General Principles and Processes of Isolation of Elements Class 12th Notes - Free NCERT Class 12 Chemistry Chapter 6 Notes - Download PDF
The chapter General Principles and Processes of isolation of elements is a very important inorganic chemistry chapter. This is a chapter with practical applications too. The NCERT Class 12 Chemistry chapter 6 notes provide a summary of the chapter General Principles and Processes of Elements Isolation. General Principles and Processes of isolation of elements Class 12 notes covers the topics occurrence of metals, concentration of ores, extraction of crude metal, Ellingham diagram, extraction of iron, refining etc. Class 12 is stressful, and many students find Chemistry to be a tough subject to study. Many students find inorganic chemistry to remember.
This Story also Contains
- NCERT Class 12 Chemistry Chapter 6 Notes-Topic 1
- Occurrence of metals
- NCERT Class 12 Chemistry Chapter 6 Notes-Topic 2
- Metallurgy
- NCERT Class 12 Chemistry Chapter 6 Notes-Topic 3
- Concentration of Metals
- NCERT Class 12 Chemistry Chapter 6 Notes-Topic 4
- Extraction of Crude Metal
- NCERT Class 12 Chemistry Chapter 6 Notes-Topic 5
- Ellingham Diagram
- NCERT Class 12 Chemistry Chapter 6 Notes-Topic 6
- Extraction of Iron
- NCERT Class 12 Chemistry Chapter 6 Notes-Topic 7
- Refining
- NCERT Class 12 Notes Chapter-Wise
- Subject Wise NCERT Exemplar Solutions
- NCERT Books and Syllabus
The NCERT Class 12 Chemistry chapter 6 notes is given in a straightforward manner, allowing students to quickly learn the entire syllabus of the chapter.
Also, students can refer,
NCERT Class 12 Chemistry Chapter 6 Notes-Topic 1
Occurrence of metals
Minerals are naturally occurring chemical components in the earth's crust that can be obtained by mining. Metals may or may not be profitable to extract from them. Ores are rocky materials that contain a significant amount of mineral to allow the metal to be mined profitably or economically. Gangue refers to the earthy or unwanted elements found in ore.
NCERT Class 12 Chemistry Chapter 6 Notes-Topic 2
Metallurgy
Metallurgy refers to the complete scientific and technological process of isolating metal from its ores. Different parts of metallurgy include
Crushing and grinding
For processing, the metal ore is first pulverised or crushed into powder. Crushing or grinding is the term for this procedure
Concentration of ores
Dressing, concentration, or benefaction are terms used to describe the process of removing unwanted contaminants from ore. Hydraulic washing, electromagnetic separation, froth flotation, and leaching are all options.
Isolation of crude metal from the ore
The unrefined metal is separated in two steps: the ore is converted to oxide and then reduced to metal. Smelting, self-reduction, metal displacement, or electrolytic reduction are all methods for converting metal oxide to metal oxide
Refining or purification of the metal
Finally, the obtained unrefined metal must be purified before it is used. Liquation, distillations, zone refining, chromatography, and electrolysis can all be used to accomplish this.
NCERT Class 12 Chemistry Chapter 6 Notes-Topic 3
Concentration of Metals
It includes a number of processes that are dependent on the physical properties of the metal complex and the presence of impurities (gangue). The type of metal, the facilities available, and environmental variables are all taken into account.
Hydraulic washing/ Gravity separation
It is based on the density differences between ore and gangue particles. Lighter impurities are washed away while heavy ores are left behind when ore is washed with a stream of water under pressure.
Magnetic separation
The difference in magnetic and non-magnetic characteristics of two ore components is used in this procedure. To extract tungsten ore particles from cassiterite, this process is utilized. It's also used to separate unwanted gangue from magnetite , chromite and pyrolusite.
Froth flotation
It is built on the idea that sulfide ores are wetted preferentially by pine oil, fatty acids, xanthates, and other similar substances, whereas gangue particles are wetted by water. Collectors are used to improve the mineral particles' non-wettability. Cresols, aniline, and other froth stabilizers are used to keep the froth stable. It is possible to separate two sulfide ores by altering the percentage of oil to water or by adding depressants if two sulfide ores are present.
Leaching (chemical separation)
It is a method of treating ore with an appropriate solvent that dissolves the ore but not the contaminants. eg : Purification of bauxite
NCERT Class 12 Chemistry Chapter 6 Notes-Topic 4
Extraction of Crude Metal


Conversion of ore into metal oxide
Oxide ore is simpler to reduce than sulfide or carbonate ore. Any of the following methods should be used to convert the provided ore into oxide.
Roasting
It is a method of converting ore into oxide ore by heating it in a regular supply of air at a temperature below the metal's melting point. Roasting converts sulfide ores into oxide. It is also utilized to get rid of contaminants like volatile oxides.
Eg: 2 ZnS + 3 O2 yields→ 2 ZnO + 3 SO2
Calcination
It is a method of converting carbonate ores into oxides by heating ore in a small amount of air. It I 's also used to get rid of moisture and volatile contaminants.
Eg : CaCO3 Heat→ CaO + CO2
Reduction of metal oxide to metal
Reduction is the process of turning metal oxide to metal. Depending on the reactivity or reducing power of the metal, an appropriate reducing agent is required. Carbon or carbon monoxide, as well as other metals such as Al, Mg, and others, are commonly utilized as reducing agents.
NCERT Class 12 Chemistry Chapter 6 Notes-Topic 5
Ellingham Diagram
Ellingham diagrams are plots of change in Gibbs free energy of formation of oxides of elements vs. temperature. It gives a good concept of how to choose a reducing agent for oxide reduction. These diagrams help to determine whether or not a thermal reduction of an ore is feasible. For a reaction to be feasible, Gibbs free energy change must be negative at a particular temperature. Ellingham Diagrams have the drawback of not taking the kinetics of reduction into account, which means that the rate of reduction cannot be predicted.
NCERT Class 12 Chemistry Chapter 6 Notes-Topic 6
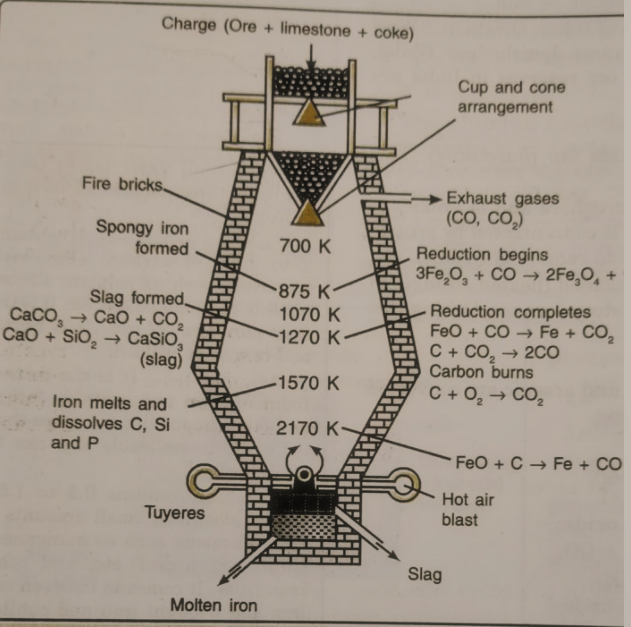
Extraction of Iron
The reduction of oxides occurs in various zones of the blast furnace.
C + 2O2 yields→ 2 CO
FeO + CO yields→ Fe + CO2
Lower temperature range ( 500 K-800 K)
3 Fe2O3 + CO yields→ 2 Fe3O4+CO2
Fe2O4 + 4 CO yields→ 3 Fe+4 CO2
Fe2O3 + CO yields→ 2 FeO+ CO2
Higher temperature range ( 900 K- 1500 K)
C + CO2 yields→ 2 CO
FeO + CO yields→ Fe + CO2
Limestone decomposes to CaO and CO2
CaCO3 yields→ CaO + CO2
Calcium silicate is formed when silica (impurity) interacts with CaO to generate slag. It floats on top of molten iron, preventing iron oxidation.
CaO + SiO2 yields→ CaSiO3
Pig iron is the iron produced in a blast furnace. It is made of impure iron and contains 4% carbon, as well as tiny amounts of S, P, Si, and Mn. It may be moulded into a wide range of shapes. Cast iron is prepared by melting pig iron, scrap iron, and coke together with a hot air blast. It has a carbon content of approximately 3%. It is exceedingly brittle and rigid. Wrought iron is the purest type of iron available for commercial use. It's also referred to as malleable iron. Pig iron is oxidatively refined in a reverberatory furnace lined with haematite, which converts carbon to carbon monoxide.
NCERT Class 12 Chemistry Chapter 6 Notes-Topic 7
Refining
It involves converting impure metal to pure based on the nature of the metal
Distillation
It is a method for purifying metals with low boiling points, such as zinc, mercury, sodium, and potassium. Impure metal is heated to convert it to vapours, which then condensate to form pure metal, which is obtained as distillate.
Liquation
This procedure can be used to purify metals with impurities with melting points higher than the metal. Sn metal can be refined using this process. Tin with impurities of iron was heated on the top of a slanting furnace. Tin melts and slides down the sloped surface, leaving no iron behind and yielding pure tin.
Electrolytic refining
Impure metal is employed as the anode, pure metal is used as the cathode, and a soluble metal salt is used as the electrolyte in this technique. Impure metal generates metal ions when an electric current is run through it, which are then discharged at the cathode, producing in pure metal.
Zone refining
It is based on the fact that impurities are more soluble in the melt than in the solid state of the metal. At one end of the impure metal rod, circular heaters are used to heat the impure metal. The molten zone continues along with the heater, picking up impurities along the way, until it reaches the other end, where it is discarded. Out of the melt, pure metal crystallizes. The procedure is done multiple times, with the heater moving in the same direction each time. It is employed in the purification of semiconductors such as B, Ge, Si, Ga, and In.
Vapour phase refining
Metal is transformed to a volatile chemical and collected in this procedure. After that, it is dissolved into pure metal.
Chromatographic method
It is based on the principle of chromatography, which is based on differential adsorption on an adsorbent for separation or purification.
These topics can be studied from General principles and processes of isolation of elements Class 12 notes pdf download.
Significance of NCERT Class 12 Chemistry Chapter 6 Notes
General Principles and Processes of isolation of elements Class 12 notes will surely assist students in tackling all little and significant problems linked to this course to a great extent Also, NCERT Class 12 Chemistry chapter 6 notes are very helpful to study the main topics of the Class 12 CBSE Chemistry Syllabus. This chapter is important for various competitive exams like VITEEE, BITSAT, JEE Main, NEET etc.
NCERT Class 12 Chemistry chapter 6 notes also helps the students for the preparations of these exams. Students can download Class 12 Chemistry chapter 6 notes pdf download and use it for the offline mode preparation.
NCERT Class 12 Notes Chapter-Wise
Subject Wise NCERT Exemplar Solutions
Subject Wise NCERT Solutions
NCERT Books and Syllabus
Frequently Asked Questions (FAQs)
Ans- The process of recovering crude metal from raw concentrated ore is known as calcination. The ore is heated below its melting point with a limited or no supply of oxygen in this method.
Ans- Electrometallurgy is the electrolysis-based separation of metal from its fused salts. Highly reactive elements such as sodium, calcium, and aluminium are extracted using this method.
Ans- Students can expect 4 to 6 marks questions from the chapter General Principles and Processes of isolation of elements.
Ans- It's a compound that's added to roasted or calcinated ores to eliminate impurities. When combined with ores, it produces slag, a flammable material. In molten metal, slag is unsolvable. It floats on the surface because it is lighter than molten metal.
Ans- The whole scientific and technological process for the extraction of pure metal from its ore is called metallurgy.
Ans- NCERT Class 12 Chemistry chapter 6 notes should contain various steps involved in the extraction of metals. One can also refer to Class 12 Chemistry chapter 6 notes pdf download.
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
