Aakash Repeater Courses
Take Aakash iACST and get instant scholarship on coaching programs.
Do you know how basic chemistry is important in various industrial and biological processes? When we observe the periodic table, we see the d- and f-block elements, which are important to a lot of reactions and their applications. These elements are also known as transition metals and inner transition metals. They show unique properties like multiple Oxidation states and the ability to form complex compounds. Understanding the mole concept helps us to predict these elements when working with reactions that involve these metals.

The general and physical properties of d-block and f-block elements have been discussed in Chapter 4. The d-block elements are elements of groups 3 to 12 of the periodic table. The elements of the f-block are those in which the 4f and 5f orbitals are filled. In NCERT Solutions for d and f block elements, you will find all the topic-wise questions given in the chapter. There are three series of the d block elements, 3d series (Sc to Zn), 4d series (Y to Cd), and 5d series (La to Hg, except Ce to Lu). The two series of the f-block elements, (4f and 5f) are known as lanthanoids and actinoids, respectively.
The d- and f-Block Elements are designed by our subject experts to offer a systematic and structured approach to these important concepts and help students to develop a clear understanding of critical concepts by the series of solved examples and conceptual explanations, these solutions provide a valuable resource to enhance performance in board exams as well as in the competitive exams like JEE Advanced, NEET, JEE Mains, etc. In this article, we will discuss detailed solutions to all the questions.
Take Aakash iACST and get instant scholarship on coaching programs.
Also Read :
| NCERT Exemplar Class 12 Chemistry Solutions Chapter 4 |
| NCERT Notes For d and f Block Elements Class 12 Chemistry |
Page 92
Question 4.1 Silver atom has completely filled d-orbitals
Answer:
Silver atom(atomic no. = 47) has completely filled d-orbital in its ground state(4
Page 95
Question 4.2 In the series
Answer:
The enthalpy of atomisation of zinc is lowest due to the absence of an unpaired electron, which is responsible for metallic bonding in the elements. Therefore, the inter-atomic bonding is weak in zinc(
Page 97
Question 4.3 Which of the 3d series of transition metals exhibits the largest number of oxidation states and why?
Answer:
In 3d series of transition metals Manganese shows largest number of oxidation states because it has highest number of unpaired electrons in its
Example-
Page 98
Question 4.4 The
Answer:
The
Copper has a high value of atomisation enthalpy and low hydration energy. Thus, as a result, the overall effect is
Page 100
Answer:
The irregular variation in ionisation enthalpies is due to the extra stability of the configuration like
In the case of chromium (
The second IE is much higher than the 1st IE. This is because it becomes difficult to remove an electron when we already did that and it already has a stable configuration (such as
Page 101
Question 4.6 Why is the highest oxidation state of a metal exhibited in its oxide or fluoride only?
Answer:
Oxygen and fluorine are strong oxidising agents and both of their oxides and fluorides are highly electronegative in nature and also small in size. Because of these properties, they can oxidise the metal to its highest oxidation states.
Page 101
Question 4.7 Which is a stronger reducing agent
Answer :
Cr+2 is a better reducing agent as compared to Fe+2, as this can be explained on the basis of standard electrode potential of Cr+2 (-0.41) and Fe+2 (+0.77).
It can also be explained on the basis of their electronic configuration achieved. Cr+2 obtained d3 configuration, whereas Fe+2 gets d5 configuration upon reduction. It is known that d3 is more stable than d5. So, Cr+2 is a better reducing agent as compared to Fe+2.
Page 103
Question 4.8 Calculate the ‘spin only’ magnetic moment of
Answer :
Atomic number (Z)= 27
So the electronic configuration cobalt (
So,
by putting the value of n= 3
we get,
Page 105
Question 4.9 Explain why
Answer :
The hydration energy released during the formation of
Page 113
Question 4.10 Actinoid contraction is greater from element to element than lanthanoid contraction. Why?
Answer :
Actinoid contraction is greater from element to element than lanthanoid contraction. The reason behind it is the poor shielding effect of 5 f$ (in actinoids) orbitals than 4
Question 4.1(i) Write down the electronic configuration of:
Answer :
Chromium has an atomic number 24. So, the nearest noble gas element is Argon (
So electronic configuration of
Question 4.1(ii) Write down the electronic configuration of:
Answer :
The atomic number of promethium is 61 and the nearest noble gas is xenon(
So, atomic configuration of
Question 4.1(iii) Write down the electronic configuration of:
Answer :
The atomic number of copper is 29 and the previous noble element is Argon (
the electronic configuration of
Question 4.1(iv) Write down the electronic configuration of:
Answer :
The atomic number of cerium (
The electronic configuration of
Question 4.1(v) Write down the electronic configuration of:
Answer :
The atomic number of cobalt (Co) is 27 and the previous noble element is Argon (
Thus electronic configuration of
Question 4.1(vi) Write down the electronic configuration of:
Answer :
The atomic number of lutetium is 71 and the previous noble element is Xe (xenon)
The electronic configuration of
Question 4.1(vii) Write down the electronic configuration of:
Answer :
The atomic number of Manganese is 25 and the previous noble element is Ar (argon)
So, the electronic configuration of
Question 4.1(viii) Write down the electronic configuration of:
Answer :
The atomic number of thorium (Th) is 90 and the previous noble gas element is Xenon (Xe)
So, the elelctronic configuration of
Question 4.2 Why are
Answer :
In +2 oxidation state of manganese has more stability than +2 oxidation state of iron, it is because half-filled and fully filled d-orbitals are more stable and
Answer :
According to our observation, except scandium, all other elements of the first row show +2 oxidation state. On moving from Sc to Mn the atomic number increases from 21 to 25 and also the increasing number of electrons in 3d orbitals from
Mn(+2) has
Answer :
Elements of the first half of the transition series exhibit many oxidation states. manganese shows the maximum number of oxidation states (+2 to +7). The stability of +2 oxidation states increases with the increase in atomic number (as more number of electrons are filled in d-orbital). However, the
Answer :
Vanadium (atomic number- 23)
E.C =
So the stable oxidation states are (+2, +3, +4, +5)
Manganese (atomic number = 25)
E.C =
So the stable oxidation states are (+2, +4, +6, +7)
Chromium (atomic number = 24)
E.C =
So the stable oxidation states are (+3, +4, +6)
No elements have
Answer :
Following oxometal anions of the first series that exhibit the oxidation state equal to its group number-
Question 4.7 What is lanthanoid contraction? What are the consequences of lanthanoid contraction?
Answer :
On moving along the lanthanoid series, the atomic number is gradually increased by one. It means the no. of electrons and protons of the atom is also increases by one. And because of it the effective nuclear charge increases (electrons are added in the same shell, and the nuclear attraction overcomes the interelectronic repulsion due to the addition of a proton). Also, with the increase in atomic number, the number of electrons in orbital also increases. Due to the poor shielding effect of the electrons, the effective nuclear charge experienced by an outer electron is increased, and also the attraction of the nucleus for the outermost electron is increased. As a result, there is a gradual decrease in the atomic size as an increase in atomic number. This is known as lanthanoid contraction.
Consequences of Lanthanoid contraction-
Answer :
Transition elements are those which have partially filled
Question 4.9 In what way is the electronic configuration of the transition elements different from that of the non-transition elements?
Answer :
Transition elements have partially filled
Non-transition elements either have fully filled d-orbitals or do not have d-orbitals. Therefore general electronic configuration is
Question 4.10 What are the different oxidation states exhibited by the lanthanoids?
Answer :
In lanthanoid +3 oxidation states are more common.
Question 4.11(i) Explain giving reasons:
(i) Transition metals and many of their compounds show paramagnetic behaviour.
Answer :
Paramagnetism is arising due to the presence of unpaired electrons. And we know that transition metals have unpaired electrons in their -orbitals. That's why they show paramagnetic behaviour.
Question 4.11(ii) Explain giving reasons:
(ii) The enthalpies of atomisation of the transition metals are high.
Answer :
Transition metals have high effective nuclear charge and also high outermost electrons. Thus they form a very strong metallic bond and due to these, transition elements have a very high enthalpy of atomisation.
Question 4.11(iii) Explain giving reasons:
(iii) The transition metals generally form coloured compounds.
Answer :
Most of the complex transition elements are coloured. This is due to the absorption of radiation from the visible light region to excite the electrons from its one position to another position in d-orbitals. In the presence of ligands, d-orbitals split into two sets of different orbital energies. Here transition of electrons takes place and emits radiation which falls on the visible light region.
Question 4.11(iv) Explain giving reasons:
(iv) Transition metals and their many compounds act as good catalyst.
Answer :
The catalytic activity of transition metals is because of two reasons-
Question 4.12 What are interstitial compounds? Why are such compounds well-known for transition metals?
Answer :
Transition metals contain lots of interstitial sites. These elements trap the other elements which are small in sizes such as Carbon, Hydrogen and Nitrogen in their interstitial site of the crystal lattice as a result forms interstitial compounds.
Answer :
In transition metals, the variation of oxidation states id from +1 to the highest oxidation number, by removing all its valence electrons. Also in transition metals, the oxidation number is differed by one unit like (
Answer :
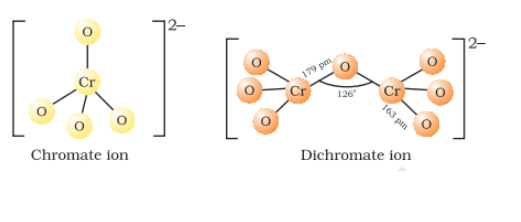
potassium dichromate is obtained from the fusion of chromite ore
Sodium chromate is filtered and acidified with sulphuric acid (
Sodium dichromate is more soluble than potassium dichromate. So, treat the solution of dichromate with the potassium chloride(
The chromate and dichromate are interconvertible in aqueous solution at pH 4
Structures of chromate and dichromate ion
Question 4.15(i) Describe the oxidising action of potassium dichromate and write the ionic equations for its reaction with:
(i) iodide
Answer :
Potassium dichromate
In the first reaction oxidation state of chromium reduced from +6 to +3
Question 4.15(ii) Describe the oxidising action of potassium dichromate and write the ionic equations for its reaction with:
(ii) iron(II) solution
Answer :
Potassium dichromate react with
----------------------------------------------------------------------------------
Question 4.15(iii) Describe the oxidising action of potassium dichromate and write the ionic equations for its reaction with:
Answer :
Potassium dichromate oxidises
The oxidizing action of dichromate ion is -
---------------------------------------------------------------------------
Question 4.16(i) Describe the preparation of potassium permanganate. How does the acidified permanganate solution react with
(i) iron
Write the ionic equations for the reactions.
Answer :
Potassium permanganate can be prepared from the fusion of pyrolusite ore(
(i)Acidified permanganate ion reacts with iron-
-------------------------------------------------------------------------------
Question 4.16(ii) Describe the preparation of potassium permanganate. How does the acidified permanganate solution react with
Write the ionic equations for the reaction.
Answer :
Reaction of acidified permanganate solution with sulphur dioxide (
Here are the reactions-
------------------------------------------------------------------------------------------------
Question 4.16(iii) Describe the preparation of potassium permanganate. How does the acidified permanganate solution react with
(iii) oxalic acid
Write the ionic equations for the reactions.
Answer :
When acidified permanganate solution react with oxalic acid (
Here are the reactions-
--------------------------------------------------------------------------------------------------
Question 4.17(i) For
Use this data to comment upon:
(i) the stability of
Answer :
The
The order of relative stabilities of different ions is-
Question 4.17(ii) For
Use this data to comment upon:
(ii) the ease with which iron can be oxidised as compared to a similar process for either chromium or manganese metal.
Answer :
From the values of
Question 4.18(i) Predict which of the following, will be coloured in aqueous solution?
Answer :
Ions which have incomplete d-orbital, they are able to do
|
|
Purple
|
|
|
green
|
|
|
colourless
|
|
|
pink
|
|
|
Yellow
|
|
|
blue pink
|
|
|
colourless
|
From the table, we notice that
Question 4.18(ii) Predict for the following, will be coloured in aqueous solution? Give reason.
Answer:
Yes,
Question 4.18(iii) Predict for the following, will be coloured in aqueous solution? Give reason.
Answer :
No,
electronic configuration of
Question 4.18(iv) Predict for the following, will be coloured in aqueous solution? Give reason.
Answer :
No, the aqueous solution of
The electronic configuration of
Question 4.18(v) Predict for the following, will be coloured in aqueous solution? Give reason.
Answer :
Yes, the aqueous solution of
The electronic configuration of
Question 4.18(vi) Predict for the following, will be coloured in aqueous solution? Give reason.
Answer :
Yes, the aqueous solution of
electronic configuration of
Question 4.18(vii) Predict for the following, will be coloured in aqueous solution? Give reason.
Answer :
Yes, the aqueous solution of
electronic configuration of
Question 4.19 Compare the stability of +2 oxidation state for the elements of the first transition series.
Answer :
According to our observation, except scandium, all other elements of the first row shows +2 oxidation state. On moving from
|
Sc
|
Ti
|
V
|
Cr
|
Mn
|
Fe
|
Co
|
Ni
|
Cu
|
Zn
|
|
+3
|
+2
+3
+4
|
+2
+3
+4
+5
|
+2
+3
+4
+5
+6
|
+2
+3
+4
+5
+6
+7
|
+2
+3
+4
+6
|
+2
+3
+4
|
+2
+3
+4
|
+1
+2
|
+2
|
Question 4.20(i) Compare the chemistry of actinoids with that of the lanthanoids with special reference to:
(i) electronic configuration
Answer :
The general electronic configuration of actinoids series is
Question 4.20(ii) Compare the chemistry of actinoids with that of the lanthanoids with special reference to:
(ii) atomic and ionic sizes
Answer :
Similar to lanthanoids, actinoids also show actinoid contraction. But the contraction is greater in actinoids because of poor shielding effects of 5f orbitals
Question 4.20(iii) Compare the chemistry of actinoids with that of the lanthanoids with special reference to:
(iii) oxidation state
Answer :
The principal oxidation state of lanthanoids is +3, but sometimes it also shows +2 and +4 oxidation states. This is due to the extra stability of fully-filled and half-filled orbitals.
Actinoids have a greater range of oxidation states due to comparable energies of and it also have a principal oxidation state is +3 but have more compounds in +3 oxidation states than lanthanoids.
Question 4.20(iv) Compare the chemistry of actinoids with that of the lanthanoids with special reference to:
(iv) chemical reactivity.
Answer :
In the lanthanoid series, an earlier member of the series is more reactive, and that is comparable to. With an increase in atomic number, lanthanoids start behaving similarly to aluminium.
Actinoids are highly reactive metals, especially when they are finally divided. When we add them into the water, they give a mixture of oxide and hydride. Actinoids combine with most of the non-metals at moderate temperatures. Alkalies have no action on these actinoid metals
Question 4.21(i) How would you account for the following:
(i) Of the
Answer :
Question 4.21(ii) How would you account for the following:
(ii) Cobalt(II) is stable in aqueous solution but in the presence of complexing reagents, it is easily oxidised.
Answer :
Cobalt (II) is more stable in aq. solution but in the presence of strong field ligand complexing agents, it gets oxidised to Cobalt (III). Though the third ionisation energy of
Question 4.21(iii) How would you account for the following:
(iii) The
Answer :
The
Question 4.22 What is meant by ‘disproportionation’? Give two examples of disproportionation reactions in aqueous solution.
Answer :
In a chemical reaction a substance gets oxidised as well as reduced simultaneously is called a disproportionation reaction. For example-
Question 4.23 Which metal in the first series of transition metals exhibits +1 oxidation state most frequently and why?
Answer :
In the first transition series, Cu (copper) exhibits +1 oxidation states most frequently. This is because
Question 4.24(i) Calculate the number of unpaired electrons in the following gaseous ions:
Answer :
The number of unpaired electron in
After losing 3 electrons, Mn has 4 electrons left.
Question 4.24(ii) Calculate the number of unpaired electrons in the following gaseous ions:
Answer :
Electronic configuration of chromium is
After losing 3 electrons, Cr has 3 electrons left d-orbital
Question 4.24(iii) Calculate the number of unpaired electrons in the following gaseous ions:
Answer :
Electronic configuration of
After losing 3 electrons, V has 2 electrons left d-orbital
Question 4.24(iv) Calculate the number of unpaired electrons in the following gaseous ions:
Answer :
Electronic configuration of
After losing 3 electrons, Ti has 1 electron left d-orbital
Question 4.24 Which one of these is the most stable in aqueous solution?
Answer :
Electronic configuration of
Question 4.25(i) Give examples and suggest reasons for the following feature of the transition metal chemistry:
(i) The lowest oxide of transition metal is basic, and the highest is amphoteric/acidic.
Answer :
The lowest oxidation states of transition metals are basic because some of their valence electrons do not participate in bonding. Thus they have free electrons, which they can donate and act as a base. In the higher oxide of transition metals, valence electrons of their participate in bonding, so they are unavailable. But they can accept electrons and behave as an acid. For example
Question 4.25(ii) Give examples and suggest reasons for the following feature of the transition metal chemistry:
(ii) A transition metal exhibits the highest oxidation state in oxides and fluorides.
Answer :
Oxygen and fluorine are a strong oxidizing agent because of their small in size and high electronegativity. So, they help transition metals to exhibit the highest oxidation states. Examples of oxides and fluorides of transition metals are
Question 4.25(iii) Give examples and suggest reasons for the following feature of the transition metal chemistry:
(iii) The highest oxidation state is exhibited in oxoanions of a metal.
Answer :
Oxygen is a strong oxidizing agent because of its small in size and high electronegativity. Thus oxo-anions of metals show the highest oxidation state.
For example-
Question 4.26(i) Indicate the steps in the preparation of:
(i)
Answer :
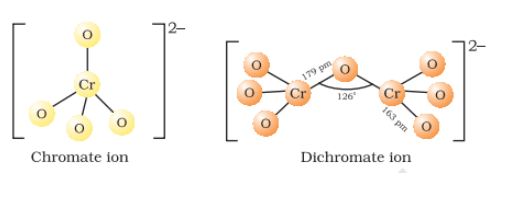
(i) Potassium dichromate is obtained from the fusion of chromite ore
(ii) Sodium chromate is filtered and acidified with sulphuric acid (
(iii) Sodium dichromate is more soluble than potassium dichromate. So, treat the solution of dichromate with the potassium chloride(
The chromate and dichromate are interconvertible in aqueous solution at pH 4
Structures of chromate and dichromate ion
Question 4.26(ii) Indicate the steps in the preparation of:
(ii)
Answer :
Potassium permanganate can be prepared from the fusion of pyrolusite ore(
This gives dark green
Question 4.27 What are alloys? Name an important alloy which contains some of the lanthanoid metals. Mention its uses.
Answer :
It is a solid solution of two or more elements in a metallic matrix. Alloys possess different physical properties than component materials.
An important alloy of lanthanoids is mischmetal.
uses-
Answer :
Inner transition metals are those in which the last electrons are filled in f-orbitals. The elements in which 4f and 5f are filled are called f-block elements. 59, 95 and 102 are the inner transition elements.
Answer :
Lanthanoid primarily shows three oxidation states +2, +3, and +4 and out of these +3 is most common in lanthanoids. they show a limited no. of oxidation states due to the large difference in energies of 4
Answer :
The last element of the actinoid series is Lawrencium (
The possible oxidation state of lawrencium is +3 because after losing 3 electrons, it becomes a stable molecule.
Answer :
Electronic configuration of
Magnetic moment can be calculated as
in Cerium n = 2
So, by putting the value of n we get
Answer :
Members of the lanthanoids which exhibit +4 oxidation states are-
Members who exhibit +2 oxidation states =
After losing 4 electrons
In the case of
Question 4.34 Write the electronic configurations of the elements with the atomic numbers 61, 91, 101, and 109.
Answer :
Atomic number = 61, Promethium
The electronic configuration is
atomic number = 91, protactinium
The electronic configuration is
Atomic number = 101, Mendelevium
The electronic configuration is
Atomic number = 109, Meitnerium
The electronic configuration is
(i) Electronic configurations
Answer :
Electronic configurations-
In 1st, 2nd and 3rd transition metal series 3
In the second transition series, different electron configurations are shown by the following metals,
In the 3rd series there are also some metals which show this type of behaviour such as;
(ii) oxidation states
Answer :
In each of the three transition series, the no. of oxidation state is minimum at the extremes and the highest at the middle of the row. In the first transition series, the +2 and +3 oxidation states are quite stable. Elements of first transition series metals form stable compounds of +2 and +3 oxidation states. But the stability of +2 and +3 oxidation state decreases in the second and third series.
Second and third transition series metals formed complexes in which their oxidation state is high (
(iii) ionisation enthalpies
Answer :
In all of the three transition series, the 1st ionisation energy increases from the left side to the right side. But, there are some exceptions like the first ionisation enthalpies of the third transition series are more significant than those of the first and second transition series. This is happening due to the weak shielding effect of 4 electrons in the third series.
Some elements in the second series have higher first IE than elements of the same column in the first transition series. There are also elements in the 2nd transition series whose first IE are lower than those of the elements corresponding to the same vertical column in the 1st transition series.
(iv) atomic sizes.
Answer : Generally, atomic sizes decrease from left to right across the period. In among the three transition series, the size of the second series element is bigger than that of the first transition element of the same vertical group. But the atomic size of the third transition element is nearly the same as the element of the second transition series element. This is because of Lanthanoid contraction.
Answer : For
In
Similarly
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Question 4.37 Comment on the statement that elements of the first transition series possess many properties different from those of heavier transition elements.
Answer : Elements of the first transition series possess many properties different from those of heavier transition elements in the following ways-
Question 4.38 What can be inferred from the magnetic moment values of the following complex species ?
Example Magnetic Moment (BM)
Answer :
Magnetic moment is given as -
Putting the value on n = 1, 2, 3, 4, 5 (number of unpaired electrons in d-orbital)
We get the value of
By comparing with our calculation we get the values n nearest to 1. It means, in the above compound d-orbital has one unpaired electron(
After comparing with our calculation the nearest value of n = 4. Here iron is in +2 oxidation state (
By observing we get the nearest value of n is 5. So, in this complex Manganese has
Question.
Solution:
X is the difference in oxidation state.
7 – 2 = 5
So X = 5
So
Hence, the correct answer is 10.
Question.
The number of unpaired electrons responsible for the paramagnetic nature of the following complex species are respectively :
1)
2)
3)
4)
Solution:
Hence, the correct answer is option (1).
The elements present in the middle of the periodic table from Group 3 to 12 are called d-block elements. The name d- blocks because the last electron enters into the d-orbital of the penultimate shell. This chapter includes both theory-based understanding and application based questions. The step by step approach to solve questions of chapter The d- and f- block elements is given below:
4.1 Position in the Periodic Table
4.2 Electronic Configurations of the d-Block Elements
4.3 General Properties of the Transition Elements (d-Block)
4.3.1. Physical Properties
4.3.2. Variation in Atomic and Ionic Sizes of Transition Metals
4.3.3. Ionisation Enthalpies
4.3.4. Oxidation States
4.3.5. Trends in the M2+/M Standard ElectrodePotentials
4.3.6. Trends in the M3+/M2+ Standard Electrode Potentials
4.3.7. Trends in Stability of Higher Oxidation States
4.3.8. Chemical Reactivity and Eo Values
4.3.9. Magnetic Properties
4.3.10. Formation of Coloured Ions
4.3.11 Formation of Complex Compounds
4.3.12 Catalytic Properties
4.3.13 Formation of Interstitial Compounds
4.3.14 Alloy Formation
4.4 Some Important Compounds of Transition Elements
4.4.1 Oxides and Oxoanions of Metals
4.5 The Lanthanoids
4.5.1 Electronic Configurations
4.5.2 Atomic and Ionic Sizes
4.5.3 Oxidation States
4.5.4 General Characteristics
4.6 The Actinoids
4.6.1 Electronic Configurations
4.6.2 Ionic Sizes
4.6.3 Oxidation States
4.6.4 General Characteristics and Comparison with Lanthanoids
4.7 Some Applications of d- and f-Block Elements
Here's a comparison table highlighting what to study beyond NCERT for JEE:
The links of the NCERT solutions of class 12 chemistry are given below:
The links of the NCERT solutions of class 12 Subject-wise, are given below:
| NCERT Solutions for Class 12 Biology |
| NCERT Solutions for Class 12 Maths |
| NCERT Solutions for Class 12 Physics |
The links of the NCERT exemplar solutions of class 12, Subject-wise, are given below:
The links to the NCERT books and syllabus are given below
| NCERT Books Class 12 Chemistry |
| NCERT Syllabus Class 12 Chemistry |
| NCERT Books Class 12 |
| NCERT Syllabus Class 12 |
Below are the some important topics of this chapter:
Variable oxidation states of transition elements
Formation of coloured ions by transition metal ions
Lanthanoid contraction
Oxidation states of actinides
Click on the link to get all NCERT Solutions. Solutions for Class 6 to 12 are available for Science And Mathematics.
1 or 2 questions can be expected for JEE Main from the NCERT syllabus Chemistry chapter d and f Block elements Class 12.
D-block elements are those in which the last electron enters the d-orbital of the penultimate (n-1) shell. They are located in the middle of the periodic table, between the s-block and p-block elements, in Groups 3 to 12. They are also known as transition elements or transition metals.
f-block elements are those in which the last electron enters the f-orbital of the antepenultimate (n-2) shell. They are located at the bottom of the periodic table as two separate series: the lanthanides and the actinides. They are also known as inner transition elements.
Changing from the CBSE board to the Odisha CHSE in Class 12 is generally difficult and often not ideal due to differences in syllabi and examination structures. Most boards, including Odisha CHSE , do not recommend switching in the final year of schooling. It is crucial to consult both CBSE and Odisha CHSE authorities for specific policies, but making such a change earlier is advisable to prevent academic complications.
Hello there! Thanks for reaching out to us at Careers360.
Ah, you're looking for CBSE quarterly question papers for mathematics, right? Those can be super helpful for exam prep.
Unfortunately, CBSE doesn't officially release quarterly papers - they mainly put out sample papers and previous years' board exam papers. But don't worry, there are still some good options to help you practice!
Have you checked out the CBSE sample papers on their official website? Those are usually pretty close to the actual exam format. You could also look into previous years' board exam papers - they're great for getting a feel for the types of questions that might come up.
If you're after more practice material, some textbook publishers release their own mock papers which can be useful too.
Let me know if you need any other tips for your math prep. Good luck with your studies!
It's understandable to feel disheartened after facing a compartment exam, especially when you've invested significant effort. However, it's important to remember that setbacks are a part of life, and they can be opportunities for growth.
Possible steps:
Re-evaluate Your Study Strategies:
Consider Professional Help:
Explore Alternative Options:
Focus on NEET 2025 Preparation:
Seek Support:
Remember: This is a temporary setback. With the right approach and perseverance, you can overcome this challenge and achieve your goals.
I hope this information helps you.
Hi,
Qualifications:
Age: As of the last registration date, you must be between the ages of 16 and 40.
Qualification: You must have graduated from an accredited board or at least passed the tenth grade. Higher qualifications are also accepted, such as a diploma, postgraduate degree, graduation, or 11th or 12th grade.
How to Apply:
Get the Medhavi app by visiting the Google Play Store.
Register: In the app, create an account.
Examine Notification: Examine the comprehensive notification on the scholarship examination.
Sign up to Take the Test: Finish the app's registration process.
Examine: The Medhavi app allows you to take the exam from the comfort of your home.
Get Results: In just two days, the results are made public.
Verification of Documents: Provide the required paperwork and bank account information for validation.
Get Scholarship: Following a successful verification process, the scholarship will be given. You need to have at least passed the 10th grade/matriculation scholarship amount will be transferred directly to your bank account.
Scholarship Details:
Type A: For candidates scoring 60% or above in the exam.
Type B: For candidates scoring between 50% and 60%.
Type C: For candidates scoring between 40% and 50%.
Cash Scholarship:
Scholarships can range from Rs. 2,000 to Rs. 18,000 per month, depending on the marks obtained and the type of scholarship exam (SAKSHAM, SWABHIMAN, SAMADHAN, etc.).
Since you already have a 12th grade qualification with 84%, you meet the qualification criteria and are eligible to apply for the Medhavi Scholarship exam. Make sure to prepare well for the exam to maximize your chances of receiving a higher scholarship.
Hope you find this useful!
hello mahima,
If you have uploaded screenshot of your 12th board result taken from CBSE official website,there won,t be a problem with that.If the screenshot that you have uploaded is clear and legible. It should display your name, roll number, marks obtained, and any other relevant details in a readable forma.ALSO, the screenshot clearly show it is from the official CBSE results portal.
hope this helps.

Take Aakash iACST and get instant scholarship on coaching programs.

This ebook serves as a valuable study guide for NEET 2025 exam.

This e-book offers NEET PYQ and serves as an indispensable NEET study material.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
As per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
As per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE