NCERT Solutions for Class 11 Physics Chapter 12 Kinetic Theory
Have you ever questioned yourself as to why gases expand or put pressure on the sides of a container? The scientific explanation is given by the Kinetic Theory of Gases, which assumes that gases are made of small particles in everlasting, rapid movement. This theory is the work of great scientists such as Boyle, Newton, Maxwell, and Boltzmann, and it is used to explain how gases act in response to changes in pressure and temperature and how they diffuse or mix.
This Story also Contains
- NCERT Solutions for Class 11 Physics Chapter 12 - Kinetic Theory: Download PDF
- NCERT Solutions for Class 11 Physics Chapter 12 - Kinetic Theory- Exercise Questions
- NCERT Solutions for Class 11 Physics Chapter 12 - Kinetic Theory - Additional Questions
- NCERT Solutions for Class 11 Physics Chapter 12 - Kinetic Theory: Higher Order Thinking Skills (HOTS) Questions
- Class 11 Physics Chapter 12 - Kinetic Theory: Topics
- Class 11 Physics Chapter 12 - Kinetic Theory: Important Formulae
- Approach to Solve Questions of the Class 11 Physics Chapter 12 - Kinetic Theory
- How Can NCERT Solutions for Class 11 Physics Chapter 12 Kinetic Theory Help in Exam Preparation?
- What Extra Should Students Study Beyond NCERT for JEE/NEET?
- NCERT Solutions for Class 11 Physics Chapter-Wise
The NCERT Solutions for Class 11 Physics Chapter 12 - Kinetic Theory are very valuable not only in the preparation for the CBSE board but also for competitive exams such as JEE and NEET. The NCERT solutions are the work of physics experts who make complex concepts simple and offer a step-by-step approach to resolving numerical and theoretical problems. These NCERT Solutions for Class 11 Physics Chapter 12 - Kinetic Theory are prepared as per the recent CBSE syllabus, making them conceptually clear and helping the students to develop their ability to solve problems. Through such NCERT solutions, students will be able to have confidence in the chapter as well as score highly in exams.
Also Read
NCERT Solutions for Class 11 Physics Chapter 12 - Kinetic Theory: Download PDF
The Class 11 Physics Chapter 12 - Kinetic Theory question answers include step-by-step solutions of the textbook questions, which help the students to have a clear picture of the behaviour of gases at the molecular scale. One can access these class 11 physics chapter 12 Kinetic Theory questions answers online and download them as a PDF solution without any payment, making practice and revision convenient.
NCERT Solutions for Class 11 Physics Chapter 12 - Kinetic Theory- Exercise Questions
Kinetic Theory class 11 question answers provide all the detailed solutions to the exercises, which allow students to understand the behaviour of gases easily at the microscopic level. The class 11 physics chapter 12 Kinetic Theory questions answers make things easy to understand, such as explaining concepts such as pressure, mean free path, gas laws, and the kinetic interpretation of temperature, developing competitive fundamentals to prepare students for exams and other competitive tests like the JEE or NEET.
Answer:
The diameter of an oxygen molecule, d = 3 Å.
The actual volume of a mole of oxygen molecules, Vactual is
$\\V_{actual}=N_{A}\frac{4}{3}\pi \left ( \frac{d}{2} \right )^{3}$ $ \\ $
$V_{actual}=6.023\times 10^{23}\times \frac{4}{3}\pi \times \left ( \frac{3\times 10^{-10}}{2} \right )^{3}\\$
$ V_{actual}=8.51\times 10^{-6}m^{3}\\ $
$V_{actual}=8.51\times 10^{-3}litres$
The volume occupied by a mole of oxygen gas at STP is V molar = 22.4 litres
$\\\frac{V_{actual}}{V_{molar}}=\frac{8.51\times 10^{-3}}{22.4}\\ $
$\frac{V_{actual}}{V_{molar}}=3.8\times 10^{-4}$
Answer:
As per the ideal gas equation
$\\PV=nRT\\ V=\frac{nRT}{P}$
For one mole of a gas at STP, we have
$\\V=\frac{1\times 8.314\times 273}{1.013\times 10^{5}}\\$
$ V=0.0224 m^{3}\\ $
$V=22.4\ litres$
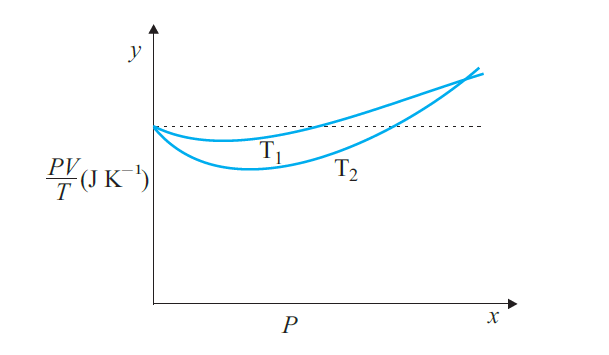
Answer:
(a) The dotted plot corresponds to the ideal gas behaviour.
(b) We know the behaviour of a real gas tends closer to that of an ideal gas as its temperature increases, and since the plot corresponding to temperature T1 is closer to the horizontal line than the one corresponding to T2 we conclude T1 is greater than T2.
(c) As per the ideal gas equation
$\frac{PV}{T}=nR$
The molar mass of oxygen = 32 g
$n=\frac{1}{32}$
R = 8.314
$\\nR=\frac{1}{32}\times 8.314$
$ nR=0.256JK^{-1}$
(d) If we obtained similar plots for $1.00\times 10^{-3}$ kg of hydrogen, we would not get the same value of $PV/T$ at the point where the curves meet on the y-axis as 1 g of Hydrogen would contain more moles than 1 g of Oxygen because of having a smaller molar mass.
Molar Mass of Hydrogen M = 2 g
mass of hydrogen
$m=\frac{PV}{T} \frac{M}{R}={0.256}\times \frac{2}{8.314}=5.48\times10^{-5}Kg$
Answer:
Initial volume, V1 = Volume of Cylinder = 30 l
Initial Pressure P1 = 15 atm
Initial Temperature T1 = 27 o C = 300 K
The initial number of moles n1 inside the cylinder is
$n_{1}=\frac{P_{1}V_{1}}{RT_{1}}$
$n_{1}=\frac{15\times 1.013\times 10^{5}\times 30\times 10^{-3}}{8.314\times 300}$
$ n_{1}=18.28$
Final volume, V2 = Volume of Cylinder = 30 l
Final Pressure P2 = 11 atm
Final Temperature T2 = 17 o C = 290 K
The final number of moles n2 inside the cylinder is
$n_{2}=\frac{P_{2}V_{2}}{RT_{2}}$
$n_{2}=\frac{11\times 1.013\times 10^{5}\times 30\times 10^{-3}}{8.314\times 290}$
$ n_{2}=13.86$
Moles of oxygen taken out of the cylinder = n 2 -n 1 = 18.28 - 13.86 = 4.42
Mass of oxygen taken out of the cylinder, m is
$\\m=4.42\times 32$
$ m=141.44g$
Answer:
Initial Volume of the bubble, V1 = 1.0 cm 3
Initial temperature, T1 = 12 o C = 273 + 12 = 285 K
The density of water is $\rho _{w}=10^{3}\ kg\ m^{-3}$
Initial Pressure is P1
Depth of the bottom of the lake = 40 m
$\begin{aligned}
& P_1=\text { Atmospheric Pressure }+ \text { Pressure due to water } \\
& P_1=P_{a t m}+\rho_w g h \\
& P_1=1.013 \times 10^5+10^3 \times 9.8 \times 40 \\
& P_1=4.93 \times 10^5 \mathrm{~Pa}
\end{aligned}$
Final Temperature, T 2 = 35 o C = 35 + 273 = 308 K
Final Pressure = Atmospheric Pressure $=1.013\times 10^{5}Pa$
Let the final volume be V 2
As the number of moles inside the bubble remains constant, we have
$\\\frac{P_{1}V_{1}}{T_{1}}=\frac{P_{2}V_{2}}{T_{2}}\\$
$ V_{2}=\frac{P_{1}T_{2}V_{1}}{P_{2}T_{1}}\\ $
$V_{2}=\frac{4.93\times 10^{5}\times 308\times 1}{1.013\times 10^{5}\times 285}\\ $
$V_{2}=5.26\ cm^{3}$
Answer:
The volume of the room, V = 25.0 m 3
Temperature of the room, T = 27 o C = 300 K
The pressure inside the room, P = 1 atm
Let the number of moles of air molecules inside the room be n
$\begin{aligned}
& n=\frac{P V}{R T} \\
& n=\frac{1.013 \times 10^5 \times 25}{8.314 \times 300} \\
& n=1015.35
\end{aligned}$
Avogadro's Number, $N_A=6.022 \times 10^{23}$
The number of molecules inside the room is N
$\begin{aligned}
& N=n N_A \\
& N=1015.35 \times 6.022 \times 10^{23} \\
& N=6.114 \times 10^{26}
\end{aligned}$
Answer:
The average energy of a Helium atom is given as $\frac{3kT}{2}$ since it is monoatomic
(i)
$\\E=\frac{3kT}{2}\\ $
$E=\frac{3\times 1.38\times 10^{-23}\times 300}{2}\\ $
$E=6.21\times 10^{-21}\ J$
(ii)
$\\E=\frac{3kT}{2}\\ $
$E=\frac{3\times 1.38\times 10^{-23}\times 6000}{2}\\ $
$E=1.242\times 10^{-19}\ J$
(iii)
$\\E=\frac{3kT}{2}\\$
$ E=\frac{3\times 1.38\times 10^{-23}\times10^{7}}{2}\\ $
$E=2.07\times 10^{-16}\ J$
Answer:
As per Avogadro's Hypothesis, under similar conditions of temperature and pressure, equal volumes of gases contain an equal number of molecules. Since the volume of the vessels is the same and all vessels are kept at the same conditions of pressure and temperature, they would contain an equal number of molecules.
Root mean square velocity is given as
$v_{rms}=\sqrt{\frac{3kT}{m}}$
As we can see, vrms is inversely proportional to the square root of the molar mass; the root mean square velocity will be maximum in the case of Neon, as its molar mass is the least.
Answer:
As we know root mean square velocity is given as $v_{rms}=\sqrt{\frac{3RT}{M}}$
Let at temperature T the root mean square speed of an atom in an argon cylinder equal to the rms speed of a helium gas atom at $-20^{0}C$
$\sqrt{\frac{3R\times T}{39.9}}=\sqrt{\frac{3R\times 253}{4}}$
$ T=2523.7\ K$
Answer:
Pressure, P = 2atm
Temperature, T = 17 o C
The radius of the Nitrogen molecule, r=1 Å.
The molecular mass of N 2 = 28 u
The molar mass of N 2 = 28 g
From the ideal gas equation
$\\PV=nRT\\ $
$\frac{n}{V}=\frac{P}{RT}\\$
The above tells us about the number of moles per unit volume; the number of molecules per unit volume would be given as
$\\n'=\frac{N_{A}n}{V}=\frac{6.022\times 10^{23}\times 2\times 1.013\times 10^{5}}{8.314\times (17+273)}\\ $
$n'=5.06\times 10^{25}$
The mean free path $\lambda$ is given as
$\\\lambda =\frac{1}{\sqrt{2}\pi n'd^{2}}\\$
$ \lambda =\frac{1}{\sqrt{2}\times \pi \times 5.06\times 10^{25}\times (2\times 1\times 10^{-10})^{2}}\\ $
$\lambda =1.11\times 10^{-7}\ m$
The root mean square velocity v rms is given as
$\\v_{rms}=\sqrt{\frac{3RT}{M}}\\ $
$v_{rms}=\sqrt{\frac{3\times 8.314\times 290}{28\times 10^{-3}}}\\ $
$v_{rms}=508.26\ m\ s^{-1}$
The time between collisions T, is given as
$\\T=\frac{1}{Collision\ Frequency}\\ $
$T=\frac{1}{\nu }\\$
$ T=\frac{\lambda }{v_{rms}}\\ $
$T=\frac{1.11\times 10^{-7}}{508.26}\\ $
$T=2.18\times 10^{-10}s$
Collision time T' is equal to the average time taken by a molecule to travel a distance equal to its diameter
$\\T'=\frac{d}{v_{rms}}\\ $
$T'=\frac{2\times 1\times 10^{-10}}{508.26}\\ $
$T'=3.935\times 10^{-13}s$
The ratio of the average time between collisions to the collision time is
$
\begin{aligned}
\frac{T}{T^{\prime}} & =\frac{2.18 \times 10^{-10}}{3.935 \times 10^{-13}} \\
\frac{T}{T^{\prime}} & =554
\end{aligned}
$
Thus, we can see that the time between collisions is much larger than the collision time.
NCERT Solutions for Class 11 Physics Chapter 12 - Kinetic Theory - Additional Questions
The Class 11 Physics Chapter 12 - Kinetic Theory Additional Questions have been provided to boost the problem-solving skills by discussing extra problems besides the NCERT textbook. The questions enhance an appreciation of such concepts as molecular motion, pressure of gases, mean free path, equipartition of energy, and are relevant in both board examinations and competitive entrance examinations such as JEE and NEET.
Answer:
Initially, the pressure of the 15 cm long air column is equal to the atmospheric pressure, P 1 = 1 atm = 76 cm of Mercury
Let the cross-sectional area of the tube be x cm 2
The initial volume of the air column, V 1 = 15x cm 3
Let's assume that once the tube is held vertical, y cm of Mercury flows out of it.
The pressure of the air column after y cm of Mercury has flown out of the column P 2 = 76 - (76 - y) cm of Mercury = y cm of mercury
Final volume of air column V 2 = (24 + y)x cm 3
Since the temperature of the air column does not change
$\\P_{1}V_{1}=P_{2}V_{2}\\ 76\times 15x=y\times (24+y)x\\ 1140=y^{2}+24y\\ y^{2}+24y-1140=0$
Solving the above quadratic equation, we get y = 23.8 cm or y = -47.8 cm
Since a negative amount of mercury cannot flow out of the column, y cannot be negative. Therefore, y = 23.8 cm.
Length of the air column = y + 24 = 47.8 cm.
Therefore, once the tube is held vertically, 23.8 cm of Mercury flows out of it, and the length of the air column becomes 47.8 cm
Answer:
As per Graham's Law of diffusion, if two gases of Molar Mass M 1 and M 2 diffuse with rates R 1 and R 2, respectively, their diffusion rates are related by the following equation
$\frac{R_{1}}{R_{2}}=\sqrt{\frac{M_{2}}{M_{1}}}$
In the given question
R 1 = 28.7 cm 3 s -1
R 2 = 7.2 cm 3 s -1
M 1 = 2 g
$\\M_{2}=M_{1}\left ( \frac{R_{1}}{R_{2}} \right )^{2}\\$
$M_{2}=2\times \left ( \frac{28.7}{7.2} \right )^{2}\\$
$M_{2}=31.78g$
The above Molar Mass is close to 32; therefore, the gas is Oxygen.
Q 3) A gas in equilibrium has uniform density and pressure throughout its volume. This is strictly true only if there are no external influences. A gas column under gravity, for example, does not have uniform density (and pressure). As you might expect, its density decreases with height. The precise dependence is given by the so-called law of atmospheres $n_{2}=n_{1}exp\left [ -mg(h_{2}-h_{1}) /k_{b}T\right ]$ where n2, n1 refer to number density at heights h2 and h1 respectively. Use this relation to derive the equation for the sedimentation equilibrium of a suspension in a liquid column: $n_{2}=n_{1}exp\left [ -mg N_{A}(\rho -\rho ^{'})(h_{2}-h_{1})/ (\rho RT)\right ]$ where $\rho$ is the density of the suspended particle, and $\rho ^{'}$, that of the surrounding medium. [ $N_{A}$ s Avogadro’s number, and R the universal gas constant.] [Hint: Use Archimedes' principle to find the apparent weight of the suspended particle.]
Answer:
$n_{2}=n_{1}exp\left [ -mg(h_{2}-h_{1}) /k_{b}T\right ]$ $(i)$
Let the suspended particles be spherical and have a radius r
The gravitational force acting on the suspended particles would be
$F_{G}=\frac{4}{3}\pi r^{3}\rho g$
The buoyant force acting on them would be
$F_{B}=\frac{4}{3}\pi r^{3}\rho' g$
The net force acting on the particles becomes
$\\F_{net}=F_{G}-F_{B}\\ $
$F_{net}=\frac{4}{3}\pi r^{3}\rho g-\frac{4}{3}\pi r^{3}\rho' g\\$
$F_{net}=\frac{4}{3}\pi r^{3}g(\rho -\rho ')$
Replacing mg in equation (i) with the above equation, we get
$\\n_{2}=n_{1}exp\left [ -\frac{4}{3}\pi r^{3}g(\rho -\rho ')(h_{2}-h_{1}) /k_{b}T\right ]\\$
$n_{2}=n_{1}exp\left [ \frac{-\frac{4}{3}\pi r^{3}g(\rho -\rho ')(h_{2}-h_{1})}{\frac{RT}{N_{A}}} \right ]\\ $
$n_{2}=n_{1}exp\left [ \frac{-mgN_{A}(\rho -\rho ')(h_{2}-h_{1})}{RT\rho '} \right ]$
The above is the equation to be derived
|
Substance
|
Atomic Mass (u)
|
Density (10 3 Kg m-3 )
|
|
Carbon (diamond)
|
12.01
|
2.22
|
|
Gold
|
197
|
19.32
|
|
Nitrogen (liquid)
|
14.01
|
1
|
|
Lithium
|
6.94
|
0.53
|
|
Fluorine
|
19
|
1.14
|
Answer:
Let one mole of a substance of atomic radius r and density $\rho$ have molar mass M
Let us assume the atoms to be spherical
Avogadro's number is $N_{A}=6.022\times 10^{23}$
$\\N_{A}\frac{4}{3}\pi r^{3}\rho =M\\ r=\left ( \frac{3M}{4N_{A}\pi \rho } \right )^{\frac{1}{3}}$
For Carbon
$\\N_{A}\frac{4}{3}\pi r^{3}\rho =M\\ r=\left ( \frac{3\times 12.01}{4\times 6.022\times 10^{23}\times \pi\times 2.22\times 10^{3} } \right )^{\frac{1}{3}}\\ r=1.29$ Å.
For gold
$\\N_{A}\frac{4}{3}\pi r^{3}\rho =M\\ r=\left ( \frac{3\times 197.00}{4\times 6.022\times 10^{23}\times \pi\times 19.32\times 10^{3} } \right )^{\frac{1}{3}}\\ r=1.59$ Å.
For Nitrogen
$\\N_{A}\frac{4}{3}\pi r^{3}\rho =M\\ r=\left ( \frac{3\times 14.01}{4\times 6.022\times 10^{23}\times \pi\times 1.00\times 10^{3} } \right )^{\frac{1}{3}}\\ r=1.77$ Å.
For Lithium
$\\N_{A}\frac{4}{3}\pi r^{3}\rho =M\\ r=\left ( \frac{3\times 6.94}{4\times 6.022\times 10^{23}\times \pi\times 0.53\times 10^{3} } \right )^{\frac{1}{3}}\\ r=1.73$ Å.
For Fluorine
$\\N_{A}\frac{4}{3}\pi r^{3}\rho =M\\ r=\left ( \frac{3\times19.00}{4\times 6.022\times 10^{23}\times \pi\times 1.14\times 10^{3} } \right )^{\frac{1}{3}}\\ r=1.88 $ Å.
NCERT Solutions for Class 11 Physics Chapter 12 - Kinetic Theory: Higher Order Thinking Skills (HOTS) Questions
The HOTS questions of Class 11 Physics Chapter 12 - Kinetic Theory aim at engaging students in the real-life application of the concept and solving the problems that challenge them beyond the core of the topic. These higher-order questions develop thinking, analytical and good conceptual clarity, which are the requirements of board examination as well as competitive examinations, such as JEE and NEET.
Q1:
Two moles of an ideal gas with $\frac{C_p}{C_v}=\frac{5}{3}$ are mixed 3 moles of another ideal gas with $\frac{C_p}{C_v}=\frac{4}{3}$. The value of $\frac{C_p}{C_v}$ for the mixture is:-
Answer:
For ideal gas:- $C_p-C_v=R$
For the first case:-
$
\frac{C_{p 1}}{C_{v 1}}=\frac{5}{3} \text { and } C_{p 1}-C_{v 1}=R
$
$
C_{p 1}=\frac{5}{3} C_{v 1} \text { and } \frac{5}{3} C_{v 1}-C_{v 1}=R$
$\Rightarrow \frac{2}{3} C_{v 1}=R$
$\Rightarrow C_{v 1}=\frac{3}{2} R
$
${ }_{\text {So, }} C_{p 1}=\frac{5}{2} R$
For the second case:-
$
\frac{C_{p 2}}{C_{v 2}}=\frac{4}{3} \text { and } C_{p 2}-C_{v 2}=R \\$
$C_{p 2}=\frac{4}{3} C_{v 2} \text { and } \frac{4}{3} C_{v 2}-C_{v 2}=R$
$\Rightarrow C_{v 2}=3 R{ } \ {\text {and }} C_{p 2}=4 R \\$
$ Y_{\text {mix }}=\frac{n_1 C_{p 1}+n_2 C_{p 2}}{n_1 C_{v 1}+n_2 C_{v 2}}=\frac{2 \times \frac{5}{2} R+3 \times 4 R}{2 \times \frac{3}{2} R+3 \times 3 R}=1.417=1.42$
Q2:
If temperature of the atmosphere varies with height as $\mathrm{T}=\left(\mathrm{T}_0-\mathrm{ah}\right)$, where a and $\mathrm{T}_0$ are positive constants, then the pressure as a function of height $h$ is (assume atmospheric pressure at sea level ( $\mathrm{h}=0$ ) is ${P_0}$ and molecule mass M of the air and acceleration due to gravity g be constant)
Answer:
$
\frac{\mathrm{dP}}{\mathrm{dh}}=-\rho \mathrm{g}=-\left(\frac{\mathrm{PM}}{\mathrm{RT}}\right) \mathrm{g}=-\left[\frac{\mathrm{PM}}{\mathrm{R}\left(\mathrm{~T}_0-\mathrm{ah}\right)}\right] g
$
$
\frac{\mathrm{dP}}{\mathrm{P}}=-\left(\frac{\mathrm{Mg}}{\mathrm{R}}\right) \frac{\mathrm{dh}}{\mathrm{~T}_0-\mathrm{ah}}
$
$
\int_{P_0}^{\mathrm{P}} \frac{\mathrm{dP}}{\mathrm{P}}=-\left(\frac{\mathrm{Mg}}{\mathrm{R}}\right) \int_0^{\mathrm{h}} \frac{\mathrm{dh}}{\left(\mathrm{~T}_0-\mathrm{ah}\right)}
$
$
\mathrm{P}=\mathrm{P}_0\left(\frac{\mathrm{~T}_0-\mathrm{ah}}{\mathrm{~T}_0}\right)^{\frac{\mathrm{M}_{\mathrm{s}}}{\mathrm{Ra}}}
$
Q3:
In two jars A and B, the pressure, volume and temperature in jar A are respectively P, V, and T, and those of B are 2P, V/4 and 2T. Then the ratio of the number of molecules in jars A and B will be,
Answer:
$\begin{aligned} & \frac{N_A}{N_B}=\frac{P_A V_A}{P_B P_B} \times \frac{T_B}{T_A} \\ & \frac{N_A}{N_B}=\frac{P \times V \times(2 T)}{2 P \times \frac{V}{4} \times T}=\frac{4}{1}\end{aligned}$
Q4:
The temperature of an open room of volume 30 m3 increases from 17 °C to 27oC due to the sunshine. The atmospheric pressure in the room remains 1×105 Pa. If $n_i$ and $n_f$ are the number of molecules in the room before and after heating, then $n_f-n_i$ will be :
Answer:
PV = nRT
$\begin{aligned} & \Rightarrow n_i=\frac{P V}{R T_i}, \quad n_f=\frac{P V}{R T_f} \\ & \Rightarrow n_f-n_i=\frac{P V}{R}\left(\frac{1}{T_f}-\frac{1}{T_i}\right)=\frac{10^5 \times 30}{8.31} \times\left(\frac{1}{300}-\frac{1}{290}\right) \\ & n_f-n_i=\frac{10^5 \times 30}{8.31} \times \frac{-10}{300 \times 290}=\frac{-10^5}{290 \times 8.31} \\ & \text { Change in the Number of molecules }=\frac{-10^5 \times 6.023 \times 10^{23}}{290 \times 8.31}=-2.5 \times 10^{25}\end{aligned}$
Q5:
A gas mixture contains 3 moles of oxygen and x moles of monoatomic gas at temperature T. Considering only translational and rotational but not vibrational modes, the total energy of the system is 15 RT, then the value of x is.
Answer:
$U=\frac{n f}{2} R T$
According to the question,
$\begin{aligned} 15 R T & =3 \times \frac{5}{2} R T+x \times \frac{3}{2} R T \\ 15 & =\frac{15}{2}+\frac{3 x}{2} \\ x & =5\end{aligned}$
Class 11 Physics Chapter 12 - Kinetic Theory: Topics
The Class 11 Physics Chapter 12 - Kinetic Theory examines how the behaviour of gases can be considered at the molecular level, in which the behaviour at the microscopic level is related to the macroscopic behaviour in the form of pressure, volume and temperature. The chapter addresses all the basic assumptions of kinetic theory, derivations and applications that describe real-life phenomena like diffusion and pressure of gases. Knowledge of these topics not only enhances clarity in concepts but also equips students to board examinations and other competitive exams such as JEE and NEET.
12.1 Introduction
12.2 Molecular Nature Of Matter
12.3 Behaviour Of Gases
12.4 Kinetic Theory Of An Ideal Gas
12.4.1 Pressure Of An Ideal Gas
12.4.2 Kinetic Interpretation Of Temperature
12.5 Law Of Equipartition Of Energy
12.6 Specific Heat Capacity
12.6.1 Monatomic Gases
12.6.2 Diatomic Gases
12.6.3 Polyatomic Gases
12.6.4 Specific Heat Capacity Of Solids
12.7 Mean Free Path
Class 11 Physics Chapter 12 - Kinetic Theory: Important Formulae
Kinetic Theory NCERT Solutions- Important Formulae contain important equations that describe the behaviour of the molecular motion in relation to the macroscopic properties of gas, such as pressure, temperature and volume. With the help of these formulas, students can solve numerical problems and see a correlation between the behaviour of microscopic particles and the observed physical phenomena.
1. Ideal Gas Equation
$
P V=n R T
$
Where:
$
\begin{aligned}
& P=\text { pressure }, \\
& V=\text { volume }, \\
& n=\text { number of moles }, \\
& R=\text { universal gas constant }, \\
& T=\text { temperature (in Kelvin) }
\end{aligned}
$
2. Average Kinetic Energy per Molecule
$
K E_{\mathrm{avg}}=\frac{3}{2} k T
$
Where $k=$ Boltzmann constant, $T=$ temperature in Kelvin
3. Total Kinetic Energy of Gas
$K E_{\text {total }}=\frac{3}{2} n R T$
4. Pressure of Ideal Gas from Kinetic Theory
$
P=\frac{1}{3} \rho \bar{v}^2
$
Where $\rho=$ mass per unit volume, $\bar{v}^2=$ mean square speed
5. Root Mean Square Speed
$
v_{\mathrm{rms}}=\sqrt{\frac{3 k T}{m}}=\sqrt{\frac{3 R T}{M}}
$
Where:
$m=$ mass of one molecule,
$M=$ molar mass
7. Relation Between Different Speeds
$
v_{\text {rms }}>v_{\text {avg }}>v_{\text {mp }}
$
Where:
- $v_{\mathrm{rms}}=\sqrt{\frac{3 R T}{M}}$
- $v_{\mathrm{avg}}=\sqrt{\frac{8 R T}{\pi M}}$
- $v_{\mathrm{mp}}=\sqrt{\frac{2 R T}{M}}$
8. Degrees of Freedom and Energy
$
E=\frac{f}{2} k T \text { per molecule }
$
Where $f=$ degrees of freedom
$
E_{\text {total }}=\frac{f}{2} n R T \text { for } n \text { moles }
$
Approach to Solve Questions of the Class 11 Physics Chapter 12 - Kinetic Theory
The Kinetic Theory of Gases has several assumptions, equations and derivations that must be understood clearly to solve some of its questions. Students are expected to learn formulas not by memorization, but to work out the logical application of formulas and interpret physical meaning underlying a formula. Organized planning assists in dealing with numerical and conceptual issues with ease.
-
Start with the revision of the fundamental postulates of the kinetic theory, including the following: gases consist of small particles in a state of perpetual random motion, the volume is insignificant, and collisions are perfectly elastic. These assumptions assist you in relating concepts to formulas.
-
Determine whether the problem is based on the derivation of pressure, mean free path, root mean square (rms) velocity or laws, such as Boyle and Charles. Saving time is achieved through categorizing and guides to the correct formula.
-
Don’t just apply formulas like $P=\frac{1}{3} \rho c^2$ or $v_{r m s}=\sqrt{\frac{3 k T}{m}} ;$ consider what they are, relation of pressure with molecular movement or temperature with speed of particles.
-
Begin with familiar values and change all the units into SI and then carefully replace them in the equation. Always verify dimensional consistency- it means that it is accurate.
-
Reasoning is involved in many questions such as pressure-temperature or volume-temperature graphs. Explanations are better understood and remembered through graphs.
-
Derivations such as the pressure exerted by the gas molecules, mean free path and degrees of freedom are common questions. The conceptual power is developed through writing them step by step.
How Can NCERT Solutions for Class 11 Physics Chapter 12 Kinetic Theory Help in Exam Preparation?
Kinetic Theory class 11 question answers help students build a clear understanding of how gases behave at the molecular level, which is essential for both conceptual and numerical questions in exams. Such Kinetic Theory class 11 question answers break down complex concepts such as pressure, temperature, mean free path, and the law of equipartition of energy into simple explanations so that students can be able to relate microscopic motion with macroscopic properties. With the help of these questions, students are well equipped to solve problems through derivations and numericals, which are often encountered in CBSE, JEE and NEET examinations. The solutions also focus on the connection between the kinetic energy and temperature to allow the students to imagine the way gas behaves in the real world. The frequent use of these NCERT solutions will boost student confidence, increase their ability to use the laws of gases and give them a solid background on other areas of physics and thermodynamics in higher classes.
What Extra Should Students Study Beyond NCERT for JEE/NEET?
NCERT textbooks are almost a must-have in studying and preparing competitive exams such as JEE and NEET, but are definitely lacking in preparing the numericals, tricky applications and deeper conceptual advancement. This is a list of what NCERT does not cover and what additional students are expected to learn in the chapter Kinetic Theory of Gases in preparation for JEE/NEET
NCERT Solutions for Class 11 Physics Chapter-Wise
Chapter-wise links of NCERT Solutions Class 11 provide an easy way to access all chapters at one stop. These solutions are aimed at allowing students to easily get explanations, solved exercises, vital formulas, and additional questions to each chapter and therefore making exam preparation and concept reviewing easier and organised.
NCERT Solutions for Class 11 Subject Wise
Also, check NCERT Books and NCERT Syllabus here
- NCERT Books Class 11 Physics
- NCERT Syllabus Class 11 Physics
- NCERT Books Class 11
- NCERT Syllabus Class 11
Subject-wise NCERT Exemplar solutions
Frequently Asked Questions (FAQs)
Common mistakes include applying calculations incorrectly and ignoring ideal gas assumptions. These mistakes can be avoided by going over the NCERT solutions.
In order to provide efficient learning and exam preparation, NCERT Solutions for Chapter 12 offer thorough explanations, methodical problem-solving strategies, and clarified concepts.
Yes, NCERT chapter-wise solutions can be found in PDF format and can be easily studied offline anytime, anywhere.
They contain all chapters of the Mechanics to the Kinetic Theory with solved exercises, HOTS questions and additional questions and formulas.
These are step-by-step solutions accompanying every exercise, HOTS, and other questions in the NCERT textbooks that allow students to comprehend new information in a proper way.
They mainly consist of step-wise descriptions, crucial formulas and illustrated solutions that make studying easier, which makes it easy to revise and obtain a good score both in the board as
They do reinforce the essential ideas in Physics, upon which the questions are set in JEE, NEET and other such competitive tests.
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters