(t)=\frac{d^2 x}{d t^2}=-\omega^2 x$
4. Angular frequency ( $\omega$ ):
$
\omega=\sqrt{\frac{k}{m}}(\text { spring system }), \omega=\sqrt{\frac{g}{l}}(\text { simple pendulum })
$
5. Time period (T) and Frequency (f):
$T=\frac{2 \pi}{\omega}, f=\frac{1}{T}$
6. Maximum values:
$v_{\max }=\omega A, a_{\max }=\omega^2 A$
7. Energy in SHM:
- Kinetic Energy:
$
K E=\frac{1}{2} m \omega^2\left(A^2-x^2\right)
$
- Potential Energy:
$
P E=\frac{1}{2} m \omega^2 x^2
$
- Total Energy:
$
E=\frac{1}{2} m \omega^2 A^2
$
8. Equation of simple pendulum (small oscillations):
$T=2 \pi \sqrt{\frac{l}{g}}$
How Can NCERT Solutions for Class 11 Physics Chapter 13 Oscillations Help in Exam Preparation?
NCERT Solutions of Class 11 Physics Chapter 13 Oscillations can be used to make students learn about the concept of periodic and simple harmonic motion (SHM) in a systematic and clear manner. They make difficult concepts such as amplitude, phase, frequency and restoring force easier, which are the foundation of most physics exam problems. NCERT Solutions of Class 11 Physics Chapter 13 Oscillations can be used to make students learn about the concept of periodic and simple harmonic motion (SHM) in a systematic and clear manner. They make difficult concepts such as amplitude, phase, frequency and restoring force easier, which are the foundation of most physics exam problems. Regular practice with these NCERT solutions helps students gain confidence in tackling derivations, graphs, and problem-based questions, which are often asked in CBSE, JEE, and NEET exams. This dedicated preparation makes the understanding of oscillatory motion good, and prepares a good foundation on which the next chapters, such as Waves and Sound, will be built.
Approach to solve the NCERT Class 11 Physics Chapter 13 - Oscillations
The right approach can go an extra mile when answering questions of Chapter 13 - Oscillations of Class 11 Physics. Rather than just going into formulas, students are expected to concentrate on picturing the motion, the correlation between restoring force and motion, and whether the system undergoes simple harmonic motion (SHM). The systematic approach to solving problems not only saves time; it also assists in preventing confusion when using formulas and working out numerical problems.
- Determine the kind of oscillation:
- Determine whether it is a simple pendulum, spring-mass system or a damped / forced oscillation.
- Identify whether it is a simple harmonic motion (SHM) or not.
-
Write the equation of motion:
-
For SHM, start with $F=-k x$ (spring) or $F=-m g \sin \theta \approx-m g \theta$ (pendulum).
-
Obtain the differential equation to ensure that it has the form: $\frac{d^2 x}{d t^2}+\omega^2 x=0$
-
Relate angular frequency, time period, and frequency:
-
Use the relation $\omega=\sqrt{\frac{k}{m}}$ for a spring or $\omega=\sqrt{\frac{g}{l}}$ for a pendulum.
-
From this, find the time period: $T=\frac{2 \pi}{\omega}$.
-
Use the energy method, where necessary:
-
Decompose the problem into potential energy ( U) and kinetic energy (K).
-
Use: $E=K+U=\frac{1}{2} k A^2$, where $A$ is amplitude.
-
This method is applied when the maximum speed, displacement or turning points are required.
-
Use initial conditions:
-
Numerical problems include initial displacement or velocity.
-
These values are to be inserted into the general solution: $x(t)=A \cos (\omega t+\phi)$.
-
Solve for constants like A (amplitude) and ϕ (phase constant).
Importance of NCERT Solutions for Class 11 Physics Chapter 13: Oscillations
NCERT Solutions for Class 11 Physics Chapter 13: Oscillations are highly important for understanding periodic motion and its mathematical description. This chapter presents the students with concepts which explain vibrations and repetitive movements that are widely found in nature and technology.
- Enables students to have a clearer understanding of the concept of oscillatory motion and simple harmonic motion (SHM).
- Defines important quantities such as amplitude, period of time, frequency, phase, velocity, and acceleration in a logical structure.
- Establishes a firm background to further discussion, such as waves, sound and alternating current.
- Plays an important role in CBSE board exams, where conceptual questions and numericals from SHM are frequently asked.
- Highly useful for competitive exams like JEE and NEET, especially questions based on SHM graphs and energy.
- Develops problem-solving ability by solving numerical problems step-by-step in the case of spring-mass systems and pendulums.
- Helps the students to make the connection between theory and life-based situations, like the pendulum clocks, musical instruments, and vibrations in machines.
- Allows proper use of formulas and concepts in various physical cases of oscillations.
What Students Learn from NCERT Solutions for Class 11 Physics Chapter 13: Oscillations?
The chapter Oscillations guides students on the idea of motions repeating themselves in a pattern. Through the assistance of NCERT Solutions of Class 11 Physics Chapter 13, students obtain both physical and mathematical interpretations of the meaning of the oscillatory motion.
- Learn the definition of oscillatory motion and how it is different to the other forms of motion.
- Know Simple Harmonic Motion (SHM) and the conditions that one must have to have a SHM motion.
- Learn how to calculate the main quantities of amplitude, time period, frequency, angular frequency and phase.
- Study the time dependence of displacement, velocity and acceleration of SHM.
- Knows the concept of energy in SHM, exchange between kinetic and potential energy.
- Examine the oscillations of systems such as a spring-mass system and a simple pendulum.
- Find out what the correlation is between uniform circular motion and SHM.
- Develop skills to solve numerical problems using correct formulas and step-by-step methods.
What Extra Should Students Study Beyond NCERT for JEE/NEET?
For JEE and NEET, just the NCERT is not enough in the Class 11 Physics Chapter 13 - Oscillations, as exams demand deeper problem-solving and application-based learning. Students should go beyond the basics and study advanced concepts, tricky derivations, and practice high-level numerical problems to strengthen their grasp for competitive exams.
NCERT Solutions for Class 11 Physics Chapter-Wise
NCERT Solutions for Class 11 Physics provide detailed, step-by-step answers to all chapters, making it easier for students to master concepts and score well in exams. With chapter-wise links, students can quickly access solutions, practice exercises, and prepare effectively for school tests, JEE, and NEET.
NCERT solutions for class 11, Subject-wise
Find the links to subject-wise NCERT Solutions below.
Also Check NCERT Books and NCERT Syllabus here
- NCERT Books Class 11 Physics
- NCERT Syllabus Class 11 Physics
- NCERT Books Class 11
- NCERT Syllabus Class 11
Subject-wise NCERT Exemplar solutions
Frequently Asked Questions (FAQs)
From the NCERT chapter oscillations, two questions can be expected for NEET exam. For more questions solve NEET previous year papers.
Yes, oscillation is important for JEE Main. One or two question can be expected from oscillations for JEE Main. It is one of the important chapter for scholarship exams like KVPY and NSEP. To solve more problems on Oscillations refer to NCERT book, NCERT exemplar and JEE main previous year papers.
Yes, you can find free and detailed Oscillations Class 11 NCERT Solutions PDF online. These solutions help you understand the chapter better and prepare well for exams.
They include step-by-step answers to all questions in the NCERT textbook for Chapter 13 – Oscillations. The solutions explain every concept clearly to help you with homework, tests, and competitive exams.
Careers360 provides precise oscillation questions and answers pdf , designed by Physics experts considering the new CBSE exam pattern. These solutions offer comprehensive knowledge for students to excel in their exams.
Chapter 13 of NCERT Physics solutions covers the topic of Simple Harmonic Motion. This includes different formulas and graphs of the motion, and the various ways to understand it using energy and circular motion.
Since Oscillations and SHM questions are commonly found in competitive exams, becoming knowledgeable with the NCERT Solutions guarantees:
- Excellent conceptual clarity
- Capacity to solve HOTS problems both conceptually and numerically
- Understanding of the format and topics of the CBSE exam
Phase is a quantity that describes the state of an SHM at any instant of time. Phase shift denotes the starting position in an SHM. It is the position of the body at t = 0.
SHM may be described as a projection of uniform circular motion on a diameter of a circle.
SHM is used to understand vibrations, wave motion, and most phenomena in nature, and it is the basis of other topics, such as waves and sound.
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters




