Mineral Nutrition Class 12th Notes - Free NCERT Class 11 Biology Chapter 12 Notes - Download PDF- Mineral Nutrition Class 11 Notes For NEET
NCERT chapter Mineral Nutrition describes several micronutrients, macronutrients, and minerals, their roles, and deficiency symptoms. The NCERT Class 11 Biology chapter 12 notes covers a brief outline of the chapter Mineral Nutrition. The main topics covered in Mineral Nutrition Class 11 notes are —ways to study the requirements of minerals by plants, essential mineral elements, absorption of elements, translocation of solutes, and soil and nitrogen metabolism.
This Story also Contains
- NCERT Class 11 Biology Chapter 12 Mineral Nutrition Notes
- Methods to Study Mineral Requirements of Plants?
- Essential Mineral Elements
- Role of Macronutrients and Micronutrients:
- Deficiency Symptoms of Various Elements:
- Mechanism of Absorption of Elements:
- Translocation of Solutes
- Soil As A Reservoir of Essential Elements:
- Metabolism of Nitrogen:
- Significance of NCERT Class 11 Biology Chapter 12 Notes
Also, students can refer,
NCERT Class 11 Biology Chapter 12 Mineral Nutrition Notes
Methods to Study Mineral Requirements of Plants?
Julius von Sachs showed for the first time that plants could be grown to maturity in a defined nutrient solution in the complete absence of soil (1860). This technique is known as hydroponics. It focuses on the culture of plants in a soil-free defined mineral solution and they require purified water and mineral nutrient salts.
Many experiments were carried out in which the roots of plants were immersed in nutrient solutions and an element was added or removed or given in varied concentrations, thus a mineral solution that is relevant for the plant growth was obtained.
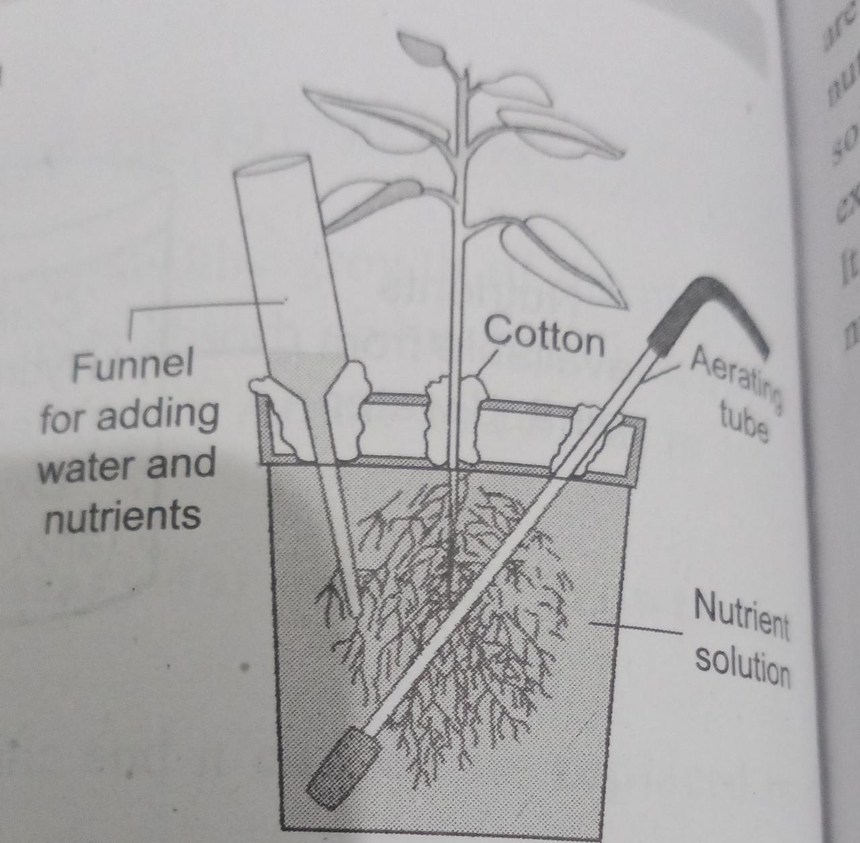
Fig.1. Setup for Nutrient Solution Culture
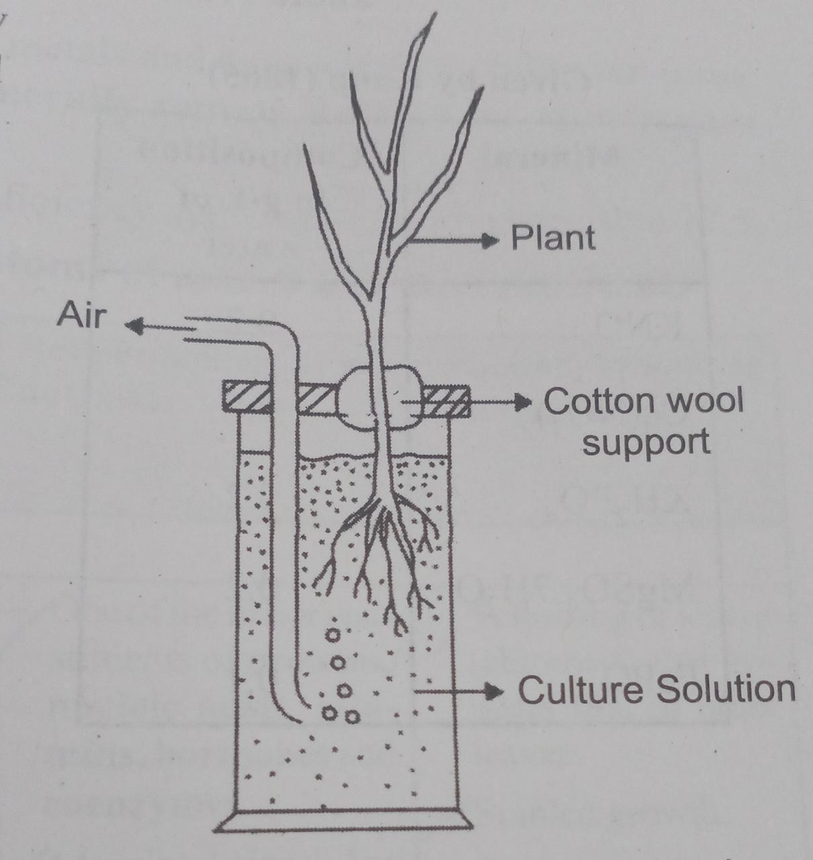
Fig.2. A Schematic Diagram Showing The Technique of Hydroponics
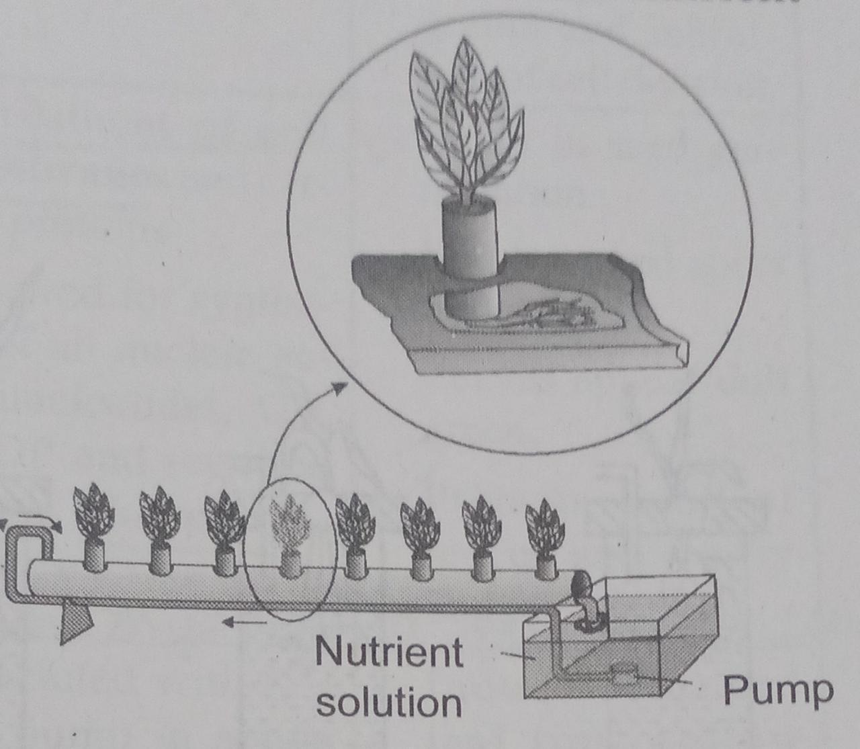
Fig.3. Hydroponic Plant Production.
Essential Mineral Elements
Many minerals present in soil enter plants via roots. Different plant species accumulate different types of minerals. There are various techniques that are able to detect the minerals even if they are present at very low concentrations.
Criteria of Essentiality of Elements:
The nutrients or elements which are important for the healthy growth of the plant are called essential nutrients or essential elements. The criteria for the essentiality of an element are
1. An essential element is very much necessary for normal growth and reproduction.
2. There is a specific requirement for every element and it cannot be replaced by another element.
3. In the metabolism and nutrition of the plant, the element must be directly involved.
Based on the above criteria, a few elements are very essential for plant growth and metabolism.
Macronutrients:
Macronutrients are required in relatively large quantities.
Some examples of macroelements include carbon, hydrogen, oxygen, nitrogen, phosphorus, sulphur, potassium calcium, and magnesium.
Micronutrients:
Micronutrients are required in minute quantities like less than 0.1 mg per gram of dry matter. These are also known as trace elements. Manganese, boron, copper, molybdenum, iron, zinc, chlorine, and nickel have been included in micronutrients.
Based on their functions essential elements can be grouped:
1. As components of biomolecules and present as structural elements of cells. (C, H, O, and N)
2. As components of energy-related chemical compounds. ( Eg. Magnesium in chlorophyll and phosphorus in ATP)
3. As activators or inhibitors of enzymes.
i. Mg2+is an activator for both enzyme RUBISCO and enzyme phosphoenolpyruvate carboxylase. Both enzymes are responsible for photosynthetic carbon fixation
ii. Zn2+ as an activator of alcohol dehydrogenase during the metabolism of nitrogen.
4. Can alter the osmotic potential of a cell ( K+) ions play an important role in opening and closing of stomata).
Role of Macronutrients and Micronutrients:
The macro or micronutrients play important roles in plants. They are an essential constituent of proteins, carbohydrates, fats, and nucleic acids and take part in various metabolic processes. Some of them act as activators or cofactors for various enzymes.
The functions of various nutrients are given below:
Nitrogen:
Required by plants in the greatest amount by all parts of the plant.
Absorbed as NO3-, some are also taken up as NO2- or NH4+
Particularly important for meristematic tissues and metabolically active cells.
Phosphorus:
It is absorbed as phosphate ions (H2PO4- or HPO4-2).
It acts as a constituent of cell membranes, proteins, all nucleic acids, and nucleotides
Potassium:
Absorbed as K+ ions.
Required in meristematic tissues, buds, leaves, and root tips.
To maintain anion-cation balance in cells.
Involved in the synthesis of proteins, opening, and closing of stomata, and in the maintenance of the turgidity of cells.
Calcium:
It is absorbed as Ca2+ ions.
It is necessary for meristematic and differentiating tissues.
During the division of cells, it is used in the formation of cell walls, particularly as calcium pectate in the middle lamella.
Helps in the formation of the mitotic spindle during cell division.
It plays an important role in regulating metabolic activities
Magnesium:
Absorbed as Mg2+ ions.
Activates the enzymes of respiration and photosynthesis
Involved in the synthesis of DNA
Helps to maintain the ribosome structure
Sulphur:
Absorbed as SO42- ions.
As a constituent of two amino acids cysteine and methionine and several proteins.
Also the main constituent of several coenzymes, vitamins ( thiamine, biotin, and Coenzyme A and ferredoxin).
Iron:
Absorbed as ferric ions ( Fe3+)
It is reversibly oxidized from Fe2+ to Fe3+ during electron transfer.
It helps in the formation of chlorophyll.
Manganese:
Absorbed as Mn2+ ions.
It activates enzymes involved in photosynthesis, respiration, and nitrogen metabolism.
It helps in photolysis (splitting water to liberate oxygen during photosynthesis).
Zinc:
Absorbed as Zn2+ ions
It activates various enzymes, specially carboxylase.
Needed in the synthesis of auxin.
Copper:
Absorbed as Cu2+ ions.
Necessary for overall metabolism in plants.
It is associated with enzymes involved in redox reactions and is reversibly oxidized from Cu+ to Cu2+.
Molybdenum:
Absorbed as MoO22+
It is a constituent of nitrogenase and nitrate reductase
Chlorine:
Absorbed as Cl- ion.
Along with Na+ and K+ elements, it helps in determining the solute concentration and the anion cation balance in the cells.
It is essential for the process of photolysis(water-splitting reaction in photosynthesis).
Deficiency Symptoms of Various Elements:
When the availability of an essential element becomes limited, the plant growth gets retarded. Hence critical concentration is defined as the concentration of the essential element below which the plant growth gets retarded. The element is set to be deficient if present below the critical concentration.
Deficiency Symptoms:
The absence or deficiency of any of the essential elements brings about certain morphological changes in plants which are called deficiency symptoms. These symptoms vary from plant to plant and element to element however if it continues it leads to the death of the plant.
When and Where do Deficiency Symptoms Occur?
1. For elements that are actively mobilized, the deficiency symptoms appear first in the older (senescent) tissues. For example, deficiency symptoms of N, K, and Mg are first visible in the senescent leaves.
2. For selectively mobile elements that are not transported out of mature organs, the deficiency symptoms appear first in the younger tissues, for example, sulphur and calcium being part of the structural component of the cell, are not easily released.
Major Deficiency Symptoms or Hunger Signs:
Chlorosis, necrosis, stunted plant growth, premature fall of leaves and buds, and inhibition of cell division are some examples of deficiency symptoms in plants.
Chlorosis:
Loss of chlorophyll leads to yellowing of leaves.
It is caused by the deficiency of elements like N, K, Mg, S, Fe, Mn, Zn, Mo.
Necrosis:
It is caused by the deficiency of elements of Ca, Mg, Cu, K.
Inhibition of cell division:
It is caused by a lack or low level of N, K, S, or Mo.
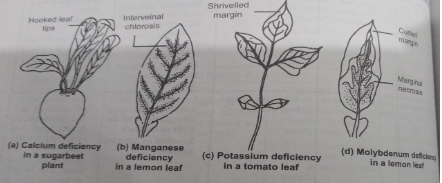
Toxicity of Micronutrients:
The micronutrients are needed in very low amounts by the plants.
The moderate decrease of micronutrients causes deficiency symptoms.
The moderate increase of micronutrients causes toxicity.
Important Aspects of Micronutrients:
There is a very narrow range of concentration at which micronutrients are optimum.
Such critical concentrations vary widely for different micronutrients.
For example, the symptom of manganese toxicity- the appearance of brown spots surrounded by chlorotic veins.
Mn competes with iron and magnesium for uptake and with Mg for binding with enzymes.
Manganese also inhibits Ca translocation in the shoot apex.
Therefore, excess of Mn induces deficiency symptoms of iron, magnesium, and calcium.
Mechanism of Absorption of Elements:
The uptake of ions into the ‘free space’ or ‘outer space’ of cells (apoplast) is passive. The ‘free space’ or ‘outer space’ of cells is the second phase. The passive movement of ions into the apoplast usually occurs through ion channels. The entry-exit of ions to and from the symplast requires energy. The movement of ions is called flux. The inward movement into the cells is influx and the outward movement is called the efflux.
Translocation of Solutes
Mineral salts are transported through the xylem. It is pulled up through the plant by transpirational pull.
Soil As A Reservoir of Essential Elements:
Due to weathering and the breakdown of rocks, nutrients become available to the roots. Soil consists of a variety of substances and minerals. It harbors nitrogen-fixing bacteria and other microbes. It acts as a matrix that stabilizes the plant.
Metabolism of Nitrogen:
The soil has limited amounts of nitrogen. Plants and microbes compete for this nitrogen.
Nitrogen Cycle:
Plants compete with microbes for the limited nitrogen that is available in the soil. So, nitrogen becomes a limiting nutrient for natural and agricultural ecosystems. The process of conversion of atmospheric nitrogen (N2) to ammonia (NH3) is ‘Nitrogen fixation. It is carried out in three ways – biological, industrial, and electrical.
Biological nitrogen fixation: It uses living organisms that reduce nitrogen to ammonia.
Industrial nitrogen fixation: As sources of nitrogen, they use industrial exhausts.
Electrical nitrogen fixation: The factors responsible for this are the forces of nature such as UV radiation.
The process of conversion of organic nitrogen to ammonia is called ‘Ammonification’ and it returns nitrogen back to the soil. Some ammonia evaporates and re-enters the atmosphere while a major part of it is converted by soil bacteria into nitrate as follows:
(i) Ammonia is oxidized to nitrite by the bacteria Nitrosomonas and/or Nitrococcus.
2NH3 + 3O2 → 2NO2- + 2H+ + 2H2O
(ii) Nitrite is further oxidised to nitrate by Nitrobacter.
2NO2_ + O2 → 2NO3_
These reactions are called ‘Nitrification’ and the nitrifying bacteria are ‘Chemoautotrophs’. Plants absorb nitrate and transport it to the leaves where it is reduced to ammonia. This ammonia forms the amine group of amino acids. Nitrates are also reduced to nitrogen during ‘Denitrification’ by Pseudomonas and Thiobacillus.
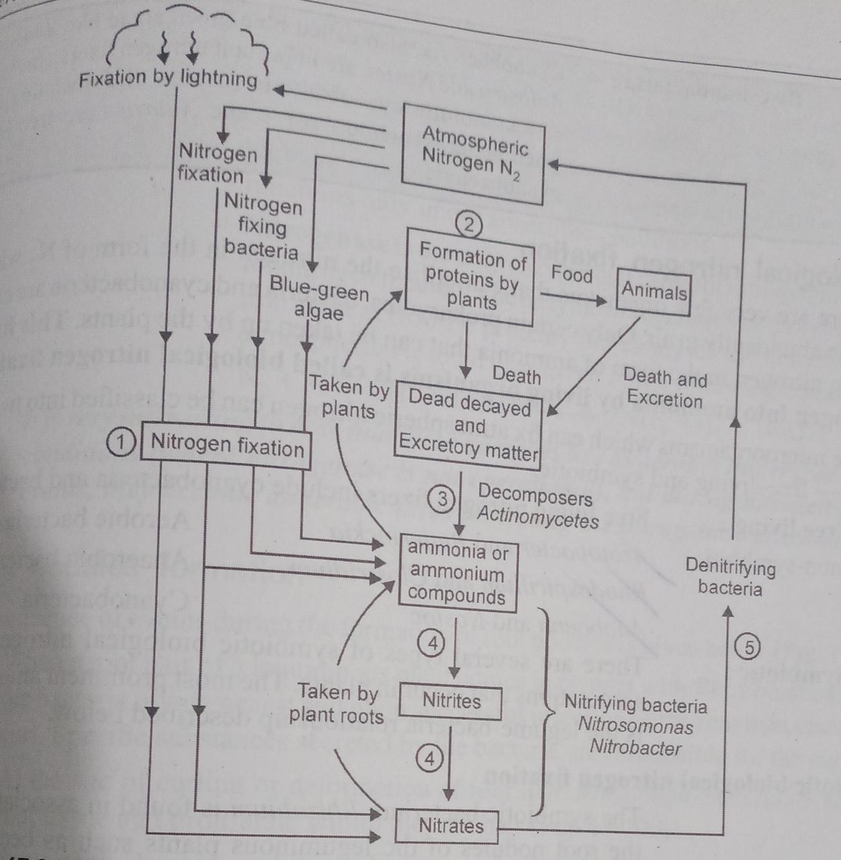
Fig.4. Nitrogen Cycle
Biological Nitrogen Fixation:
- Only a few prokaryotic organisms can use the nitrogen in the form of N2, available in the atmosphere. Biological nitrogen fixation is the reduction of nitrogen to ammonia by living organisms. They’re also called Nitrogen fixers.
- The nitrogen-fixing bacteria could be free-living or symbiotic.
- An example of free-living nitrogen-fixing bacteria is Azotobacter.
Symbiotic Biological Nitrogen Fixation:
An example of symbiotic biological nitrogen fixation is the legume-bacteria relationship. Rhizobium has a symbiotic relationship with the roots of several legumes such as sweet pea, lentils, garden pea, etc. The most common association is root nodules which are small outgrowths on the roots.
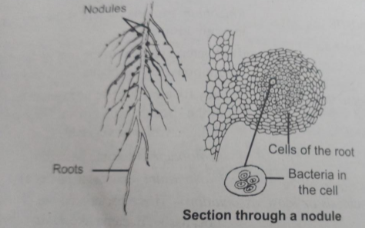
Fig.5. Leguminous Root Nodules Showing Bacterium Rhizobium
Nodule Formation:
The nitrogenase enzyme acts as a catalyst in the conversion of atmospheric nitrogen to ammonia.
The reaction is:
N2 + 8e- + 8 H+ + 16 ATP → 2NH3 + H2 + 16 ADP + 16P1
The energy required for the above reaction (8ATP for each NH3 produced) comes from the respiration of host cells. Leg-haemoglobin, also known as an oxygen scavenger, protects nitrogenase from oxygen in the nodules. This microbe lives as an aerobic, free-living organism where nitrogenase is not functional.
The Fate of Ammonia:
The ammonia is protonated to form NH4+ (ammonium) ions by protonation. NH4+ is used to synthesize amino acids in plants. It occurs by
(i) Reductive amination: Ammonia reacts with α-ketoglutaric acid to form glutamic acid in the presence of glutamate dehydrogenase.
α-ketoglutaric acid + NH4+ + NADPH → glutamate + H2O + NADP
(ii) Transamination: It is the process of transfer of an amino group from one amino acid to the keto group of a keto acid. It is facilitated by the enzyme transaminase.

Significance of NCERT Class 11 Biology Chapter 12 Notes
The NCERT Notes of Class 11 Biology chapter 12 Mineral Nutrition helps to revise the chapter and to get an idea about the main topics covered in the chapter. Also, these Notes for Class 11 Biology chapter 12 are useful to cover the main topics of Class 11 Biology chapter 12 and also for competitive exams like AIPMT, NEET etc. Class 11 Biology chapter 12 notes pdf file can be used to prepare in offline mode.
NCERT Class 11 Notes Chapter-Wise
NCERT Class 11 Biology Chapter 12 Notes |
Subject Wise NCERT Exemplar Solutions
- NCERT Exemplar Class 11 Solutions
- NCERT Exemplar Class 11 Maths
- NCERT Exemplar Class 11 Physics
- NCERT Exemplar Class 11 Chemistry
- NCERT Exemplar Class 11 Biology
Subject Wise NCERT Solutions
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
