NCERT Exemplar Solutions for Class 10 Science Chapter 1 provide detailed, step-wise answers to advanced questions that help students understand reaction types, balancing equations, and application-based problems.
NCERT Exemplar Class 10 Science Solutions Chapter 4 Carbon And Its Compounds
Do you know why carbon forms millions of compounds, what makes diamond so hard while graphite made up of the same element so soft, and how carbon compounds form the basis of life on earth? The answer to all these questions lies in NCERT Exemplar Class 10 Science Solutions Chapter 4, Carbon is an essential element in our life. It is the core of all living things and non-living materials we use. Carbon plays a significant role in every aspect of our existence from the energy we consume to the air we breathe.
The board follows a centralised evaluation system where answer sheets of the students are coded to maintain anonymity and ensure fair marking across centres.
This Story also Contains
- NCERT Exemplar Class 10 Science Solutions Chapter 4 (MCQ)
- NCERT Exemplar Class 10 Science Solutions Chapter 4 (Short Answer)
- NCERT Exemplar Class 10 Science Solutions Chapter 4 (Long Answer)
- Important Question From NCERT Exemplar Class 10 Science Chapter 4
- Approach to Solve Class 10 Science Chapter 4 Questions
- Advantages of Using Class 10 Science NCERT Exemplar Chapter 4 Carbon And Its Compound Solutions
- NCERT Exemplar Class 10 Science Chapter 4 Topic And Subtopics:
- NCERT Class 10 Science Exemplar Solutions for Other Chapters
- NCERT Solutions for Class 10 Science Chapter-wise
- NCERT Solutions for Class 10 Subject Wise
- NCERT Notes for Class 10 Subject Wise
- NCERT books and syllabus

NCERT Exemplar solutions of class 10 Science provides a detailed explanation of the properties of carbon and the principles and theories that govern their behavior. This chapter includes many key concepts of carbon such as its ability to form strong covalent bonds, long chains, and rings which makes it essential in our daily life and organic chemistry. These NCERT exemplar solutions follow the CBSE 2025 syllabus to properly train students for their board exams and their performances in competitive examinations.
NCERT Exemplar Class 10 Science Solutions Chapter 4 (MCQ)
MCQ questions are covered in the NCERT Exemplar Class 10 Science Chapter 4 Carbon And Its Compounds to enhance your knowledge. The concepts are explained in detail in Carbon And Its Compounds Class 10 Science notes available on our website. Students can also check NCERT Solutions to all questions chapter-wise.
Question 1. Carbon exists in the atmosphere in the form of
(a) carbon monoxide only
(b) carbon monoxide in traces and carbon dioxide
(c) carbon dioxide only
(d) coal
Answer: (b)
Solution:
Carbon is present in the atmosphere mainly as carbon dioxide $\left(\mathrm{CO}_2\right)$, which is released through respiration, combustion, and volcanic activity. Carbon monoxide (CO) is found in trace amounts usually produced from incomplete combustion of fuels. Coal is a solid form of carbon and is not found in the atmosphere.
Question 2. Which of the following statements are usually correct for carbon compounds? These
(i) are good conductors of electricity
(ii) are poor conductors of electricity
(iii) have strong forces of attraction between their molecules
(iv) do not have strong forces of attraction between their molecules
(a) (i) and (iii) (b) (ii) and (iii)
(c) (i) and (iv) (d) (ii) and (iv)
Answer:(d)
Solution:
Carbon predominantly forms covalent compounds, due to valency 4. Generally, carbon is a poor conductor of electricity as its compounds are predominantly covalent in nature. The forces of attraction in between their molecules are weak as they are not covalent in nature.
Statements (ii) and (iv) are correct.
Therefore, option (d) is correct.
Question 3. A molecule of ammonia $(NH_3)$has
(a) only single bonds
(b) only double bonds
(c) only triple bonds
(d) two double bonds and one single bond
Answer:(a)
Solution:
A molecule of ammonia (NH3) has only single bonds and these are covalent bonds.
Therefore, option (a) is correct.
Question 4. Buckminsterfullerene is an allotropic form of
(a) phosphorus
(b) sulphur
(c) carbon
(d) tin
Answer:(c)
Solution:
Buckminsterfullerene is an allotrope of carbon-containing clusters of 60 carbon atoms joined together to form spherical molecules. Its formula is C60, (C-Sixty)
It has 20 hexagons and 12 pentagons. Other allotropic forms of carbon are graphite, diamond, coal, coke…etc.
Therefore, option (c) is correct.
Question 5. Which of the following are correct structural isomers of butane?
(a) (i) and (iii)
(b) (ii) and (iv)
(c) (i) and (ii)
(d) (iii) and (iv)
Answer:(c)
Solution: Structural isomers: two or more organic compounds having the same molecular formulas but different structures.
Butane: $C_4H_{10}$
Structure (i) is n-butane. It is a structural isomer of butane.
Structure (iii) is iso-butane. It is a structural isomer of butane.
Other structures, i.e., (ii) and (iv) have different molecular formulas, hence incorrect
Therefore, option (c) is correct.
Question 6. $CH_{3}-CH_{2}-OH\xrightarrow[Heat]{Alkaline KMnO_{4}}CH_{3}-COOH$
In the above given reaction, alkaline $KMnO_4$ acts as
(a) reducing agent
(b) oxidizing agent
(c) catalyst
(d) dehydrating agent
Answer:(b)
Solution: Here ethanol is converted to ethanoic acid.
$KMnO_4$ acts as an oxidizing agent as it removes hydrogen from $CH_3CH_2OH$ and adds one oxygen to it.
Therefore, option (b) is correct.
Question 7. Oils on treating with hydrogen in the presence of palladium or nickel catalyst form fats. This is an example of
(a) Addition reaction
(b) Substitution reaction
(c) Displacement reaction
(d) Oxidation reaction
Answer: (a)
Solution:
Oils are unsaturated compounds containing double bonds. Unsaturated compounds are the only ones that can undergo addition reactions.
The reaction is known as the hydrogenation reaction. For example:
Hydrogenation reactions are addition reactions.
Therefore, option (a) is correct.
Question 8. In which of the following compounds, $-OH$ is the functional group?
(a) Butanone
(b) Butanol
(c) Butanoic acid
(d) Butanal
Answer: (b)
Solution:
(a) Butanone: $C_4H_8O$
The functional group is $-C=O$
(b) Butanol: $CH_3-CH_2-CH_2-CH_2-OH$
The general formula of alcohols is $C_nH_{(2n+1)}- OH.$
For Butanol, n = 4
Butanol Formula = $C_4H_9OH$
The functional group is $-OH$.
(c) Butanoic acid: $C_4H_8O_2$
The functional group is $-COOH.$
(d) Butanal: $C_4H_8O$
The functional group is $-CHO$.
Therefore, option (b) is correct.
Question 9. The soap molecule has a
(a) hydrophilic head and a hydrophobic tail
(b) hydrophobic head and a hydrophilic tail
(c) hydrophobic head and a hydrophobic tail
(d) hydrophilic head and a hydrophilic tail
Answer: (a)
Solution:
A soap molecule is made up of two parts: A long hydrocarbon part and a short ionic part $-COO-Na^+$ group. The long hydrocarbon chain is hydrophobic (water repelling) and the ionic portion is hydrophilic (water-attracting).
Therefore, option (a) is correct.
Question 10. Which of the following is the correct representation of electron dot structure of nitrogen?
Answer: (d)
The electronic configuration of N (atomic number 7) is 2, 5
Therefore, it needs three more electrons to complete its octet. Each nitrogen atom shares three electrons to form a molecule of $N_{2}$ as:
$N\equiv N$
Hence the correct electron dot structure is:
Therefore, option (d) is correct.
Question 11. Structural formula of ethyne is
Answer: (a)
Ethyne: $C_2H_2$
‘eth’ shows the presence of two carbon atoms and ‘yne' shows the presence of a triple bond,
Thus, the ethyne structure is:
$H-C\equiv C-H$
It is also known as acetylene.
Therefore, option (a) is correct.
Question 12. Identify the unsaturated compounds from the following
(i) Propane
(ii) Propene
(iii) Propyne
(iv) Chloropropane
(a) (i) and (ii) (b) (ii) and (iv)
(c) (iii) and (iv) (d) (ii) and (iii)
Answer:
A hydrocarbon in which the two carbon atoms are connected by a ‘double bond’ or ‘triple bond' is categorized as unsaturated hydrocarbon
(i) Propane: $C_3H_8$
(ii) Propene: $C_3H_6$
(iii) Propyne: $C_3H_4$
$CH_3-C\equiv CH$
(iv) Chloropropane: $C_3H_7Cl$
So, Propene and propyne have double bonds and triple bonds respectively which means they are unsaturated.
Propane and chloropropane are saturated hydrocarbons which contain only single bonds.
Therefore, option (d) is correct.
Question 13. Chlorine reacts with saturated hydrocarbons at room temperature in the
(a) absence of sunlight
(b) presence of sunlight
(c) presence of water
(d) presence of hydrochloric acid
Answer: (b)
Chlorine reacts with saturated hydrocarbon at room temperature in the presence of sunlight,
$CH_{4}+Cl_{2}\overset{sunlight}{\rightarrow}CH_{3}Cl+HCl$
$CH_3Cl+Cl_{2}\overset{sunlight}{\rightarrow}CH_{2}Cl_{2}+HCl$
$CH_2Cl_{2}+Cl_{2}\overset{sunlight}{\rightarrow}CHCl_{3}+HCl$
$CHCl_{3}+Cl_{2}\overset{sunlight}{\rightarrow}CCl_{4}+HCl$
Therefore, option (b) is correct.
Question 14. In the soap micelles
(a) the ionic end of soap is on the surface of the cluster while the carbon chain is in the interior of the cluster.
(b) ionic end of soap is in the interior of the cluster and the carbon chain is out of the cluster.
(c) both ionic end and carbon chain are in the interior of the cluster
(d) both ionic end and carbon chain are on the exterior of the cluster
Answer:
A group of soup molecules aggregated in a spherical arrangement surrounding the dirt or oil in the soap solution in water is called a ‘micelle’. In a soap micelle, the soap molecules are arranged readily with hydrocarbon ends directed towards the center and ionic ends directed outwards.
Therefore, option (a) is correct.
Question 15. Pentane has the molecular formula $C_5 H_{12}$. It has
(a) 5 covalent bonds
(b) 12 covalent bonds
(c) 16 covalent bonds
(d) 17 covalent bonds
Answer:
Structure of pentane:
It contains 16 covalent bonds.
Therefore, option (c) is correct.
Question 16. Structural formula of benzene is
Answer: (c)
Benzene molecules contains alternate single and double bonds in hexagonal carbon chains. Each carbon is attached to a hydrogen atom which makes the formula of benzene $C_6H_6$ in structure.
Therefore, option (c) is correct.
Question 17. Ethanol reacts with sodium and forms two products. These are
(a) sodium ethanoate and hydrogen
(b) sodium ethanoate and oxygen
(c) sodium ethoxide and hydrogen
(d) sodium ethoxide and oxygen
Answer: (c)
Ethanol reacts with sodium to form sodium ethoxide and releases hydrogen gas.
$C_2H_5OH + 2Na \rightarrow 2C_2H_5O^{-}Na^{+} + H_2$
Therefore, option (c) is correct.
Question 18. The correct structural formula of butanoic acid is:
Answer: (d)
Butanoic acid formula = $C_4H_8O_2$
$C_3H_7COOH$
The general formula of carboxylic acid is $R-COOH$ where R is an alkyl group.
General formula of carboxylic acid = $C_{n-1}H_{2n-1}COOH$
Butanoic acid means 4 carbons
Therefore, option (d) is correct.
Question 19. Vinegar is a solution of
(a) 50% – 60% acetic acid in alcohol
(b) 5% – 8% acetic acid in alcohol
(c) 5% – 8% acetic acid in water
(d) 50% – 60% acetic acid in water
Answer: (c)
Vinegar: $CH_3COOH$
Vinegar is 5%-8% solution of acetic acid in water.
It is also known as acetic acid.
Therefore, option (c) is correct.
Question 20. Mineral acids are stronger acids than carboxylic acids because
(i) mineral acids are completely ionised
(ii) carboxylic acids are completely ionised
(iii) mineral acids are partially ionised
(iv) carboxylic acids are partially ionised
(a) (i) and (iv) (b) (ii) and (iii)
(c) (i) and (ii) (d) (iii) and (iv)
Answer: (a)
Solution:
Mineral acids are strong acids that ionizes almost completely and carboxylic acids are weak acids as they don’t ionize easily and only a small percentage of it get ionized.
Therefore, option (a) is correct.
Question 21. Carbon forms four covalent bonds by sharing its four valence electrons with four univalent atoms, e.g. hydrogen. After the formation of four bonds, carbon attains the electronic configuration of
(a) helium
(b) neon
(c) argon
(d) krypton
Answer: (b)
Electronic configuration of carbon (C) = 2, 4
When it forms four covalent bonds by sharing its four valence electrons with hydrogen, it forms CH4
So it has a total of 10 electrons which is the configuration of Neon.
Therefore, option (b) is correct.
Question 22. The correct electron dot structure of a water molecule is
Answer: (c)
The oxygen atom has 6 valence electrons, out of which 2 are involved in bonding with the 2 hydrogen atoms. Therefore water molecule has 2 bonded pairs of electrons and 2 lone pairs.
Hence the answer is
Therefore, option (c) is correct.
Question 23 Which of the following is not a straight chain hydrocarbon?
Answer: (d)
We can see that all the carbon atoms are attached by covalent bonds in a continuous straight chain.
But in structure (d), 5 carbons in a chain with another carbon connected to 2nd carbon from the right.
Therefore, option (d) is correct.
Question 24. Which among the following are unsaturated hydrocarbons?
(a) (i) and (iii)
(b) (ii) and (iii)
(c) (ii) and (iv)
(d) (iii) and (iv)
Answer: (c)
Unsaturated hydrocarbons have double or triple bonds in their structures.
We can see that structure (ii) has a triple bond.
Structure (iv) has a double bond
Therefore, option (c) is correct.
Question 25. Which of the following does not belong to the same homologous series?
(a) $CH_4$
(b) $C_2 H_6$
(c) $C_3 H_8$
(d) $C_4 H_{8}$
Answer: (d)
A homologous series is a group of organic compounds having similar structures and similar chemical properties in which the successive compounds differ by $CH_2$ group.
So, a homologous series of alkanes is
Methane $CH_4$
Ethane $C_2 H_6$
Propane $C_3 H_8$
Butane $C_4 H_{8}$
So, $C_4H_8$ does not belong to the homologous series
Therefore, option (d) is correct.
Question 26. The name of the compound $CH_3 - CH_2 - CHO$ is
(a) Propanal
(b) Propanone
(c) Ethanol
(d) Ethanal
Answer: (a)
The functional group present in $CH_3 - CH_2 - CHO$ is an aldehyde.
The compound has 3 carbons so -Prop is the prefix
Aldehyde has a suffix of -al
Prefix + an + Suffix = Propanal
Therefore, option (a) is correct.
Question 27. The heteroatoms present in
$CH_3 - CH_2 - O - CH_2 - CH_2Cl$ are
(i) oxygen
(ii) carbon
(iii) hydrogen
(iv) chlorine
(a) (i) and (ii)
(b) (ii) and (iii)
(c) (iii) and (iv)
(d) (i) and (iv)
Answer: (d)
Atoms other than C and H, if present in organic compounds, are called heteroatoms.
Oxygen and chlorine are the elements found in the given compound apart from carbon and hydrogen.
Therefore, option (d) is correct.
Question 28. Which of the following represents saponification reaction?
(a) $CH_3COONa + NaOH \overset{CaO}{\rightarrow} CH_4 + Na_2CO_3$
(b) $CH_3COOH + C_2H_5OH \overset{H_2SO_{4}}{\rightarrow}CH_3 COOC_2H_5 + H_2O$
(c) $2CH_3COOH + 2Na \rightarrow 2CH_3COONa + H_2$
(d) $CH_3COOC_2H_5 + NaOH \rightarrow CH_3 COONa + C_2H_5OH$
Answer: (d)
When an ester is heated with sodium hydroxide solution, ester gets hydrolyzed (breaks down) to form the parent alcohol and sodium salt of carboxylic acid. This process is called saponification. Equation (d) has alcohol and sodium salt of carboxylic acid.
Therefore, option (d) is correct.
Question 29. The first member of alkyne homologous series is
(a) ethyne
(b) ethene
(c) propyne
(d) methane
Answer: (a)
A homologous series is a group of organic compounds having similar structures and similar chemical properties in which the successive compounds differ by $CH_2$ group.
The general formula of alkyne is $C_nH_{2n-2}$ where n is the number of carbon atoms. The first member of the alkyne homologous series is ethyne $C_2H_2$ as you need 2 carbons minimum to have a carbon connected with a double bond.
Therefore, option (a) is correct.
NCERT Exemplar Class 10 Science Solutions Chapter 4 (Short Answer)
NCERT Exemplar Class 10 Science Solutions Chapter 4 Carbon And Its Compounds short answer type questions are given for practice. This section contains important questions that are asked in the exams.Practice short answer types from the questions below.
Question 30. Draw the electron dot structure of ethyne and also draw its structural formula.
Answer:
The molecular formula of ethyne is $C_2H_2$.
Electronic configuration of C= 2, 4
Electronic configuration of H = 1
Electron dot structure is:
Structural formula is
$H-C\equiv C-H$
Question 31. Write the names of the following compounds
Answer:
(a) Pentane —e + oic acid = Pentanoic acid
The structure has 5 carbons and a carboxylic acid group.
(b) Butane—ane + yne = Butyne
The structure has 5 carbons and a triple bond.
(c) Heptane —e +al = Heptanal
As the structure has 7 carbons and aldehyde group
(d) Pentane —e + ol = Pentanol
As the structure has 5 carbon atoms and one alcohol group
Question 32. Identify and name the functional groups present in the following compounds.
Answer:
(a) —OH (alcohol)
(b)
(carboxylic acid)
(c)
(ketone)
(d) —C= C—
(alkene)
Carboxylic acid is ethanoic acid
Alcohol is ethanol
X is ethyl ethanoate
Reaction involved:
$CH_{3}COOH(l)+C_{2}H_{5}OH(l)\overset{Conc. H_{2}SO_{4}}{\rightarrow}CH_{3}COOC_{2}H_{5}(l)+H_{2}O(l)$
Question 34. Why detergents are better cleansing agents than soaps? Explain.
Answer:
Detergents are better cleansing agents than soaps because they can be used even with hard water. The charged ends of detergents do not form insoluble precipitates with calcium and magnesium ions in hard water but soap doesn’t work in hard water. Hard water contains Ca2+ and Mg2+ ions which react with soap to form Insoluble salts of calcium and magnesium.
Detergents have a stronger cleansing action than soaps and are more soluble in water than soaps.
Question 35. Name the functional groups present in the following compounds
(a) $CH_3 CO CH_2 CH_2 CH_2 CH_3$
(b) $CH_3 CH_2 CH_2 COOH$
(c) $CH_3 CH_2 CH_2 CH_2 CHO$
(d) $CH_3 CH_2 OH$
Answer:
(a) Ketone
(b) Carboxylic acid, —COOH
(c) Aldehyde, —CHO
(d) Alcohol, —OH
Question 36. How is ethene prepared from ethanol? Give the reaction involved in it.
Answer:
Ethene is prepared by the dehydration of ethyl alcohol in the presence of $conc. H_{2}SO_{4}$ at $160^{\circ}C$.
$C_2H_5OH \overset{conc. H_{2}SO_{4}}{\rightarrow} CH_2 = CH_2 + H_2O$
Question 37. Intake of small quantity of methanol can be lethal. Comment.
Answer:
Methanol is very poisonous because it gets oxidized to methanol (formaldehyde) in the liver. Methanal reacts aggressively with the components of cells and causes the protoplasm to get coagulated (In the same way, as an egg gets coagulated on boiling). Methanol also affects the optic nerve causing blindness.
$CH_{3}OH+[O]\xrightarrow[H_{2}SO_{4}]{Na_{2}Cr_{2}O_{7}}CH_{2}O+H_{2}O$
Answer:
When ethanol reacts with sodium, the gas evolved is hydrogen.
$2C_2H_5OH + 2Na \rightarrow 2C_2H_5O^-Na^+ + H_2$
When ethanol is heated with excess of concentrated sulphuric acid at $170^{\circ}C$, it gets dehydrated to form ethene.
The reaction is as follows:
$CH_3CH_2OH \overset{Conc. H_{2}SO_{4}}{\rightarrow} CH_2 = CH_2 + H_2O$
Concentrated sulphuric acid removes water from ethanol, thereby, acting as a dehydrating agent.
(a) Carbon forms carbon tetrachloride when reacted with chlorine in the presence of sunlight.
Carbon tetrachloride: $CCl_4$
(b) Carbons form carbon dioxide or carbon monoxide when reacted with oxygen based on the availability of oxygen.
Carbon dioxide: $CO_2$
Carbon monoxide: CO
(a) Electronic configuration of CI (atomic number 17)
2 8 7
(b) The electron dot structure of chlorine molecule is
Both carbon (C) and silicon (Si) have similar valence shell electronic configuration
C (atomic number 6) – Electronic configuration 2, 4
Si (atomic number 14) – Electronic configuration 2, 8, 4
Both have four electrons in the valence shell and hence show the phenomenon of catenation.
Carbon exhibits catenation much more than silicon or any other element due to its smaller size which makes the C—C bonds strong while the Si—Si bonds are comparatively weaker due to their large size.
Thus, due to the greater strength of C—C over Si—Si bonds, carbon shows catenation to a greater extent than silicon.
Question 43. Unsaturated hydrocarbons contain multiple bonds between the two C-atoms and show addition reactions. Give the test to distinguish ethane from ethene.
Answer:
When an unsaturated hydrocarbon reacts with bromine the double or triple bonds between the carbons get broken and bromine gets attached to those carbons.
Conformation test:
Ethane when burnt in the air will give a clear flame whereas ethene will give lots of smoke.
$2 C_2H_6 + 7O_2 \rightarrow 4CO_2 + 6H_2O$
$C_{2}H_{4}(g)+3O_{2}(g) \rightarrow 2CO_{2}(g)+2H_{2}O(l)+Smoke$
Question 44. Match the reactions given in Column (A) with the names given in column (B).
|
Column A |
Column B |
|
(a) $CH_3OH + CH_3COOH \overset{H^{+}}{\rightarrow} CH_3COOCH_3 + H_2O$ |
(i) Addition reaction |
|
(b) $CH_2 = CH_2 + H_2 \overset{Ni}{\rightarrow} CH_3 - CH_3$ |
(ii) Substitution reaction |
|
(c) $CH_4 + Cl_2 \overset{sunlight}{\rightarrow} CH_3Cl + HCl$ |
(iii) Neutralisation reaction |
|
(d) $CH_3COOH+NaOH \rightarrow CH_3COONa+H_2O$ |
(iv) Esterification reaction |
Answer:
A – iv
B – i
C – ii
D – iii
Solution:
Addition reaction - a reaction in which one molecule combines with another to form a larger molecule with no other products.
$CH_2 = CH_2 + H_2 \overset{Ni}{\rightarrow} CH_3 - CH_3$
Substitution reaction – in which one functional group in a chemical compound is replaced by another functional group.
$CH_4 + Cl_2 \overset{sunlight}{\rightarrow} CH_3Cl + HCl$
Neutralization reaction – in which an acid and base react to give salt and water
$CH_3COOH+NaOH \rightarrow CH_3COONa+H_2O$
Esterification reaction – in which an organic acid reacts with an alcohol to form an ester and water
$CH_3OH + CH_3COOH \overset{H^{+}}{\rightarrow} CH_3COOCH_3 + H_2O$
Question 45. Write the structural formulae of all the isomers of hexane.
Answer:
(a) Hexane
(b) 2-methyl pentane
(c) 2,2-dimethyl butane
(d) 2,3-dimethyl butane
(e) 3-methyl pentane
(a) Nickel (Ni) acts as the catalyst during the reaction. It first absorbs the hydrogen molecule on its surface as hydrogen atoms and then the alkene molecule side by side. The two hydrogen atoms then add across the double bond of the alkene to form the addition product, i.e., 2, 3- dimethyl butane.
(b) Conc. $H_{2}SO_{4}$ increases the rate of the forward reaction by removing $H_{2}O$ formed during the reaction. In other words, conc. $H_{2}SO_{4}$ acts as a dehydrating agent.
(c) Alkaline $KMnO_{4}$ acts as an oxidizing agent and oxidizes ethanol to ethanoic acid
NCERT Exemplar Class 10 Science Solutions Chapter 4 (Long Answer)
The following are the long-answer type questions that needs more practice. These NCERT Exemplar Class 10 Science Chapter 4 Carbon And Its Compounds important questions are frequently asked in the exams. Long-answer type questions are covered to improve your subject knowledge and conceptual thinking:
When sodium hydrogen carbonate reacts with ethanoic acid, it forms sodium ethanoate salt, water, and carbon dioxide.
$CH_3COOH + NaHCO_3\rightarrow CH_3COONa + H_2O + CO_2$
So, salt X is Sodium ethanoate = $CH_3COONa$
Activity
1. Take sodium hydrogen carbonate in a test tube and add ethanoic acid
2. Carbon dioxide is evolved with brisk effervescence.
3. Pass the gas into lime water and if it turns white (milky), it means carbon dioxide gas is present. This is the confirmation test for carbon dioxide.
$Ca(OH)_2 (aq) + CO_2 (g) \rightarrow CaCO_3 (s) + H_2O (l)$
a) Hydrocarbons are compounds made of carbon and hydrogen.
Example- Methane: $CH_4$
Ethene: $CH_2 = CH_2$
Benzene: $C_6H_6$
b) In saturated hydrocarbons, all the bonds of carbon are single bonds but in unsaturated hydrocarbon compounds, the carbon chain has either a double bond or triple bond or both in some cases.
Example
Saturated hydrocarbons:
Methane
Ethane
Unsaturated hydrocarbons:
Ethene
Ethyne
$H-C\equiv C-H$
Propene
c) A functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions.
Example:
Alcohol -OH
Carboxylic Acid -COOH
Chlorine -Cl
Fluorine -F
Iodine -I
Aldehyde -CHO
Cyanide -CN
Question 49. Name the reaction which is commonly used in the conversion of vegetable oils to fats. Explain the reaction involved in detail.
Answer:
The conversion of vegetable oil to fat is called a hydrogenation reaction.
Vegetable oils (unsaturated hydrocarbons) are treated with hydrogen and passed over nickel and palladium at 200$^{\circ}C$. The unsaturated compounds turn into saturated.
Example:
$H_{2}C=CH_{2}+H_{2}\overset{Ni}{\rightarrow}C_{2}H_{6}$
This process is used in industries to convert oils to vanaspati ghee as it is more stable and has a lot of shelf-life.
Question 50. (a) Write the formula and draw electron dot structure of carbon tetrachloride.
(b) What is saponification? Write the reaction involved in this process.
Answer:
a) Carbon tetrachloride
CCl4
(b) When an ester is heated with sodium hydroxide solution, ester gets hydrolyzed (breaks down) to form the parent alcohol and sodium salt of carboxylic acid.
Example:
$CH_3COOCH_3 + NaOH \rightarrow CH_3COONa + CH_3OH$
Question 51. Esters are sweet-smelling substances and are used in making perfumes. Suggest some activity and the reaction involved for the preparation of an ester with well labeled diagram.
Answer:
Reaction Set up:
Take 1 mL ethanol (absolute alcohol) 1 mL glacial acetic acid and a few drops of concentrated sulphuric acid in a test tube.
Warm the test tube in a water bath for at least five minutes.
Pour the contents of the test tube into a beaker containing 20-50 mL of water and smell the resulting mixture.
The sweet smell confirms the presence of an ester.
Reaction mixture:
Ethanoic acid + Alcohol in the presence of concentrated Sulphuric Acid
Reaction:
$CH_3COOH + CH_3CH_2OH \overset{H_{2}SO_4}{\rightarrow}CH_3COOC_2H_5 + H_2O$
Confirmation test for ester formation is a sweet smell.
Since Compound C has two oxygen atoms it can be an ester or carboxylic acid.
Given that the gas evolved (on the reaction with sodium) burns with a pop sound in an indication that the evolved gas is hydrogen.
Compound R is going to be a salt with sodium being the positive ion and the rest of the compound C which lost hydrogen must be negatively charged. This is only possible with carboxylic acid and not ester.
So C is a carboxylic acid and based on the number of carbons it is Ethanoic acid.
Compound C is $CH_3COOH.$
Compound R is $CH_3COONa.$.
Compound A is $CH_3OH$.
Compound S is $CH_3COOCH_3$
Reactions involved:
$2CH_3 COOH + 2Na \rightarrow 2CH_{3}COONa+H_{2}$
(C) (R)
$CH_3COOH + CH_{3}OH \rightarrow CH_3COOCH_{3}+H_2O?$
(C) (A) (R)
$CH_3COOOH +NaOH\rightarrow CH_{3}COONa?+H_{2}O$
(C) (R)
$CH_3COOCH_{3}+NaOH\rightarrow CH_{3}COONa +CH_{3}OH?$
(R) (A)
(a) Calcium hydroxide or Limewater $(Ca(OH)_2)$ taken in tube B turns milky when carbon dioxide passes through it as it forms Calcium carbonate $(CaCO_3)$
$Ca(OH)_2 (aq) + CO_2 (g) \rightarrow CaCO_3 (s) + H_2O (l)$
(b) Test Tube A
Sodium Carbonate reacts with ethanoic acid to form sodium ethanoate, carbon dioxide, and water
$Na_2CO_3 + CH_3COOH \rightarrow CH_3COONa + CO_2 + H_2O$
Test Tube B
Calcium hydroxide turns milky white due to the formation of Calcium carbonate (CaCO3) when Carbon dioxide is passed through it.
$Ca(OH)_2 (aq) + CO_2 (g) \rightarrow CaCO_3 (s) + H_2O (l)$
(c) No change will take place when we take ethanol instead of ethanoic acid.
$CH_3CH_2OH + Na_2CO_3 \rightarrow \text{ NO REACTION}$
(d) Take a small piece of Calcium oxide in a test tube. Add water to it, part of it gets dissolved but the majority of it gets suspended. So pass the complete solution into a filter paper and collect it in a test tube and this solution is lime water.
$CaO + H_2O \rightarrow Ca(OH)_2$
Question 54. How would you bring about the following conversions? Name the process and write the reaction involved.
(a) ethanol to ethene.
(b) propanol to propanoic acid.
Write the reactions.
Answer:
(a) When Ethanol is reacted with concentrated Sulphuric acid in the presence of heat it will lead to the removal of water molecules and form ethene. This type of reaction is called dehydrogenation reaction.
$CH_3CH_2OH \overset{conc. H_{2}SO_{4}}{\rightarrow} CH_2=CH_2 + H_2O$
(b) When propanol is heated in the presence of potassium permanganate, it will convert to propanoic acid. This type of reaction is called an oxidation reaction.
$CH_3CH_2CH_2OH \overset{Alk. KMnO_{4}}{\rightarrow} CH_3CH_2COOH$
Question 55. Draw the possible isomers of the compound with molecular formula $C_3H_6O$ and also give their electron dot structures.
Answer:
For $C_3H_6O$
1) Propanal
2) Acetone
3) Methoxyethane
$H_{2}C=CH-O-CH_{3}$
4) trans-1-propenol
5) cis-1-propenol
6) 2-propen-2-ol
7) 2-propen-1-ol
8) oxacyclobutane
9) cyclopropanol
10 and 11) Enantiomers of methyl oxirane:
(S)-Methyloxirane (R)-Methyloxirane
Question 56. Explain the given reactions with the examples
(a) Hydrogenation reaction
(b) Oxidation reaction
(c) Substitution reaction
(d) Saponification reaction
(e) Combustion reaction
Answer:
(a) Hydrogenation reaction is the addition of hydrogen to the unsaturated molecule of a hydrocarbon.
Oils are unsaturated compounds containing double bonds. Unsaturated compounds are the only ones which can undergo addition reaction.
The reaction is known as the hydrogenation reaction. For example:
Hydrogenation reactions are additional reactions
(b) Oxidation reaction: A reaction in which an organic compound is reacted with an oxidizing agent where hydrogen is removed or oxygen is added to the compound.
For example:
Alcohol $\rightarrow$ Aldehyde/Ketone $\rightarrow$ Carboxylic acid
$CH_{3}CH_{2}OH \overset{Alk. KMnO_{4}}{\rightarrow}CH_{3}COOH$
(c) Substitution reaction is when an atom or group of atoms is replaced with another atom or group of atoms.
$CH_4 + Cl_2 \overset{hv}{\rightarrow} CH_3Cl + HCl$
(d) When an ester is heated with sodium hydroxide solution, it gets hydrolyzed (breaks down) to form the parent alcohol and sodium salt of carboxylic acid.
Example:
$CH_3COOCH_3 + NaOH \overset{Alk. KMnO_{4}}{\rightarrow} CH_3COONa + CH_3OH$
(e) Combustion Reaction is where an organic compound reacts with oxygen to form carbon dioxide and water vapor.
$CH_4 + 2O_2 \rightarrow CO_2 + 2H_2O + Heat + Light$
As the compound on combustion forms two moles of carbon dioxide and 3 moles of water, it means that 2 carbon atoms and 6 hydrogen atoms are present. So the molecular formula is C2H6 (ethane).
$C_2H_6 + 3.5 O_2 \rightarrow 2CO_2 + 3H_2O$
Compound A is ethanol (CH3CH2OH). On dehydrogenation, it gives ethene.
$C_2H_5OH \overset{Hot conc. H_{2}SO_{4}}{\rightarrow}C_{2}H_{4}+H_{2}O$
Compound B is ethene $(CH_2=CH_2)$
The compound C is obtained by the addition of 1-mole hydrogen in the presence of nickel to B which means C is ethane
$C_{2}H_{4}+H_{2}\overset{Ni}{\rightarrow}C_{2}H_{6}$
Compound C is $CH_3-CH_3$
$C_{2}H_{6}+7O_{2}\rightarrow4CO_{2}+6H_{2}O+Heat+Light$
Important Question From NCERT Exemplar Class 10 Science Chapter 4
Some Class 10 Science NCERT Exemplar Chapter 4 Carbon And Its Compounds questions and answers are given below that will help you tackle complex problems. The questions below will help you evaluate your understanding of the concepts.
Question 1: The correct IUPAC name of the following alkane is
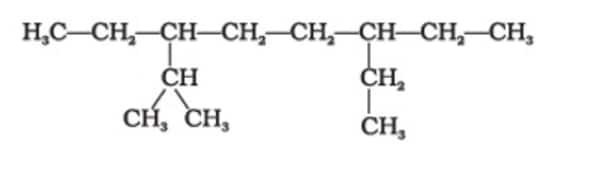
(1) 3,6 – Diethyl – 2 – methyloctane
(2) 5 – Isopropyl – 3 – ethyloctane
(3) 3 – Ethyl – 5 – isopropyloctane
(4) 3 – Isopropyl – 6 – ethyloctane
Answer:
Since the alkane has 8 carbon atoms, it is octane, viz., the longest chain. There is a methyl group on carbon 2, ethyl groups on carbon 3 & 6. Since 2 ethyl groups are present, it will be diethyl, and alphabetically it comes before methyl. The lowest sum rule is followed by side chains present on the carbon atoms 2, 3 & 6.
Hence, the answer is the option (1).
Question 2: What is the name of this functional group formula?
$-\mathrm{OH}$
(1) Ester
(2) Hydroxyl
(3) Amino
(4) All of them
Answer:
Hydroxyl is the correct functional group name of
$-\mathrm{OH}$
Hence, the answer is the option (2).
Question 3: Each carbon atom in a diamond is covalently bonded to four other carbons in a tetrahedron.
(a) True
(b) False
Answer: True
In a diamond crystal structure, each carbon atom forms strong covalent bonds with four neighboring carbon atoms, arranged in a tetrahedral fashion. This results in a tightly packed, three-dimensional network of carbon atoms. Each carbon atom shares its valence electrons with its neighboring atoms, creating a stable and rigid structure, characteristic of the diamond's exceptional hardness and optical properties.
Hence, the given statement is (True).
Question 4: Allotropy or allotropism is the property of some chemical elements to exist in two or more different forms, known as allotropes of these elements.
(a) True
(b) False
Answer: True
Allotropy refers to the property of certain chemical elements to exist in two or more distinct forms, called allotropes. These allotropes possess different physical and chemical properties while consisting of the same element. Carbon, for example, exhibits allotropy with forms such as graphite, diamond, and fullerene. This phenomenon is essential in understanding the diverse behaviors and applications of these elements in various contexts.
Hence, the given statement is (True).
Question 5: Which of following prefix is used for ester?
(1) Alkoxy
(2) Carboxylate
(3) Both (1) and (2)
(4) None of these
Answer:
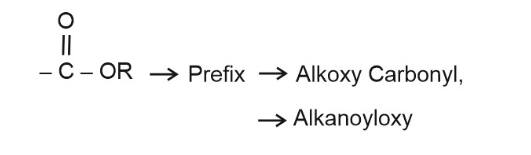
Hence, the answer is the option (4).
Approach to Solve Class 10 Science Chapter 4 Questions
To solve Class 10 Science NCERT Exemplar Chapter 4 Carbon And Its Compounds questions, it is important to follow a systematic approach. It is recommended to strategies your study plan to solve the questions of this chapter. Approach is crucial to solving the questions effectively. The following are some points that can help you build a good strategy:
1). Before solving questions, first make sure that you have a good understanding of the topics like:
- Covalent bonding in carbon
- Nature of carbon and properties like catenation, tetravalency
- Homologous series
- Various functional groups like alcohol, carboxylic acid, aldehydes, and ketones.
- Must have a good command of the nomenclature of organic compounds and IUPAC rules.
2). Identify which concept is applicable to solve that particular question. Students can also follow Carbon And Its Compounds Class 10 Science notes to understand all these concepts better.
- Identify keywords in questions like name the compound, identify the functional group, write the reaction, etc.
- Then identify the topic and answer accordingly
3). Practice more and more questions based on reactions, as they are more likely to be asked in board exams
- Combustion reaction
- Oxidation and reduction reaction questions
- Addition and substitution questions
- Esterification
4). Questions from structures and nomenclature of compounds are asked frequently in exams practice Electron dot structure, Open and branched chain structure and IUPAC nomenclature
5). Practice again and again, as it will help in mastering NCERT Exemplar Class 10 Science Chapter 4 Carbon And Its Compounds. Start with simpler problems then gradually move to difficult ones. Questions from this chapter are asked directly in the CBSE and State board exams.
Advantages of Using Class 10 Science NCERT Exemplar Chapter 4 Carbon And Its Compound Solutions
NCERT Exemplar Class 10 Science Solutions Chapter 4 Carbon And Its Compounds helps students to understand the nature of carbon and its compounds. Given below some points on the advantages of these solutions:
- Students can use these solutions to understand the topics like covalent bonding, homologous series, functional groups, nomenclature, chemical properties of ethanol, ethanoic acid, soaps, and detergents.
- These NCERT Exemplar Solutions for Class 10 are prepared by subject experts in a very clear and comprehensive manner that help students to understand how different organic compounds are formed and react.
- Every question of NCERT is covered in theseClass 10 Science NCERT Exemplar Chapter 4 Carbon And Its Compounds .
- They provide solved examples and chemical equations that are helpful in both CBSE board and competitive exams.
NCERT Exemplar Class 10 Science Chapter 4 Topic And Subtopics:
Class 10 Science NCERT Exemplar Chapter 4 Carbon And Its Compounds explores the following topics:
1. Bonding in Carbon- The covalent bond
2. Versatile nature of carbon
- Saturated and Unsaturated Carbon Compounds
- Chains, Branches, and Rings
- Homologous Series
- Nomenclature of Carbon Compounds
3. Chemical Properties of Carbon Compounds
- Combustion
- Addition Reaction
- Substitution Reaction
4. Some important carbon compounds- Ethanol and Ethanoic acid
- Properties of Ethanol
- Properties of Ethanoic Acid
NCERT Class 10 Science Exemplar Solutions for Other Chapters
NCERT Solutions for Class 10 Science Chapter-wise
The NCERT Solutions for all Class 10 science chapters are given below-
NCERT Solutions for Class 10 Subject Wise
Students can refer to the links given below for the NCERT subject-wise solutions:
NCERT Notes for Class 10 Subject Wise
Students can refer to the links given below for the NCERT subject-wise notes:
NCERT books and syllabus
Students can refer to the links given below for the NCERT Books and Syllabus of class 10:
Frequently Asked Questions (FAQs)
Carbon is fundamental to organic chemistry because it can form stable bonds with other carbon atoms as well as with a variety of other elements, such as hydrogen, oxygen, and nitrogen. This unique ability to bond in various ways allows for the formation of diverse organic compounds, making carbon the backbone of life on Earth.
Hydrocarbons are organic compounds that consist entirely of hydrogen and carbon atoms. They can be classified into two main categories: aliphatic hydrocarbons, which include alkanes, alkenes, and alkynes, and aromatic hydrocarbons, which contain at least one aromatic ring. Hydrocarbons serve as the primary constituents of fossil fuels and are essential for producing energy and various industrial products.
Saturated hydrocarbons contain only single bonds between carbon atoms and have the maximum number of hydrogen atoms attached to their carbon skeleton. In contrast, unsaturated hydrocarbons contain one or more double or triple bonds between carbon atoms, which means they have fewer hydrogen atoms.
Isomerism refers to the phenomenon where compounds have the same molecular formula but different structural arrangements of atoms. In carbon compounds, isomerism can be structural or stereoisomerism. Isomerism is important in organic chemistry because different isomers can have distinct physical and chemical properties despite having the same molecular formula.
Common functional groups discussed in the NCERT Exemplar Chapter 4 Science are given below-
1. Hydroxyl Group (-OH), Found in alcohols.
2. Carbonyl Group (C=O), Found in ketones and aldehydes.
3. Carboxyl Group (-COOH), Found in carboxylic acids.
4. Amino Group (-NH₂), Found in amines.
5. Ester Group (-COO-), Found in esters.
6. Alkyl Group (-C₆H₅), Found in aromatic compounds.
With the help of the radioactive form of carbon which is also known as radiocarbon, the age of any organic material is calculated. This method is called carbon dating
The chapter covers various types of carbon compounds, including hydrocarbons and functional groups containing other atoms such as alcohols, acids, and esters. It highlights their structures, properties, and the significance of functional groups in determining the behavior of these compounds.
A functional group is a specific group of atoms within a molecule that determines the compound's characteristic reactions and properties. For instance, the -OH group denotes alcohols, while the -COOH group represents carboxylic acids.
Carbon compounds are ubiquitous in our daily lives. They are found in fuels, plastics, pharmaceuticals, food, and more. For instance, hydrocarbons are key ingredients in household products, vehicles, and even bioactive components in our food.
Alkanes are saturated hydrocarbons containing only single C-C bonds, alkenes are unsaturated hydrocarbons with at least one C=C double bond, and alkynes have at least one C≡C triple bond. This structural difference leads to varying chemical reactivities, with alkenes and alkynes being more reactive than alkanes due to the presence of double or triple bonds.
Questions related to CBSE Class 10th
On Question asked by student community
The CBSE Board Exam Class 10 English Question Paper can be downloaded in PDF format here .
Students can download the CBSE 10th Maths question paper from below.
Download CBSE Class 10 Maths Question Paper Here
The CBSE 10th maths exam follows the 80 (Theory) + 20 (Internal) format.
-
Question 1 (8 Marks):
-
(A) 4 MCQs (1 mark each).
-
(B) 4 Very Short Answer questions (1 mark
-
You can check the SST 2026 question paper here .
Hi Student
Check out the link below to get the Central Board of Secondary Education question paper 2026 Class 10 for all subjects.
Hi,
Please open the link below to find the CBSE Class 10th Maths 2026 question papers and answer key - https://school.careers360.com/boards/cbse/cbse-class-10-maths-question-paper-2026
https://school.careers360.com/boards/cbse/cbse-10th-maths-answer-key-2026
Popular CBSE Class 10th Questions
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters




























































