Surface Chemistry Class 12th Notes - Free NCERT Class 12 chemistry Chapter 5 Notes - Download PDF
The chapter, surface chemistry is the continuation of the NCERT chapter, chemical kinetics. The ncert Class 12 Chemistry chapter 5 notes covers a brief profile of the surface chemistry chapter. The main topics covered in Class 12 Chemistry chapter 5 notes are mechanism of adsorption, introduction to adsorption, adsorption isotherms, the difference between absorption and adsorption, types of adsorption, adsorption from solution phase, applications of adsorption.
This Story also Contains
- Surface Chemistry :
- Surface Chemistry Class 12 Notes - Topic 1:
- Adsorption:
- NCERT Class 12 Chemistry Chapter 5 Notes- Topic 2:
- Catalysis:
- Surface Chemistry Class 12 Notes- Topic 3:
- Colloids:
- NCERT Class 12 Chemistry Chapter 5 Notes - Topic 4
- Emulsions:
- NCERT Class 12 Notes Chapter-Wise
- NCERT Books and Syllabus
NCERT Class 12 Chemistry chapter 5 notes also include a brief introduction to catalysis, types of catalysis, adsorption theory of heterogeneous catalysis, enzyme catalysis. Class 12 Chemistry chapter 5 notes also cover the basic equations in the chapter. The basics of colloids, classification of colloids, preparations of colloids, purification of colloids, properties of colloids, and emulsions are also covered in the CBSE Class 12 Chemistry chapter 5 notes. These Surface Chemistry Class 12 notes also include some solved examples related to mentioned topics. All these topics can be downloaded from Class 12 Chemistry chapter 5 notes pdf download.
Also, students can refer,
Surface Chemistry :
The branch of chemistry that deals with surface/interface occurring phenomenon is called surface chemistry. The bulk phases can be liquid or solid. There is no existence of interface in gases due to their complete miscibility with other gases.
Surface Chemistry Class 12 Notes - Topic 1:
Adsorption:
The phenomenon of accumulation or retention of molecular species on the surface of solids or liquids rather than in bulk is known as adsorption.
Adsorbate - The substance which accumulates or retains at the surface is called adsorbate.
Adsorbent - The substance on which adsorbate accumulates is called adsorbent. For example - alumina gel, charcoal, silica gel, colloids, clay, etc.
Desorption:
The phenomenon in which adsorbate is removed from the adsorbent is called desorption. It is the opposite of adsorption.
Difference Between Absorption and Adsorption:
Absorption | Adsorption |
The phenomenon of assimilation in which the constituent of a substance enters into the bulk phase of another substance. | The phenomenon in which the adhesion of constituents of a substance takes place on the surface of another substance. |
Bulk phenomenon | Surface phenomenon |
Uniform distribution of absorbate | Adsorbate concentrates only on the surface |
Endothermic process | Exothermic process |
Independent of temperature | Temperature-dependent |
The rate of reaction is uniform for absorption | The rate of reaction increases slowly and then achieves equilibrium. |
Example- absorption of liquid by a sponge. | Example- adsorption of pollutants by pollution masks. |
Mechanism of adsorption:
The surface constituents of the adsorbent are in a different environment than the bulk constituents.
The forces acting between bulk constituent and its neighbors are mutually balanced.
The forces exerted by surface constituents are unbalanced and thus called residual forces.
Residual forces attract the particles of adsorbate towards the surface of the adsorbent.
At a given temperature and pressure, the increase of surface area per unit mass of the adsorbent increases the extent of adsorption.
ΔH is negative, ΔS is negative and thus ΔG is negative.
Types of adsorption :
There are two types of adsorption on the basis of the type of forces acting between constituents.
Physisorption | Chemisorption |
Arises due to Van Der Waal’s forces | Arises due to the formation of chemical bonds |
Non-specific in nature | Highly specific in nature |
Reversible | Irreversible |
Depends on the nature of gas | Depends on the nature of gas |
Low enthalpy of adsorption | High enthalpy of adsorption |
Low temperature is favorable | High temperature is favorable |
No appreciable activation energy needed | Sometimes high activation energy is needed |
Depends on the surface area | Depends on the surface area |
Multimolecular layers are formed | The unimolecular layer is formed |
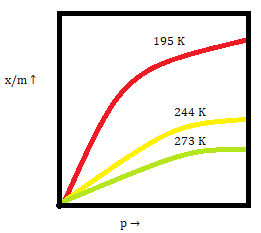
Adsorption isotherms :
At constant temperature, the variation of the amount of gas adsorbed by the adsorbent with pressure is expressed via adsorption isotherms.
Freundlich adsorption isotherm:
The quantity of gas adsorbed by unit mass of solid adsorbent and pressure at a particular temperature is expressed as:
![]()
x - the mass of gas adsorbate
m - the mass of adsorbent
p - pressure
n & k - constants that depend on the nature of the adsorbent & the gas at a particular temperature
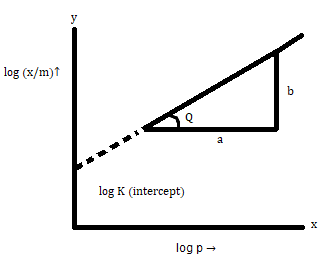
Take log on both sides :
![]()
On comparing with y = mx + c,
y = log (x/m)
m = 1/n
x = log p
c = log k
The graph for Freundlich isotherm can be plotted as below:
Adsorption from solution phase
Solids can also adsorb solutes from solutions. Some observations from solid-liquid adsorption are given below:
As temperature increases ↑ the extent of adsorption decreases ↓
As the surface area of the adsorbent increases ↑ the extent of adsorption increases ↑
The extent of adsorption is dependant on the concentration of solute.
The extent of adsorption is dependant on the nature of adsorbate and adsorbent.
Freundlich equation for adsorption from solution by solid adsorbate:
![]()
Take log on both sides :
![]()
Comparing the above equation with y = mx+c we get a straight line graph.
Applications of adsorption :
Gas masks, Heterogeneous catalysis, Removal of coloring matter from solutions, Separation of inert gases, In curing diseases, Froth floatation process, Control of humidity, Adsorption indicators, Production of high vacuum, Chromatographic analysis, etc.
NCERT Class 12 Chemistry Chapter 5 Notes- Topic 2:
Catalysis:
The process of altering the rate of reaction of a chemical reaction by introducing a catalyst.
Catalysts are substances that accelerate or decelerate the rate of a reaction and is retrieved unchanged after the completion of the reaction.
Promoters: Enhance activity of the catalyst
Poisons: Diminish activity of the catalyst
Classification of catalysis:
Homogeneous catalysis: The catalysis in which both reactants and catalyst(s) are in the same phase. Examples -
Oxidation of sulfur dioxide
![]()
Hydrolysis of methyl acetate
![]()
Hydrolysis of sugar
![]()
Heterogeneous catalysis: The catalysis in which both reactants and catalyst(s) are in different phases. Examples -
Oxidation of sulfur dioxide
![]()
Haber’s Process
![]()
Ostwald’s process
![]()
Hydrogenation of vegetable oil
![]()
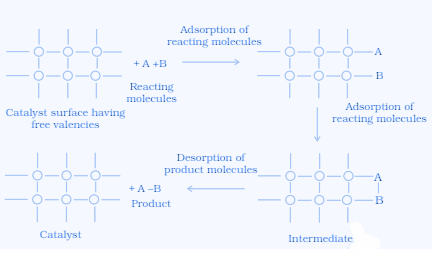
Adsorption theory of heterogeneous catalysis:
The adsorption theory of heterogeneous catalysis explains the mechanism of heterogeneous catalysis. The mechanism can be explained in 5 steps:
Reactants diffuse on the surface of the catalyst.
Reactant molecules get adsorbed on the surface of the catalyst.
A chemical reaction occurs via the formation of an intermediate.
Reaction products are desorbed from the surface of the catalyst.
Diffusion of products away from the surface of the catalyst.
This adsorption theory gives an explanation for the unchanged composition of catalyst at the end of the reaction.
Shape selective catalysis by zeolites:
Shape-selective catalysis is a catalytic reaction in which the pore structure of the catalyst and the size of the reactant and product molecules are major factors to carry out the reaction.
Due to their honeycomb-like structures, zeolites are good shape-selective catalysts.
Zeolites are microporous aluminosilicates with a 3D network of silicates in which sometimes aluminium atoms replace silicon atoms giving an Al-O-si framework.
Enzyme catalysis:
Enzymes are proteins of high molecular mass. They form colloidal solutions in water. They are very effective catalysts in natural processes. Enzymes are also called biochemical catalysts. Enzyme catalyzed reactions are also called biochemical catalysis. Some examples are discussed below:
Inversion of cane sugar
![]()
Conversion of glucose into ethyl alcohol
![]()
‘Conversion of starch into maltose
![]()
Conversion of maltose into glucose
![]()
Decomposition of urea into ammonia and carbon dioxide
![]()
A short summary of enzyme-catalyzed reactions is tabulated below:

Characteristics of enzyme catalysis:
Highly efficient
Highly specific in nature
Highly active under optimum temperature (298-310K)
Highly active under optimum pH (5-7)
Increased activity in the presence of activators & co-enzymes
Decreased activity due to influence of inhibitors and poisons
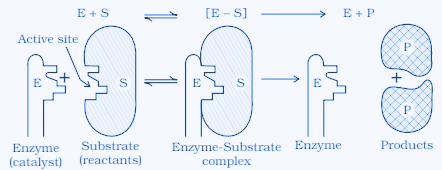
Mechanism of enzyme catalysis:
Step 1: Enzyme binds to substrate to form an activated complex.
![]()
Step 2: Activated complex is decomposed to form the product.
![]()
Surface Chemistry Class 12 Notes- Topic 3:
Colloids:
A heterogeneous system in which very fine particles of one substance called dispersed phase is dispersed in another substance is termed as the dispersion medium.
Classification of colloids:
Colloids can be classified on the basis of:
Physical state
Nature of interaction
Type of particles
Classification based on the physical state of the dispersed phase and dispersion medium:
Dispersed phase | Dispersion medium | Type of colloid | Examples |
Solid | Solid | Solid sol | Colored glass, gemstones |
Solid | Liquid | Sol | Paints, cell fluids |
Solid | Gas | Aerosol | Smoke, dust |
Liquid | Solid | Gel | Cheese, butter, jellies |
Liquid | Liquid | Emulsion | Milk, hair cream |
Liquid | Gas | Aerosol | Fog, mist, cloud, insecticide sprays |
Gas | Solid | Solid sol | Pumice stone, foam rubber |
Gas | Liquid | Foam | Froth, whipped cream, soap lather |
Classification based on the nature of the interaction between the dispersion medium and dispersed phase:
Colloids can be classified on the basis of interaction in 2 categories:
Property | Lyophobic | Lyophilic |
Preparation | Can not be prepared easily | Can be prepared easily |
Stability | Less stable | More stable |
Reversibility | Irreversible | Reversible |
Viscosity | Nearly the same viscosity as the solvent | Higher viscosity than solvent |
Surface tension | Nearly the same surface tension as a solvent | Higher surface tension than solvent |
Hydration / solvation | Less solvated | Highly solvated |
Charge | Positive / negative | No charge |
Visibility | Can be seen under a microscope | Can not be seen under a microscope |
Coagulation/Precipitation | Precipitated by low concentration of electrolytes | Precipitated by high concentration of electrolytes |
Tyndall effect | More scattering | Less scattering |
Migration in an electric field | Migrate towards cathode/anode | May/ may not migrate |
Nature | Mostly inorganic | Mostly organic |
Classification based on the type of particles of the dispersed phase and dispersion medium:
Multimolecular colloids: On dissolution, a large number of atoms and smaller molecules aggregate to form colloidal species called multimolecular colloids.
Multimolecular colloids: In suitable solvents, macromolecules forms colloidal solutions.
Associated colloids: substances that act as normal strong electrolytes at low concentration but show colloidal behavior at high concentration due to the formation of aggregate molecules called micelles.
Kraft temperature - temperature above which micelle formation takes place.
Critical micelle concentration - concentration above which micelle formation takes place.
Micelle formation and cleansing action of soap -
Micelle consists of a hydrophobic hydrocarbon–like central core.
Soap molecules form micelle around the oil droplet.
The hydrophobic part of the stearate ions is in the oil droplet and the hydrophilic part extends out of the droplet.
The polar groups interact with water.
The oil droplet surrounded pulled in water and removed from the dirty surface.
Soap helps in emulsification and washing away of oils & dirt.
The negatively charged sheath around the oil droplets prevents them from aggregating.
Preparation of colloids:
Chemical methods
Molecules formed by chemical reactions aggregate to form sols. Example-
![]()
Electrical disintegration or Bredig’s Arc method
The intense heat produced between the electrodes vapourises the metal. This metal then condenses to form particles of colloidal size.
Peptization
The process of converting a precipitate in the presence of a small amount of electrolyte into colloidal sol by shaking it with a dispersion medium is called peptization.
Purification of colloidal solutions:
Purification can be done by (These processes are not discussed in detail in class 12 chemistry chapter 5 notes.) :
Dialysis
Electrodialysis
Ultrafiltration
Properties of colloidal solution:
colligative properties are of small order than true solutions.
Tyndall effect - Colloidal solutions show a mild to strong opalescence when viewed at right angles to the passage of light.
Colour - the color of the colloidal solution changes with the perspective of the viewer.
Brownian movement - the state of continuous zig-zag motion.
A charge is always present on colloidal particles.
Electrophoresis.
Coagulation and precipitation.
NCERT Class 12 Chemistry Chapter 5 Notes - Topic 4
Emulsions:
Emulsions are formed by the dispersion of finely divided droplets of one liquid in another liquid. They are liquid-liquid colloidal systems.
Types of emulsions:
Oil-in-water: In this system, water is a dispersion medium, and oil is a dispersed phase. Example - butter.
Water-in-oil: In this system, oil is a dispersion medium, and water is a dispersed phase.
Emulsifying agent - A substance that forms an interfacial film between the dispersed phase and dispersed medium.
Applications of colloids:
Electrical precipitation of smoke
Purification of drinking water
Medicines
Tanning
The cleansing action of soap
Photographic films
Rubber industry
Industrial Products
Significance of NCERT Class 12 Chemistry chapter 5 notes
surface chemistry Class 12 notes will be helpful to revise the chapter and to get an idea about the main topics covered in the chapter. Also, this ncert class 12 chemistry chapter 5 notes are useful to cover the main topics of the Class 12 CBSE Chemistry syllabus and also for competitive exams like VITEEE, BITSAT, JEE Main, NEET, etc. Class 12 Chemistry chapter 5 notes pdf download can be used to prepare in offline mode.
NCERT Class 12 Notes Chapter-Wise
Subject wise NCERT Exampler solutions
- NCERT Exemplar Class 12 Solutions
- NCERT Exemplar Class 12 Maths
- NCERT Exemplar Class 12 Physics
- NCERT Exemplar Class 12 Chemistry
- NCERT Exemplar Class 12 Biology
Subject Wise NCERT Solutions
NCERT Books and Syllabus
Frequently Asked Questions (FAQs)
Ans- The main differences as covered in the NCERT book are given in the tabular form.
Absorption | Adsorption |
The phenomenon of assimilation in which the constituent of a substance enters into the bulk phase of another substance. | The phenomenon in which the adhesion of constituents of a substance takes place on the surface of another substance. |
Bulk phenomenon | Surface phenomenon |
Uniform distribution of absorbate | Adsorbate concentrates only on the surface |
Endothermic process | Exothermic process |
Independent of temperature | Temperature-dependent |
The rate of reaction is uniform for absorption | The rate of reaction increases slowly and then achieves equilibrium. |
Example- absorption of liquid by a sponge. | Example- adsorption of pollutants by pollution masks. |
Ans- Students can expect 2 to 4 mark questions (including numerical questions) from the chapter Surface Chemistry.
Ans- As given in ncert notes for class 12 Chemistry chapter 5, Colloids can be classified on the basis of:
Physical state
Nature of interaction
Type of particles
Ans- As stated in Class 12 Surface Chemistry notes, There are two types of adsorption on the basis of the type of forces acting between constituents.
Physisorption | Chemisorption |
Arises due to Van Der Waal’s forces | Arises due to the formation of chemical bonds |
Non-specific in nature | Highly specific in nature |
Reversible | Irreversible |
Depends on the nature of gas | Depends on the nature of gas |
Low enthalpy of adsorption | High enthalpy of adsorption |
Low temperature is favorable | High temperature is favorable |
No appreciable activation energy needed | Sometimes high activation energy is needed |
Depends on the surface area | Depends on the surface area |
Multimolecular layers are formed | The unimolecular layer is formed |
These topics can also be downloaded from surface chemistry class 12 notes pdf download.
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
