NCERT Solutions for Class 12 Chemistry Chapter 10 Biomolecules
Have you ever wondered how wounds heal, how genetics work, and how the food we eat provides us energy? The only reason behind all these phenomena is Biomolecules. They play a very important role in our daily lives as they are the essential building blocks of life. From simply eating food to the occurrence of all the processes inside our body, biomolecules play an important role.
The CBSE Class 12 Physical Education paper will have five sections, which will contain 37 questions. Of the 37 questions, 18 will be multiple-choice questions and will carry one mark each.
This Story also Contains
- NCERT Solutions for Class 12 Chemistry Chapter 10 Download PDF
- NCERT Solutions for Class 12 Chemistry Biomolecules (Intext Questions From 10.1 to 10.8)
- NCERT Solutions for Class 12 Chemistry Biomolecules (Exercise Questions )
- Class 12 Chemistry NCERT Chapter 10: Higher Order Thinking Skills (HOTS) Questions
- Approach to Solve Questions of Chapter 10 Biomolecules
- Topics of the NCERT Book Class 12 Chemistry Biomolecules
- What Extra Should Students Study Beyond NCERT for JEE/NEET?
- What Students Learn from NCERT Solutions for Class 12 Chemistry Chapter 10 Biomolecules
- Importance of Class 12 Chemistry Chapter 10 Biomolecules Solutions
- NCERT Solutions Class 12 Chemistry Chapter-wise

This chapter is important from the exam point of view as it carries good weightage in CBSE board exams. The NCERT solutions for class 12 chemistry are designed by our subject experts in a very comprehensive and structured manner. These solutions of NCERT will help the students to improve their accuracy and speed. The detailed solutions and higher-order thinking skills (HOTS) questions and approaches to solve questions are included in the article that will help you master the topics.
NCERT Solutions for Class 12 Chemistry Chapter 10 Download PDF
Students can download the class 12 chemistry chapter 10 biomolecules solutions pdf for free. These solutions of NCERT are designed to help you understand the fundamental concepts and solve textbook questions with ease.
Also Read
NCERT Solutions for Class 12 Chemistry Biomolecules (Intext Questions From 10.1 to 10.8)
These biomolecules ncert solutions for Intext Questions give step by step explanations to make concepts easier to understand and improve exam preparation. These NCERT solutions also help students build a strong conceptual foundation by clarifying common doubts and highlighting important points.
Page no 290
Answer :
We know that water is a polar molecule. It is also known that "like dissolves like". So either a polar molecule or the compounds that can form hydrogen bonds with water molecules will get dissolved in water.
Glucose and sucrose have five and eight -OH groups, respectively. So they are likely to form hydrogen bonds with molecules with water. In the case of cyclohexane and benzene, these are non-polar molecules because of a very low electronegativity difference. So these are not soluble in water.
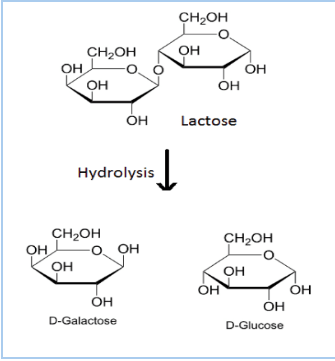
Question 10.2 What are the expected products of hydrolysis of lactose?
Answer :
The expected products in the hydrolysis of lactose are Galactose and Glucose.
Question 10.3 How do you explain the absence of aldehyde group in the pentaacetate of D-glucose?
Answer :
When pentaacetate of glucose is made to react with hydroxylamine, no reaction occurs, which indicates the absence of a free CHO group.
In aqueous solution, the cyclic structure having -OH group at C-1 should get converted to an open chain structure, which has an aldehyde group at C-1. It should then react with hydroxylamine and give glucose oxime. But such a case is not observed. This suggests that in aqueous solution open chain structure doesn't exist and as a result, the aldehyde group is absent in the pentaacetate of glucose.
Page no 295
Answer :
Amino acids have both acidic and basic groups, which lead to the formation of zwitterions and make them dipolar compounds. Due to this property, they form strong molecular bonds themselves and also with water. This results high melting point and good solubility in water as compared to haloacids.
Halo acids don't exhibit the property of dipolar compounds. Only the carboxyl group of the haloacid is involved in H-bonding, not the halogen atom. That is why they have low melting points and less solubility than amino acids.
Question 10.5 Where does the water present in the egg go after boiling the egg?
Answer :
Due to denaturation of proteins, globules unfold and helix gets uncoiled, which changes their biological activity. In denaturation, secondary and tertiary structures are destroyed, whereas primary structure remains the same.
Due to this process denaturation of proteins, coagulation of egg takes place while boiling. In an egg, the globular protein changes into a rubber-like structure, which is responsible for the absorption of water.
Page no 301
Question 10.6 Why cannot vitamin C be stored in our body?
Answer :
Vitamin C is a water-soluble vitamin that is excreted from the body in the form of urine and cannot be stored. So they must be supplied in the diet regularly.
Question 10.7 What products would be formed when a nucleotide from DNA containing thymine is hydrolysed?
Answer :
When a nucleotide from the DNA containing thymine is hydrolyzed, the products are thymine $\beta$-D-2-deoxyribose and phosphoric acid.
Answer :
When RNA is hydrolysed, there is no relationship among the quantities of different bases obtained; this fact suggests that RNA is a single-stranded structure. Unlike DNA, which is a double-stranded structure in which pairing of bases occurs e.g., adenine pairs with thymine. Thus, on hydrolysis, the amount of adenine produced will be the same as the amount produced by thymine. In RNA, there is no relationship with the quantities of bases, meaning bases don't occur in pairs, or it is a single-stranded structure.
NCERT Solutions for Class 12 Chemistry Biomolecules (Exercise Questions )
These class 12 chemistry chapter 10 biomolecules solutions for exercise questions give easy answers that help in revising the chapter well and scoring better in exams. These NCERT solutions also enhance problem-solving skills by providing clear explanations and practical examples for each question.
Question 10.1 What are monosaccharides?
Answer :
Monosaccharides are the carbohydrates that cannot be hydrolysed further to give simpler units of polyhydroxy aldehyde or ketone. Nearly 20 monosaccharides are known to occur in nature. The general formula of monosaccharides is $(CH_{2}O)_{}n$ where, n =3 to 7. Some common examples of monosaccharides are glucose, fructose.
Question 10.2 What are reducing sugars?
Answer :
The carbohydrate that can reduce Fehling’s solution and Tollens’ reagent is referred to as a reducing sugar. Also, all monosaccharides, whether ketose or aldose, are reducing sugars.
Question 10.3 Write two main functions of carbohydrates in plants.
Answer :
The importance of carbohydrates in plants:-
(i) Carbohydrates are used as storage molecules as starch, in plants.
(ii) The cell wall of the plants is made up of cellulose
Question 10.4 (1) Classify the following into monosaccharides and disaccharides.
(1) Ribose
Answer :
Ribose is a monosaccharide carbohydrate since it doesn't give simpler units upon hydrolysis.
Question 10.4(2) Classify the following into monosaccharides and disaccharides.
Answer:
It is a monosaccharide carbohydrate because upon hydrolysis it doesn't break into simpler ketone or aldehyde.
Question 10.4(3) Classify the following into monosaccharides and disaccharides.
Answer :
Maltose is a disaccharide carbohydrate, i.e., upon hydrolysis it yields two monosaccharide units.
Question 10.4(4) Classify the following into monosaccharides and disaccharides.
Answer :
Galactose is a monosaccharide carbohydrate.
Question 10.4(5) Classify the following into monosaccharides and disaccharides.
Answer :
It is a monosaccharide carbohydrate because upon hydrolysis, it doesn't break into simpler compounds of ketone and aldehyde.
Question 10.4(6) Classify the following into monosaccharides and disaccharides.
Answer :
Lactose is a disaccharide carbohydrate since it breaks into two simpler monosaccharide units.
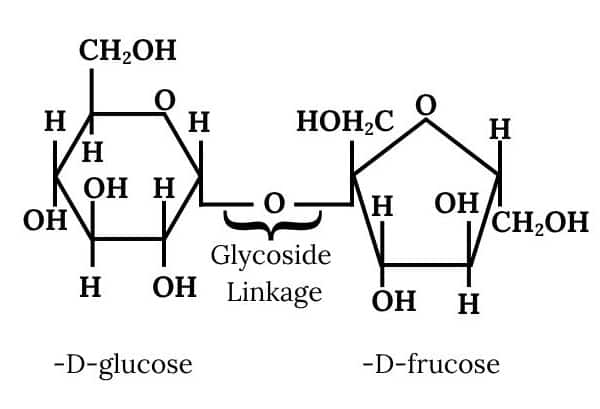
Question 10.5 What do you understand by the term glycosidic linkage?
Answer :
It is the linkage by which two monosaccharide units are joined together by an oxygen atom. This linkage is formed by the loss of a water molecule.
Question 10.6 What is glycogen? How is it different from starch?
Answer :
Glycogen is a polysaccharide carbohydrate that is stored in the body of animals or human beings. Whenever our body requires energy, glycogen breaks down to glucose with the help of the enzyme. It is present in the liver, brain and muscles. Whereas starch is a polysaccharide carbohydrate stored in plants.
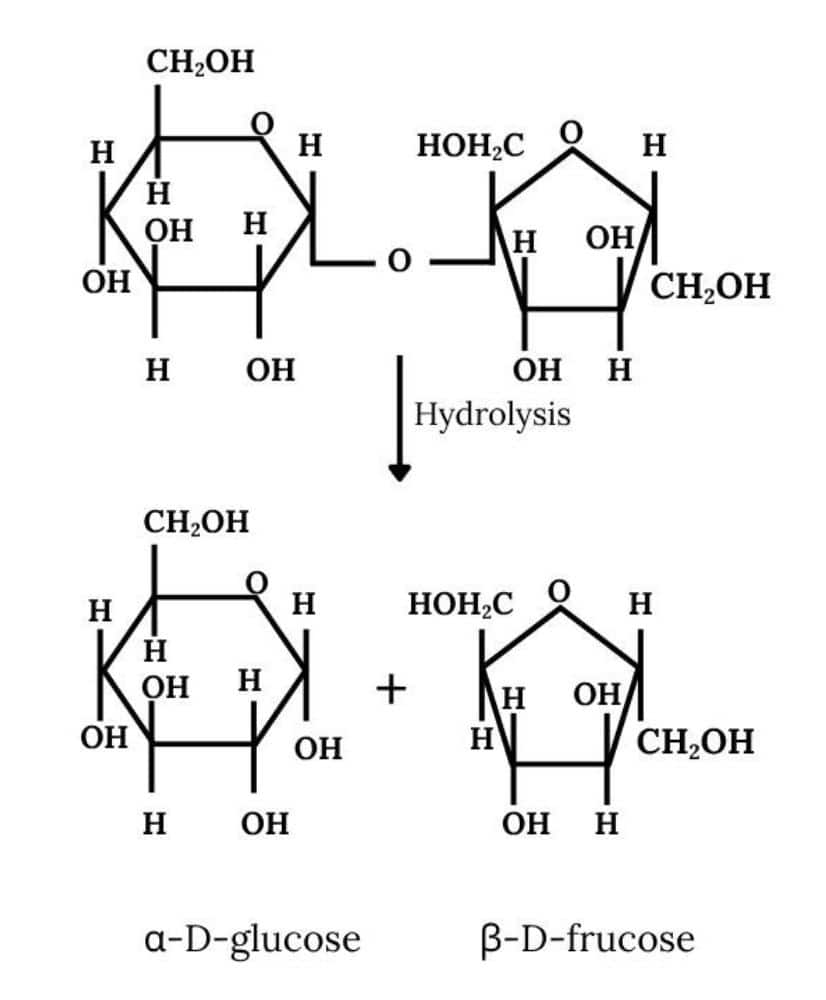
Question 10.7 (i) What are the hydrolysis products of
Answer :
Upon hydrolysis, sucrose breaks into $\beta$-D- glucose and $\beta$-D-fructose.
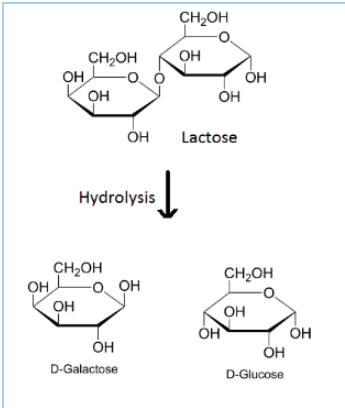
Question 10.7 (ii) What are the hydrolysis products of
Answer :
Hydrolysis of lactose gives $\beta$-D-galactose and $\beta$-D-glucose
Question 10.8 What is the basic structural difference between starch and cellulose?
Answer :
Cellulose is a long, straight-chain polysaccharide made of $\beta$-D-glucose units and has a 1,4-glycoside linkage.
Whereas starch is made up of 2 components:- Amylose and amylopectin.
Amylose is a long straight chain made of $\alpha$ - D - glucose units and joined by a 1,4-glycosidic linkage.
Amylopectin is a branched structure, and chains are formed at 1,4-glycosidic linkage, and branching occurs at 1,6-glycosidic linkage.
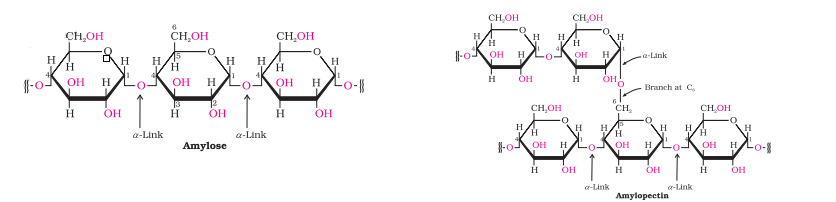
Question 10.9(i) What happens when D-glucose is treated with the following reagents?
Answer :
When glucose is treated with HI for a long time, n-hexane is formed.
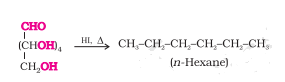
Question 10.9(ii) What happens when D-glucose is treated with the following reagents?
Answer :
Since bromine water is an oxidising agent so it will oxidise the aldehyde group to the carboxylic acid group, thus, glucose is converted to gluconic acid.
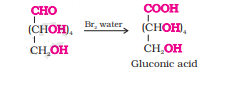
Question 10.9(iii) What happens when D-glucose is treated with the following reagents?
Answer :
On reacting glucose with nitric acid, oxidation takes place and glucose is converted into saccharic acid.
Question 10.10 Enumerate the reactions of D-glucose which cannot be explained by its open chain structure.
Answer :
Below are a few reactions that cannot be explained by the open structure of glucose:-
1. Even if glucose has an aldehyde group, it does not give Schiffs test, and as a result, it does not form the hydrogen sulphite addition product with $\mathrm{NaHSO}_3$.
2. The pentaacetate of glucose does not react with hydroxylamine, which indicates the absence of a free CHO group.
3. It is found that glucose exists in two different crystalline forms, which are named as $\alpha$ and $\beta$. This property can only be explained by the cyclic structure of glucose.
Question 10.11 What are essential and non-essential amino acids? Give two examples of each type.
Answer :
The amino acids that are required for the body and can be synthesised in the body are known as non-essential amino acids. E.g., Glycine, Alanine. Whereas, the amino acids that are required for our body but cannot be synthesised by our body and should be taken through diet are known as essential amino acids. E.g., Valine, Leucine
Question 10.12(i) Define the following as related to proteins
Answer :
Peptide linkage is an amide bond formed between COOH group and $\mathrm{NH}_2$ group. The reaction between two molecules of either different or similar amino acids proceeds by the combination of the amino group of one molecule with the carboxyl group of the other molecule. This will result in the elimination of a water molecule and the formation of a peptide bond –CO–NH–.
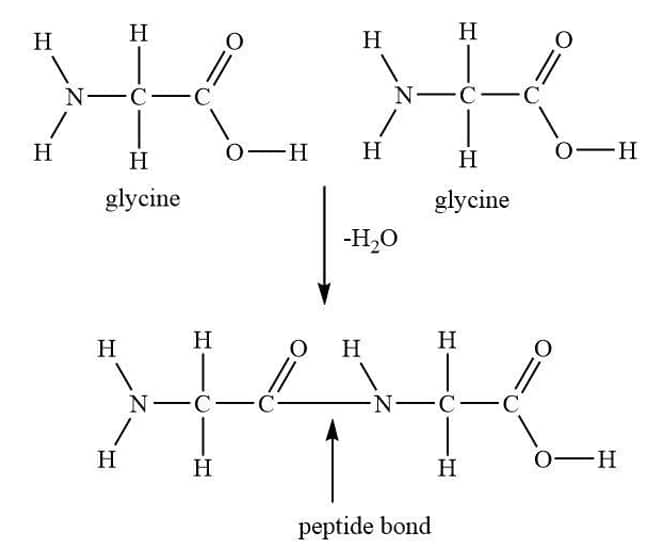
Question 10.12(ii) Define the following as related to proteins
Answer :
Primary structure of proteins: Each and every polypeptide present in a protein has amino acids linked with each other in a specific sequence and it is this sequence of amino acids that is known to be the primary structure of that protein. Any change in this primary structure, that is, in the sequence of amino acids, creates a different protein.
Question 10.12(iii) Define the following as related to proteins
Answer :
When a protein in its raw form is subjected to a change, like a change in temperature, e.g boiling in hot water or a chemical change like a variation in pH, the hydrogen bonds are disturbed. This results in the unfolding of globules and the uncoiling of the helix, and thus protein loses its biological activity. This is called denaturation of the protein.
Question 10.13 What are the common types of secondary structure of proteins?
Answer :
Secondary structures of proteins are found to exist in two types of structure:-
(i) $\alpha -$ Helix structure:- α-Helix is a way in which a polypeptide chain forms all possible hydrogen bonds by twisting into a right-handed screw or helix with the NH group of each amino acid and residue hydrogen bonded to the of C=O an adjacent turn of the helix.
(ii) $\beta$-pleated sheet:- In this structure, all peptide chains are stretched out to maximum extension and then laid side by side, which is held together by strong intermolecular hydrogen bonds.
Question 10.14 What type of bonding helps in stabilising the a-helix structure of proteins?
Answer :
Hydrogen bonding is the intermolecular bonding that helps in stabilising the $\alpha$-helix structure of proteins. Hydrogen bonds are formed between the -NH- of the amino acid and $C = O$ of the adjacent turn of helix.
Question 10.15 Differentiate between globular and fibrous proteins.
Answer :
The difference between fibrous protein and globular protein is given below :-
|
Fibrous Protein
|
Globular Protein
|
|
When the polypeptide chains run parallel and are held together with the help of hydrogen and disulphide bonds.
|
In case of a globular protein, the chains of polypeptides coil around and give a spherical shape
|
|
These proteins are generally insoluble in water.
|
These proteins are generally soluble in water.
|
|
E.g. keratin, myosin
|
E.g., Insulin, albumins
|
Question 10.16 How do you explain the amphoteric behaviour of amino acids?
Answer :
Amino acid has both acidic group $(C=O)$ and basic group $(N-H)$. Thus, in aqueous solution, the carboxyl group can lose a proton and the basic group (amine group) can accept a proton. In this way, it forms a zwitter ion which can act in both ways i.e., acidic as well as basic. Hence, amino acids are amphoteric in nature.
Question 10.17 What are enzymes?
Answer :
Enzymes are the biocatalysts i.e., they catalyse biological reactions. Enzymes are very specific in nature and a particular enzyme can be used for a particular substrate. They are generally named after the name of a compound on which they act. For example conversion of maltose to glucose requires the enzyme maltase.
Question 10.18 What is the effect of denaturation on the structure of proteins?
Answer :
In the denaturation of protein, globules get unfolded and helix gets uncoiled, and globular protein converts into fibrous protein. The primary structure remains the same but the secondary and tertiary structures of the protein are destroyed, so its biological activity changes.
Question 10.19 How are vitamins classified? Name the vitamin responsible for the coagulation of blood.
Answer :
On the basis of their solubility in water, vitamins are classified as:-
- Water-soluble vitamins: Water-soluble vitamins such as Vitamin C need to be taken through diet regularly as they are excreted in the form of urine.
- water-insoluble and fat-soluble vitamins: Since these vitamins are not soluble in water so we do not need to take them regularly through diet. They are stored in the liver and adipose tissue. E.g. Vitamin D
Vitamin K is responsible for the coagulation of blood.
Question 10.20 Why are vitamin A and vitamin C essential to us? Give their important sources.
Answer :
Vitamin A: Sources of this vitamin are carrots, butter and milk. Its deficiency causes Xerophthalmia (hardening of the cornea of the eye).
Vitamin C: Sources of this vitamin are citrus fruits, amla, and green vegetables. Its deficiency causes Scurvy (bleeding gums).
Question 10.21 What are nucleic acids? Mention their two important functions.
Answer :
Nucleic acids are biomolecules found in the nuclei of cells. They are of two types
(i) DNA - Deoxyribonucleic acid
(ii) RNA - Ribonucleic acid.
The two important functions of nucleic acid are:-
(i) DNA present in chromosomes is responsible for heredity by transferring genes.
(ii) Both nucleic acids are responsible for protein synthesis in a cell.
Question 10.22 What is the difference between a nucleoside and a nucleotide?
Answer :
|
Nucleoside
|
Nucleotide
|
|
1. It has a base and sugar.
|
1. It has base, sugar and phosphoric acid.
|
|
2. The base is attached to the 1' position of the sugar.
|
2. In this, the base is attached to 1' position and phosphoric acid to 5' position of the sugar moiety.
|
Question 10.23 The two strands in DNA are not identical but are complementary. Explain.
Answer :
The two strands in DNA are not identical, but they are held together because they form hydrogen bonds with each other. So they are not identical but complementary to each other. Cytosine forms a hydrogen bond with guanine, and adenine forms a hydrogen bond with thymine. So, that is why the two strands act as complementary to each other.
Question 10.24 Write the important structural and functional differences between DNA and RNA.
Answer :
Structural differences between DNA and RNA:-
(i) The sugar moiety in DNA is D-2-deoxyribose, whereas the sugar moiety in RNA is D-ribose.
(ii) DNA contains thymine, but it is absent in RNA.
(iii) The helical structure of DNA is double-stranded, whereas it is single-stranded in the case of RNA.
Functional differences between DNA and RNA: The main function of DNA is to provide heredity, but the main function of RNA is the synthesis of protein in a cell.
Question 10.25 What are the different types of RNA found in the cell?
Answer :
The three types of RNA found in the cell are:-
(i) messenger RNA (mRNA)
(ii) ribosomal RNA (rRNA)
(iii) transfer RNA (tRNA)
Class 12 Chemistry NCERT Chapter 10: Higher Order Thinking Skills (HOTS) Questions
Given below the class 12 chemistry chapter 10 biomolecules question answer that make you think harder and understand biomolecules in a simple and clear way. These NCERT Solutions for Class 12 also encourage critical thinking by connecting theoretical concepts with real-life biological applications.
Question 1. A dipeptide, " $x$ " on complete hydrolysis gives " $y$ " and " $z$ ". " y " on treatment with aq. $\mathrm{HNO}_2$ produces lactic acid. On the other hand " $z$ " on heating gives the following cyclic molecule.

Based on the information given, the dipeptide X is:
1) valine-glycine
2) alanine-glycine
3) valine-leucine
4) alanine-alanine
Answer:
Hence, the correct answer is option (2).
Question 2. Given below are two statements:
Statement-I: D-(+)- Glucose and D-(+)- fructose are formed on hydrolysis of sucrose.
Statement II : Invert sugar is formed during sucrose hydrolysis.
In light of the above statements, choose the correct answer from the options given below-
1) Both Statement I and Statement II are true.
2) Statement I is false but Statement II are true.
3) Statement I is true but Statement II is false.
4) Both Statement I and Statement II are false.
Answer:
On hydrolysis of sucrose gives $\mathrm{D}-(+)$-glucose and D-(-)-fructose while in St. (1) D-(+)-fructose is given; hence, Statement-(1) is incorrect.

St. II - It is correct because sucrose on hydrolysis gives invert sugar
Hence, the correct answer is option (2).
Question 3. The total number of hydrogen bonds of a DNA double Helix strand whose one strand has the following sequence of bases is _________.
5’ – G – G-C-A-A-A-T-C-G-G-C-T-A-3’.
Answer:
Two nucleic acid chains are wound about each other and held together by H bonds between pairs of bases.
Adenine forms two hydrogen bonds with thymine, and guanine forms three hydrogen bonds with cytosine.
5' G–G–C–A–A–A–T–C–G–G–C–T–A–3'
In the given DNA strand, there are a total of seven guanine and cytosine bases, which form a total of 21 H-bonds, and six adenine and thymine bases, which will form a total of 12 H-bonds with the other DNA strand.
Total no. of H bonds = 7 × 3 + 6 × 2 = 33
Hence, the answer is 33.
Question 4. Given below are two statements :
Statement (I) : On hydrolysis, oligo peptides give rise to fewer number of $\alpha$-amino acids while proteins give rise to a large number of $\beta$-amino acids.
Statement (II) : Natural proteins are denatured by acids which convert the water soluble form of fibrous proteins to their water insoluble form.
In the light of the above statements, choose the most appropriate answer from the options given below :
(1) Both statement I and statement II are correct
(2) Statement I is incorrect but Statement II is correct
(3) Both statement I and statement II are incorrect
(4) Statement I is correct but Statement II is incorrect
Answer: Protein give rise to alpha amino acid, so the above statement is incorrect.
Natural proteins are denatured by acid. Due to this, globules unfold and helices get uncoiled.
Hence, the correct answer is option (3).
Question 5. Identify the pair of reactants that upon reaction, with elimination of HCl will give rise to the dipeptide Gly-Ala.
(1)

(2)

(3)

(4)

Answer:

Hence, the correct answer is option (1).
Question 6: In Maltose, the type of glycosidic linkage present is
(1) $(1,4) \alpha$-glycosidic linkage.
(2) $(1,4) \beta$-glycosidic linkage.
(3) $(1,2) \alpha$-glycosidic linkage.
(4) $(1,2) \beta$-glycosidic linkage.
Answer:

Hence, the correct answer is option (1).
Question 7: Match List I with List II
| LIST I | LIST II | ||
| A. | Glucose $+\mathrm{Br}_2 / \mathrm{H}_2 \mathrm{O}$ | I. | Gluconic acid |
| B. | Glucose $/ conc.\mathrm{HNO}_3 $ | II. | Saccharic acid |
| C. | Glucose $+\mathrm{NaBH}_4$ | III. | Sorbitol |
| D. | Glucose + HI / Red P / | IV. | $n$-Hexane |
Choose the correct answer from the options given below:
(1) $\mathrm{A}-\mathrm{I}, \mathrm{B}-\mathrm{II}, \mathrm{C}-\mathrm{III}, \mathrm{D}-\mathrm{IV}$
(2) A-II, B-I, C-IV, D-III
(3) A-I, B-III, C-II, D-IV
(4) A-III, B-II, C-I, D-IV
Answer:
$\mathrm{Br}_2 / \mathrm{H}_2 \mathrm{O} \rightarrow$ mild oxidation of $-\mathrm{CHO} \rightarrow$ gluconic acid conc. $\mathrm{HNO}_3 \rightarrow$ oxidation of $-\mathrm{CHO} \&-\mathrm{CH}_2 \mathrm{OH} \rightarrow$ saccharic acid $\mathrm{NaBH}_4 \rightarrow$ reduction of aldehyde → sorbitol $\mathrm{HI} /$ Red $\mathrm{P} / \Delta \rightarrow$ complete reduction $\rightarrow$ n-hexane
Hence, the correct answer is option (1).
NCERT Exemplar Solutions
- NCERT Exemplar Class 12 Solutions
- NCERT Exemplar Class 12th Maths Solutions
- NCERT Exemplar Class 12th Physics Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Class 12th Biology Solutions
Approach to Solve Questions of Chapter 10 Biomolecules
To effectively solve biomolecules class 12 question answer, one should follow a structured approach. Here are some points that can help you with problem-solving strategies
1. Before attempting any question, make sure that you clearly understand the basic concepts like
- Carbohydrates- Monosaccharides, disaccharides, polysaccharides; classification and examples
- Proteins- Amino acids, peptide bond formation, levels of protein structure, enzymes.
- Nucleic Acids- DNA vs. RNA, nucleotide structure, base pairing.
- Vitamins and Enzymes- Types, functions, coenzymes, enzyme action mechanisms.
2. Make flowcharts for classifications such as types of carbohydrates, and use mnemonics for vitamin types or amino acids. Revise reactions like hydrolysis of sucrose, peptide formation, and DNA replication basics. Practice previous year questions to understand trends and frequently asked areas. Highlight NCERT in text questions that are frequently asked in exams. Students can also refer to class 12 chemistry chapter 10 biomolecules solutions.
3. Read the questions carefully and look out for terms like structure, difference between, or mechanism. Underline the keywords when solving long-answer questions.
4. Use these NCERT Solutions for Class 12 Chemistry Chapter 10 Biomolecules as questions from these are asked directly in exams, along with this also refer exemplar problems that are great for higher-order thinking skills.
Topics of the NCERT Book Class 12 Chemistry Biomolecules
Given below the topics covered in class 12 chemistry chapter 10 biomolecules solutions. They cover important concepts that help in understanding the role and behaviour of different biomolecules in living organisms.
10.1 Carbohydrates
10.2 Proteins
10.3 Enzymes
10.4 Vitamins
10.5 Nucleic Acids
10.6 Hormones
NCERT Books and NCERT Syllabus
What Extra Should Students Study Beyond NCERT for JEE/NEET?
Given below the comparison table between topics covered in NCERT and JEE. This table helps you see the similarities and differences between the NCERT syllabus and the topics asked in JEE. Students can refer biomolecules ncert solutions for understanding these concepts better.
What Students Learn from NCERT Solutions for Class 12 Chemistry Chapter 10 Biomolecules
These class 12 chemistry chapter 10 biomolecules question answer help students to understand complex biological concepts in a simple way. Given below some points on what students will learn using these solutions:
- These solutions help students to understand the structure and functions of carbohydrates, proteins, nucleic acids, and vitamins.
- Here they learn about the classification and properties of biomolecules based on their chemical composition.
- The role of enzymes and their mechanisms in biochemical reactions are well explained in these biomolecules class 12 question answers.
- Using these solutions students can easily understand the biological significance and practical applications of biomolecules.
Importance of Class 12 Chemistry Chapter 10 Biomolecules Solutions
The biomolecules class 12 question answer are important as they connect chemistry with biology. Given below some points on importance of these solutions:
- These solutions help students understand how biomolecules and vitamins plays an important role in living organisms.
- The questions related to the structure, classification, and functions of biomolecules are frequently asked in CBSE and competitive exams like NEET, JEE.
- The class 12 chemistry biomolecules question answer help students understand the topics like glycosidic linkage, peptide bond formation, and enzyme catalysis.
NCERT Solutions for Class 12
NCERT Solutions Class 12 Chemistry Chapter-wise
Along with NCERT Solutions for Class 12 Chemistry Chapter 10 Biomolecules you can also find links to solutions for other Class 12 NCERT chapters below.
Frequently Asked Questions (FAQs)
Class 12 Chemistry Chapter 10 Biomolecules NCERT Solutions are detailed, step-by-step answers to all the intext and exercise questions from this chapter, designed to help students understand concepts clearly and prepare effectively for exams.
You can access NCERT answers for Class 12 Chemistry Chapter 10 from the official NCERT website, educational platforms, or reliable study resources that provide step-by-step solutions for all intext and exercise questions.
NCERT Solutions should include the 20 standard amino acids, focusing on their classification as essential or non-essential, and as acidic, basic, or neutral based on side chains. Examples like glycine, alanine, valine, leucine, serine, cysteine, aspartic acid, glutamic acid, lysine, and arginine are typically covered, along with their general structure, zwitterionic form, and isoelectric point.
A peptide bond is a covalent chemical bond formed between two amino acid molecules when the carboxyl group of one amino acid reacts with the amino group of the other, releasing a molecule of water.
Nucleic acids are polymers that store and transmit genetic information. The two main types are
- DNA (Deoxyribonucleic acid)
- RNA (Ribonucleic acid)
Glycogen is a polysaccharide that serves as the storage form of glucose in animals. It is primarily stored in the liver and muscles.
he chapter covers several key topics, including the classification and structure of biomolecules, their functions in living organisms, and the chemical reactions they undergo. It also touches on enzymes and their role as biological catalysts.
One common example of a biomolecule is glucose, a simple sugar that serves as a primary energy source for cells. It is involved in cellular respiration, where it is converted into energy that powers various cellular activities.
Enzymes are proteins that accelerate chemical reactions in biological systems by lowering the activation energy needed for reactions to occur. They are specific to substrates and help increase the rate of reactions, facilitating metabolic processes in the body.
Proteins are composed of amino acids linked by peptide bonds, and their structure can be categorized into four levels: primary, secondary, tertiary, and quaternary. Their specific structure determines their function in processes such as catalysis, transport, and cell signaling.
Questions related to CBSE Class 12th
On Question asked by student community
Hello
You will be able to download the CBSE Previous Year Board Question Papers from our official website, careers360, by using the link given below.
https://school.careers360.com/boards/cbse/cbse-previous-year-question-papers
I hope this information helps you.
Thank you.
Hello
You will be able to download the CBSE Pre-Board Class 12 Question Paper 2025-26 from our official website by using the link which is given below.
https://school.careers360.com/boards/cbse/cbse-pre-board-class-12-question-paper-2025-26
I hope this information helps you.
Thank you.
Hello,
Yes, it's completely fine to skip this year's 12th board exams and give them next year as a reporter or private candidate, allowing you to prepare better; the process involves contacting your current school or board to register as a private candidate or for improvement exams during the specified
HELLO,
Yes i am giving you the link below through which you will be able to download the Class 12th Maths Book PDF
Here is the link :- https://school.careers360.com/ncert/ncert-book-for-class-12-maths
Hope this will help you!
Hello,
Here is your Final Date Sheet Class 12 CBSE Board 2026 . I am providing you the link. Kindly open and check it out.
https://school.careers360.com/boards/cbse/cbse-class-12-date-sheet-2026
I hope it will help you. For any further query please let me know.
Thank you.
Popular CBSE Class 12th Questions
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters


