NCERT Class 12 Chemistry Chapter 1 Notes The Solid State- Download PDF Notes
The solid state is a very important chapter of physical chemistry in Class 12 from an exam point of view. The NCERT Class 12 Chemistry Chapter 6 notes give you a basic idea of the solid state. The topics covered in NCERT Class 12 Chemistry notes are: definitions, general characteristics of solid state, difference between amorphous and crystalline solids, classification of crystalline solids, crystal lattices and unit cells, number of atoms in a unit cell, close packed structures, packing efficiency, calculations involving unit cell dimensions, imperfections in solids, and electric and magnetic properties of solids. Download the CBSE Notes for Class 12 Chemistry, Chapter 1, The Solid State, PDF to use offline anywhere. Students must go through each topic in the solid state class 12 notes in the easiest and most effective way possible with the help of NCERT Notes for Class 12.
This Story also Contains
- NCERT Class 12 Chapter 1 Class Notes
- The Solid State
- General Characteristics of Solid State
- Types of Solids: Amorphous and Crystalline Solids
- Classification of Crystalline Solids
- Unit Cells
- Number of Atoms in a Unit Cell
- Close Packed Structures
- Formula of a Compound and Number of Voids Filled
- Packing Efficiency
- Calculations Involving Unit Cell Dimensions
- Imperfections in Solids
- Electrical Properties and Magnetic Properties of Solids
Class 12 chemistry chapter 1 notes also cover all the important concepts related to this chapter, which are useful in various competitive exams. The solid state NCERT Notes for Class 12 Chemistry help you revise these major concepts given in the NCERT Book in a short period of time during CBSE Class 12 Board exam preparation. CBSE, the solid state notes class 12 will help you with quick revision. The Solid State chapter covers all headings of the NCERT textbook. CBSE Class 12 chemistry chapter 1 notes also contain important formulas that have been frequently asked in the various exams. Having revision notes and NCERT Solutions for Chapter 1 Chemistry Class 12 notes handy is beneficial to save you time.The NCERT Class 11 notes PDF can be downloaded through the link given below.
Also, students can refer,
NCERT Class 12 Chapter 1 Class Notes
The Solid State
Definition of Solids: Solids are states of matter that have definite volume, shape, and mass due to the short distance between the fixed positions of particles and strong interactions between them.
General Characteristics of Solid State
The characteristics and properties of the solid state are listed below:
(i) Solids have definite mass, volume and shape.
(ii) Short Intermolecular distances.
(iii) Strong Intermolecular forces.
(iv) Constituent particles (atoms, molecules or ions) in solids have fixed positions and can only oscillate about their mean positions.
Types of Solids: Amorphous and Crystalline Solids
On the basis of the nature of order present in the arrangement of their constituent particles, Solids can be classified as crystalline or amorphous.
Amorphous solids has short-range order of constituent particles, and they are isotropic in nature, having no sharp melting point. whereas Crystalline solids have a characteristic shape, having the arrangement of constituent particles of long-range order, anisotropic in nature and a sharp melting point.
Classification of Crystalline Solids
The crystalline property of a solid depends on the nature of interactions between the constituent particles, and crystalline solids are divided into four different categories:
1. Ionic solids
2. Covalent or Network solids
3. Molecular solids
4. Metallic solids
Unit Cells
Definition: it is defined as the smallest repeating unit of the crystal lattice is the unit cell, the building block of a crystal which when repeated forms the 3-D crystal.
Types Of Unit Cell
There are different varieties of the unit cell:
- Primitive Cubic Unit Cell
- Body-centered Cubic Unit Cell
- Face centered cubic unit cell
Number of Atoms in a Unit Cell
1. Primitive Cubic unit Cell
The primitive cubic unit cell has atoms only at its corner. Each atom at a corner is shared between eight adjacent unit cells. Therefore, only 1/8th of an atom effectively belongs to a particular unit cell.
2. Body-Centred Cubic unit Cell
In a body-centred cubic unit cell, atoms are at each of its corners and also one atom at its body centre.
Number of Atoms in BCC Cell:
- 8 corners × 1/8 per corner atom = 8 × 1/8 = 1 atom
- 1 body centre atom = 1 × 1 = 1 atom
Therefore, the total number of atoms present per unit cell = 2 atoms.
3. Face-Centred Cubic unit Cell
In a face-centred cubic unit cell, atoms are present at all the corners and at the centre of all the faces of the cube. The atom which is present at the face-centre is shared between 2 adjacent unit cells and only 1/2 of each atom effectively belongs to an individual cell.
Number of Atoms in FCC unit Cell
- 8 corners × 1/8 per corner atom = 8 × 1/8 = 1 atom
- 6 face-centered atoms × 1/2 atom per unit cell = 3 atoms
Therefore, the total number of atoms in a unit cell = 4 atoms.
Close Packed Structures
(a) Close Packing in One Dimension
There is only one way of arranging spheres in a one dimensional close packed structure, that is to arrange them in a row and touching each other.
Coordination number is defined as the no. of nearest neighbour particles directly in contact with each atom. In this case of one dimension close packing, the coordination number is equal to two.

(b) Close packing in Two Dimensions
In two-dimensional close packing, 1-D rows of closed packed spheres are stacked to obtain a two-dimensional pattern.
This stacking can be done in two ways:
1. Square close packing
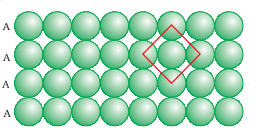
2. Hexagonal close packing
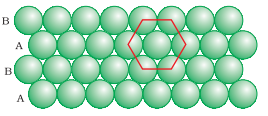
(c) Close Packing in Three Dimensions
All real structures are three dimensional structures. They can be obtained by stacking two dimensional layers one above the other. Three-dimensional closed packing are as follows:
(i) Three-dimensional close packing from two-dimensional square close-packed layers
(ii) Three-dimensional close packing from two-dimensional hexagonal close-packed layers.
Formula of a Compound and Number of Voids Filled
Voids are gaps between the constituent particles. Voids in solid states are the vacant space between the constituent particles in a closed packed structure.
There are two types of interstitial voids in a 3D structure:
a. Tetrahedral voids
b. Octahedral voids
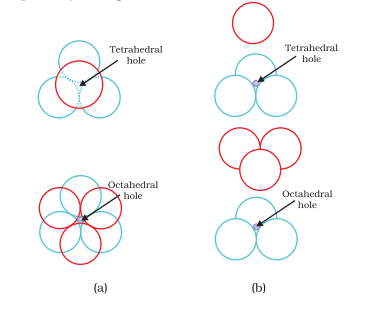
Packing Efficiency
Packing Efficiency is defined as the percentage of total space filled by the particles.
1. Packing Efficiency in hcp and ccp Structures
Hexagonal close packing (hcp) and cubic close packing (ccp) have the same packing efficiency which is equal to 74%.
2. Efficiency Packing in Body-Centred Cubic Structures
In body centered cubic unit cell, one atom is located at body center apart from corners of the cube its packing efficiency is equal to 68%.
3. Packing Efficiency in Simple Cubic Lattice
In the simple cubic unit cell, atoms are located at the corners of the cube having packing density of 524% .
Calculations Involving Unit Cell Dimensions
The unit cell is a three-dimensional structure containing one or more atoms. We can determine the volume of this unit cell with the knowledge of the dimensions of the unit cell.
Mass of unit cell = number of atoms in unit cell × mass of each atom = z × m
Where, z = number of atoms in the unit cell, m = Mass of each atom
Mass of an atom can be given with the help of Avogadro number and molar mass as: M/NA
Where M = molar mass
NA = Avogadro’s number
Volume of the unit cell, V = a3
Since, Density of unit cell = mass of unit cell/ volume of the unit cell
=> Density of unit cell = m/V = z×ma/a3 = z×M/a3×NA
Imperfections in Solids
Deviation in the arrangement of constituting particles in a solid is known as imperfections in solids. The defects are of two types:
Point defects: Point defects arises due to deviations from ideal arrangement around a point or an atom in a crystalline substance.
Line Defects: Line defects arises due to the irregularities or deviations from an ideal arrangement in entire rows of lattice points. These irregularities are called crystal defects.
Types of Point Defects
1. Stoichiometric defect – These are the point defects that do not disturb the stoichiometry of the solid. They are also called intrinsic or thermodynamic defects. These are of two types: Vacancy defect and Interstitial defect
2. Frenkel defect – In ionic solids, the smaller ion which is mostly cation, dislocates from its place and occupies an interstitial site. Hence, a vacancy defect is created at its original position and the interstitial defect is experienced at its new position where the cation is dislocated.
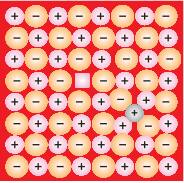
3. Schottky defect – In Ionic Solids, an equal number of anions and cations are missing from the compound. It reduces the density of the substance. In Schottky defect, the size of cations and anions are of nearly same.
Electrical Properties and Magnetic Properties of Solids
On the basis of their electrical conductivity, solids can be classified into three types.
(i) Conductors
(ii) Insulators
(iii) Semiconductors
Magnetic properties
Solids show many types of magnetic properties which are mentioned below. These properties are used in audio, video and other recording devices. All these properties can be correlated with their electronic configurations or structures.
(i) Diamagnetic materials
(ii) Paramagnetic materials
(iii) Ferromagnetic materials
(iv) Antiferromagnetic materials
(v) Ferrimagnetic materials
Chapter-Wise NCERT Class 12 Notes Chemistry
Chemistry Book: Part 1
Chapter 1: Solutions
Chapter 2: Electrochemistry
Chapter 3: Chemical Kinetics
Chapter 4: The d & f block Element
Chapter 5: Coordination Compounds
CBSE Chemistry Notes For Class 12 Book: Part 2
Chapter 6: Haloalkanes and Haloarenes
Chapter 7: Alcohols, Phenols, and Ethers,
Chapter 8: Aldehydes, ketones and Carboxylic acids
Chapter 9: Amines
Chapter 10; Biomolecules
Deleted/Removed Chapter From Class 12 Chemistry
A total of six chapters have been removed from the chemistry syllabus. The chapters removed from the NCERT Class 12 Chemistry textbook 2023–24 are listed below:
- The solid-state
- Surface Chemistry
- General Principles and Processes of Isolation of Elements
- The p-block Elements
- Polymers
- Chemistry in Everyday Life
Significance of NCERT Class 12 Chemistry Chapter 1 notes
The Solid State class 12 notes can be of great help for students to revise the chapter in less amount of time and to get an idea about their level of coverage of topics from these articles. Also, this The solid state notes class 12 is important for quick recap for competitive exams like VITEEE, BITSAT, JEE Main, NEET etc. The solid state notes class 12 are highly recommended for serious students aspiring to score well in their examination. The cbse class 12 chemistry ch 1 notes provided here are good source for last minute revision before the exam. Students can use chemistry class 12 chapter 1 notes pdf to highlight important concepts of the chapter.
Other CBSE NCERT Study Resources
Subject-wise NCERT notes
Subject Wise NCERT Solution
| NCERT Solution Class 12 Physics | NCERT Solution Class 12 Chemistry |
| NCERT Solution Class 12 Mathematics | NCERT Solution Class 12 Biology |
Subject Wise NCERT Exemplar
Frequently Asked Questions (FAQs)
- Number of octahedral voids
- Coordination Entity
- Central Atom or Ion
- Ligands
- Coordination Number
- Coordination Sphere
- Coordination Polyhedron
- Oxidation Number of Central Atom.
- Imperfections in solids
For complete solutions : https://school.careers360.com/ncert/ncert-solutions-class-12-chemistry For further reading on the topics, students can refer to ch 1 chemistry class 12 notes
4 marks, for more information students can refer chemistry class 12 chapter 1 notes pdf.
Solid state hold weightage of 2%.
6 marks
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
