Chapter 3 explains the physical and chemical properties of metals and non-metals, their reactions, methods of extraction, corrosion, and the importance of alloys, helping students understand their uses and significance in daily life.
NCERT Solutions For Class 10 Science Chapter 3 "Metals and Non-Metals"
Why do some elements conduct electricity while others do not? What makes gold so shiny and iron so rusty? Who decided that hitting something with a hammer should be the test of whether it's metal? NCERT Solutions for Class 10 Science Chapter 3 Metals and Non-Metals answer all these questions. We use different elements in our everyday life, from small table spoons to large Rockets, and all of them are made up of elements that are very important for making our lives easy and comfortable. All these elements are divided into two categories, i.e., Metals and non-metals.
This Story also Contains
- NCERT Solutions for Class 10 Science Chapter 3: Download PDF
- NCERT Solution For Class 10 Science Chapter 3 (Intext Question)
- NCERT Solution for Class 10 Science Chapter 3 (Exercise Questions)
- Practice Questions of NCERT Class 10 Science Chapter 3
- Approaches To Solve Questions of Chapter 3 Class 10 Science
- Topics and Sub-Topics Covered in NCERT Class 10 Science Chapter 3
- NCERT Class 10 Science Chapter 3: Important Reactions and E-book
- What Students Learn from NCERT Solutions for Class 10 Science Chapter 3
- NCERT Solutions for Class 10th Science - Chapter-wise

Properties of metals and non-metals help us to understand why some metals are hard and others are soft. NCERT Solutions not only help students to score well in their CBSE board and other state board exams but also in competitive exams. All the NCERT Solutions for Class 10 Science are updated chapter-wise following the latest CBSE Class 10 syllabus and the guidelines provided.
NCERT Solutions for Class 10 Science Chapter 3: Download PDF
Students can download the metals and non-metals ncert solutions in pdf format for free. These solutions are designed to help you understand the fundamental concepts and solve textbook questions with ease. They also serve as an excellent resource for quick revision and exam preparation.
NCERT Solution For Class 10 Science Chapter 3 (Intext Question)
To develop an understanding of the concepts, in-text questions are important. In this article, at first we are covering the class 10 science chapter 3 metals and non-metals question answer, later we are covering the exercise solutions of NCERT.
Topic 3.1 - Physical Properties Page no- 40
Question 1(i) Give an example of a metal which is a liquid at room temperature.
Answer:
Generally, metals are solid at room temperature as they have a strong metallic bond that binds them close.
Question 1(ii) Give an example of a metal which can be easily cut with a knife.
Answer:
Metal is generally hard in nature due to its metallic bond between each other. But sodium is a metal which is so soft that it can even be cut with a knife.
Question 1(iii) Give an example of a metal which is the best conductor of heat.
Answer:
Most metals are a good conductor of heat and electricity but silver is considered the best conductor of heat and electricity among them (gold and copper are also very good conductors).
Question 1(iv) Give an example of a metal which that poor conductor of heat.
Answer:
Generally, metals are considered a good conductor of heat and electricity. But mercury and lead show slight exceptions as they are poor conductors of heat.
Question 2 Explain the meanings of malleable and ductile.
Answer:
Malleable substances are substances that can be beaten in order to convert them into sheets. Generally, all metals are considered to be malleable in nature.
For example silver, iron, etc. Gold has the highest malleable property as one single gram of gold can be beaten into a sheet of 1 square meter.
Ductile substances are substances that can be drawn into wires. All metals like gold and copper are ductile in nature. Moreover, the wires for the transfer of electricity are made of copper.
Topic 3.2: Chemical properties of metals Page no- 46
Question 1 Why is sodium kept immersed in kerosene oil?
Answer:
Sodium is a very reactive metal. It reacts rapidly with oxygen and thus cannot be kept in air as it will explode. So it is better to store it in a liquid.
Further, the density of sodium is less than that of water so it will float on the surface. So water is not a good solvent for the purpose. So sodium is kept in kerosene oil.
Question 2(i) Write equations for the reactions of iron with steam
Answer:
Iron reacts with steam and forms iron oxide. The reaction is shown below : $3 \mathrm{Fe}_{(s)}+4 \mathrm{H}_2 \mathrm{O}_{(g)} \longrightarrow \mathrm{Fe}_3 \mathrm{O}_{4(g)} \quad+4 \mathrm{H}_{2(g)}$
Question 2(ii) Write equations for the reactions of calcium and potassium with water
Answer:
Calcium and potassium react with water to give their respective hydroxides. The reactions are shown below :
$
\mathrm{Ca}+2 \mathrm{H}_2 \mathrm{O} \longrightarrow \mathrm{Ca}(\mathrm{OH})_2 \quad+\mathrm{H}_3 \quad+\text { Heat }
$
$
2 \mathrm{~K}+2 \mathrm{H}_2 \mathrm{O} \longrightarrow 2 \mathrm{KOH}+\mathrm{H}_2+\text { Heat }
$
Use the Table above to answer the following question about metals A, B, C and D.
(i) Which is the most reactive metal?
|
Metal |
Iron (II) sulphate |
Copper (II) sulphate |
Zinc sulphate |
Silver nitrate |
|
A |
No reaction |
Displacement | ||
|
B |
Displacement |
No reaction | ||
|
C |
No reaction |
No reaction |
No reaction |
Displacement |
|
D |
No reaction |
No reaction |
No reaction |
No reaction |
Answer:
From the table, it can be seen that no metal is able to displace zinc from its solution, thus zinc is the most reactive among given metals. Further, only metal B is able to displace iron from the iron sulphate solution. And element A can displace copper. Similarly, element C can displace silver from its solution.
So the reactivity order obtained is : D < C < A < B
Hence B is most reactive.
Use the Table above to answer the following questions about metals A, B, C and D.
(ii) What would you observe if B is added to a solution of Copper(II) sulfate?
|
Metal |
Iron (II) sulfate |
Copper (II) sulphate |
Zinc sulphate |
Silver nitrate |
|
A |
No reaction |
Displacement | ||
|
B |
Displacement |
No reaction | ||
|
C |
No reaction |
No reaction |
No reaction |
Displacement |
|
D |
No reaction |
No reaction |
No reaction |
No reaction |
Answer:
As we have seen in part (a) that element A can displace copper from its solution. Also, B is more reactive than element A.
So element B will also displace copper from copper sulphate solution.
Use the table above to answer the following question about metals A, B, C, and D.
(iii) Arrange the metals A, B, C, and D in the order of decreasing reactivity.
|
Metal |
Iron (II) sulfate |
Copper (II) sulphate |
Zinc sulphate |
Silver nitrate |
|
A |
No reaction |
Displacement | ||
|
B |
Displacement |
No reaction | ||
|
C |
No reaction |
No reaction |
No reaction |
Displacement |
|
D |
No reaction |
No reaction |
No reaction |
No reaction |
Answer:
As stated in part (a):-
No metal is able to displace zinc from its solution, thus zinc is the most reactive among given metals.
Further, only metal B is able to displace iron from the iron sulphate solution. And element A can displace copper. Similarly, element C can displace silver from its solution.
So the reactivity order obtained is : D < C < A < B
Answer:
In the case when dilute hydrochloric acid is added to a reactive metal, we obtain hydrogen gas. The reaction when sulphuric acid reacts with a reactive metal is shown below: $\mathrm{Fe}+\mathrm{H}_2 \mathrm{SO}_4 \quad \longrightarrow \mathrm{FeSO}_4 \quad+\mathrm{H}_2$
In this reaction, we obtain an iron sulfate solution with the release of hydrogen gas.
Answer:
By reactivity, we know that zinc is more reactive than iron. Thus zinc will displace iron from the iron sulphate solution and form zinc sulphate. The reaction is shown below:- $\mathrm{Zn}+\mathrm{FeSO}_4 \quad \longrightarrow \mathrm{ZnSO}_4 \quad+\mathrm{Fe}$
Topic- 3.3: How do metals and non-metals react? Page no- 49
Question 1(i) Write the electron-dot structures for sodium, oxygen, and magnesium.
Answer:
The electron dot structure for sodium, oxygen, and magnesium is shown below:-

Question 1(ii) Show the formation of and by the transfer of electrons.
Answer:
The formation of Na2O and MgO is shown below:-
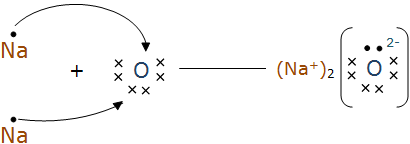
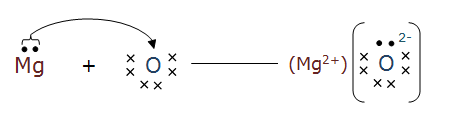
Question 1(iii) What are the ions present in the compounds?
Answer:
In $\mathrm{Na}_2 \mathrm{O}$ the ions present are $\mathrm{Na}^{+}$and $\mathrm{O}^{2-}$, whereas in MgO the ions are $\mathrm{Mg}^{2+}$ and $\mathrm{O}^{2-}$.
Question 2. Why do ionic compounds have high melting points?
Answer:
The ionic compounds have a very strong electrostatic force of attraction. To overcome these forces very high energy is needed. Thus it is generally seen that the melting point of ionic compounds is quite high.
Topic-3.4: Occurrence of metals Page no- 53
Question 1(i) Define the following terms - Mineral.
Answer:
In nature, the elements are present in a combined state known as minerals. Minerals may be defined as the solid chemical compound that occurs naturally in pure form. The chemical composition of minerals is the same throughout.
Question 1(ii) Define the following term - Ores
Answer:
Ores are defined as the minerals which can be extracted to make profits. Only economically extractable elements (generally metals) are considered to be ores.
Question 1(iii) Define the following term: Gangue.
Answer:
Gangue is the impurities present in the ores such as sand, silt soil. To make the element efficient and more useful, we need to remove the gangue from the ores.
Question 2. Name two metals which are found in nature in the free state.
Answer:
The metals which are very low reactive elements may be found in free states. Two such metals are gold and silver . These metals are so unreactive that they don't form any oxidme that whichs or sulphides.
Question 3. What chemical process is used for obtaining a metal from its oxide?
Answer:
We can obtain metal from its oxide by using the reduction process. The metal oxides are reduced using reducing agents such as hydrogen, which reacts with the reactive metals so that they can displace the metal from its oxide.
For e.g., From zinc oxide, we can get zinc by using a reducing agent, carbon.
$\mathrm{ZnO}+\mathrm{C} \rightarrow \mathrm{Zn}+\mathrm{CO}$
Topic 3.5 Corrosion Page no- 55
Question 1. Metallic oxides of zinc, magnesium, and copper were heated with the following metals
In which cases will you find displacement reactions taking place?
|
Metal |
Zinc |
Magnesium |
Copper |
|
Zinc oxide | |||
|
Magnesium oxide | |||
|
Copper oxide |
Answer:
The reactivity order of the given elements are: Mg > Zn > Cu
Thus the following result is observed :
|
Metal |
Zinc |
Magnesium |
Copper |
|
Zinc oxide |
No reaction |
Displacement |
No reaction |
|
Magnesium oxide |
No reaction |
No reaction |
No reaction |
|
Copper oxide |
Displacement |
Displacement |
No reaction |
Question 2. Which metals do not corrode easily?
Answer:
The low reactive metals such as gold, don't corrode easily, whereas highly reactive elements are very easily corroded. This is why these metals (highly reactive) are given gold plating in order to protect them from corrosion.
Question 3. What are alloys?
Answer:
Alloys are defined as the homogeneous mixture of two or more elements. Alloys are prepared to give specific features of all the constituent elements. It is prepared by melting one metal and then diffusing the other into.
For example: steel is an alloy, another one, and carbon.
NCERT Solution for Class 10 Science Chapter 3 (Exercise Questions)
All questions from the chapter-end exercise have been covered in the metals and non-metals class 10 question answer, which are given below. NCERT Solutions for Class 10 provide clear and step by step answers to all the exercise questions from the chapter.
Question 1. Which of the following pairs will give displacement reactions?
a) NaCl solution and copper metal
b) $\mathrm{MgCl}_2$ solution and aluminum metal
c) $\mathrm{FeSO}_4$ solution and silver metal
d) $\mathrm{Ag} \mathrm{NO}_3$ solution and copper metal
Answer:
(d) $\mathrm{Ag} \mathrm{NO}_3$ solution and copper metal.
In all other options, the solution metal is more reactive than the given metal.
For e.g. in (b) $\mathrm{MgCl}_2$ solution and aluminum metal, Mg metal is more reactive than aluminum thus reactive will not take place.
Question 2. Which of the following methods is suitable for preventing an iron frying pan from rusting?
(a) Applying grease
(b) Applying paint
(c) Applying a coating of zinc
(d) All of the above.
Answer:
(c) Applying a coating of zinc
It will be most suitable method to prevent it. Paint and grease can also be applied but these are not suitable methods as the pan will be heated and washed again and again.
(a) calcium
(b) carbon
(c) silicon
(d) iron.
Answer:
The element is Calcium. As calcium oxide is soluble in water and has a high melting point.
Question 4. Food cans are coated with tin and not with zinc because
(a) Zinc is costlier than tin.
(b) Zinc has a higher melting point than tin
(c) Zinc is more reactive than tin.
(d) Zinc is less reactive than tin.
Answer:
This is because zinc is more reactive than tin. So, zinc may react with food items and make food unhealthy to consume.
Question 5(a) You are given a hammer, a battery, a bulb, wires, and a switch.
How could you use them to distinguish between samples of metals and non-metals?
Answer:
To distinguish between metals and non-metals, we can use two properties of metals:-
(i) Most of the metals are malleable, i.e., they can be converted into sheets. So with the help of a hammer, we can test and distinguish.
(ii) Metals are a good conductor of electricity whereas non-metals are poor conductors.
Question 5(b) You are given a hammer, a battery, a bulb, wires, and a switch.
Assess the usefulness of these tests in distinguishing between metals and non-metals.
Answer:
These are useful as there is no chemical reaction involved in identifying the metals and non-metals. These are just the physical tests which can be carried out at any level. As in the first test, the hammer is easily available at home. And in the second test, we used wires which are easily available in the market.
Question 6 What are amphoteric oxides? Give two examples of amphoteric oxides.
Answer:
Amphoteric oxides are those oxides of metals that behave in both acidic and basic manner. These oxides react with acids and bases to produce salt and water as the major products. E.g. Aluminium oxide (Al2O3), Zinc oxide (ZnO).
Question 7 Name two metals which will displace hydrogen from dilute acids, and two metals which will not.
Answer: Clearly, the metals which are more reactive than hydrogen will displace it from the dilute acids.
For e.g. potassium and sodium. But metals like silver and gold are less reactive than hydrogen so they will not be able to displace hydrogen.
Answer:
The configuration for electrolytic refining will be:-
Electrolyte:- Salt solution of metal M
Anode:- Impure metal M
Cathode:- Pure metal M (Thin strips)
What will be the action of gas on dry litmus paper?
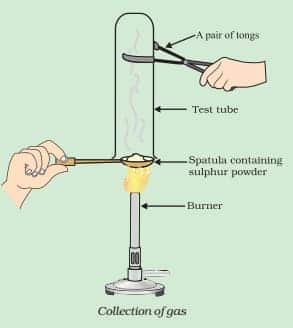
Answer:
In the given procedure, sulphur dioxide is formed.
(i) Thus gas (sulphur dioxide) has no effect on dry litmus paper.
(ii) SO2 converts moist blue litmus paper to red due to the formation of sulphurous acid. (as sulphur dioxide reacts with moisture to produce acid).
Write a balanced chemical equation for the reaction taking place.
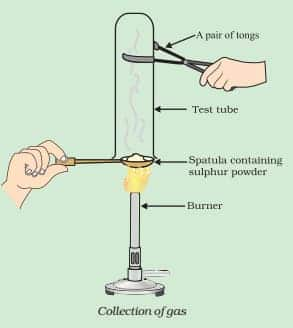
Answer:
In the reaction, sulphur dioxide is formed. The chemical reaction is shown below:-
$\mathrm{S}+\mathrm{O}_2 \rightarrow\mathrm{SO}_2(\mathrm{~g})$
Question 10 State two ways to prevent the rusting of iron.
Answer:
The two ways to prevent rusting of iron are:-
(a) By making alloys of the elements.
(b) By using the electroplating method such as galvanization.
Question 11 What type of oxides are formed when non-metals combine with oxygen?
Answer:
When non-metals combine with the oxygen they form acidic oxides.
For e.g., SO2 is an acidic oxide formed when sulphur combines with oxygen.
Question 12(a) Give reasons:
Platinum, gold, and silver are used to make jewellery.
Answer:
There are three main reasons for why platinum, gold, and silver are used to make jewellery:-
(i) They are lustrous in nature.
(ii) They are very low reactive elements, thus they don't corrode easily.
(iii) They are found in low quantity so that makes them costly and not available for all. Hence these are used as a sign to show status in society.
Question 12(b) Give reasons:
Sodium, potassium, and lithium are stored under oil.
Answer:
The elements like sodium, potassium, and lithium are very reactive metals. They react with oxygen vigorously and thus are dangerous to keep them in contact with air and moisture. Hence they are generally kept under oil.
Question 12(c) Give reasons:
Aluminum is a highly reactive metal, yet it is used to make utensils for cooking.
Answer:
This is true that aluminum is a highly reactive element, but it has a property of non-corrosion. That's why it is used to make cooking utensils. The property comes from the fact that aluminum reacts with oxygen and forms a thin layer of aluminum oxide which acts as a protective coating layer to resist further corrosion.
Question 12(d) Give reasons:
Carbonate and sulphide ores are usually converted into oxides during the process of extraction.
Answer:
This is because the extraction of metal is easier from their oxides rather than their carbonates or sulphides. As in the case of oxides, we have to simply use a metal more reactive than the impure metal which needs to be extracted.
Answer:
The lemon and tamarind contain citric acid, which neutralizes the basic carbonic acid and thus dissolves the greenish layer. This is why lemon juice and tamarind is used to clean tarnished copper vessels.
Question 14 Differentiate between metal and non-metal on the basis of their chemical properties.
Answer:
The major differences between metals and non-metals are given below:-
|
Metals |
Non-metals |
|
1. They have ionic bonds. |
They have covalent bonds |
|
2. They are electropositive. |
They are electronegative. |
|
3. They form basic oxides. |
They form acidic oxides. |
|
4. They react with dilute acids to form salt and evolve hydrogen gas. |
Since they cannot replace hydrogen, thus they cannot react with dilute acids. |
Answer:
The solution man used must be aqua regia. It is a solution of a 3:1 mixture of conc. HCl and conc. HNO3 . It has the property that gold dissolves in this solution.
After dipping the gold in the solution, the outer layer of gold dissolves and the new surface is exposed, which appears to be shiny. This is why the weight of ornament is decreased.
Question 16 Give reasons why copper is used to make hot water tanks and not steel (an alloy of iron).
Answer:
This is because steel contains iron which reacts with steam and forms iron oxide. Whereas copper doesn't react with cold water, hot water, and steam.
That's why hot water tanks are made up of copper, not steel.
$3 \mathrm{Fe}+4 \mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{Fe}_3 \mathrm{O}_4+4 \mathrm{H}_2 \mathrm{O}$
Practice Questions of NCERT Class 10 Science Chapter 3
Some important practice questions related to the class 10 science chapter 3 metals and non-metals solutions are given below. These questions have a high probability of being asked in future board exams.
Question 1. Which of the following is the correct arrange-ment of the given metals in descending order of their reactivity?
Zinc, Iron, Magnesium, Sodium
(1) Zinc > Iron > Magnesium > Sodium
(2) Sodium > Zinc > Magnesium > Iron
(3) Sodium > Magnesium > Iron > Zinc
(4) Sodium > Magnesium > Zinc > Iron
Answer:
The decreasing order of chemical reactivity is given below.
(most active) Sodium > Magnesium > Zinc > Iron (least active)
Hence, the answer is the option (4).
Question 2. Non-metals are not lustrous. Which of the following non-metal is lustrous?
(1) Nitrogen
(2) Sulphur
(3) Iodine
(4) Oxygen
Answer:
Shining metals are also called lustrous metal. For example, gold. Non-metals such as sulphur, oxygen, nitrogen are non-lustrous but iodine is a greyish black solid and crystals have a metallic lustre.
Hence , the answer is the option (3).
Question 3. Magnetism is most beneficial for separating
(1) Non- magnetic solids from non- magnetic liquids
(2) Gases and non- metallic liquids
(3) Magnetic solids and solids such as sulfur
(4) Non- metallic solids and solids such as sulfur
Answer:
Magnetic solids and solids such as sulfur can be separated using magnetic separation.
Hence, the answer is the option (3).
Question 4. Which of the following pairs will give displacement reactions?
(1) NaCl solution and copper metal
(2) MgCI2 solution and aluminium metal
(3) FeSO4 solution and silver metal
(4) AgNO3 solution and copper metal
Answer:
(a)Cu cannot displace $\mathrm{Na} \rightarrow$ No reaction
(b) Al cannot displace Mg $\rightarrow$ No reaction
(c) Ag cannot displace Fe $\rightarrow$ No reaction
(d) Cu can displace Ag $\rightarrow$ Reaction takes place
Hence, the answer is the option (4).
Question 5. Which of the following statement is NOT correct about non-metals
(1) Non-metals are poor conductor of electricity
(2) Non-metals are neither malleable nor ductile.
(3) Non-metals are poor conductor of heat
(4) Oxides of non-metals are basic in nature
Answer:
Oxides of non-metals are acidic in nature
$\mathrm{S}+\mathrm{O}_2 \rightarrow \mathrm{SO}_2$ (acidic)
Hence, the answer is the option (4).
Question 6: Which of the following metals is stored in kerosene because it reacts vigorously with oxygen and moisture in air?
(1) Copper
(2) Aluminium
(3) Sodium
(4) Iron
Answer:
Sodium is highly reactive and catches fire when exposed to air or moisture. To prevent this reaction, it is stored under kerosene.
Hence, the correct answer is option (3).
Question 7: A non-metal that is a good conductor of electricity is:
(1) Sulphur
(2) Phosphorus
(3) Carbon
(4) Chlorine
Answer:
Graphite, an allotrope of carbon, has free electrons that allow electricity to pass through it. Most non-metals are poor conductors, but graphite is a major exception.
Hence, the correct answer is option (3)
Question 8: Which of the following methods is suitable for preventing an iron frying pan from rusting ?
(1) applying grease
(2) applying paint
(3) applying a coat of zinc
(4) all of the above
Answer:
Since the frying pan is used to cook food, applying of paint and grease shall contaminate the food when heated. However, applying a coat of zinc (also called galvanization) is the most suitable method for preventing an iron frying pan from rusting.
Hence, the answer is the option (3).
Question 9: If copper is kept exposed to damp air for a considerable time, it gets a green coating on its surface. This is due to the formation of :
(1) hydrated copper sulphate
(2) copper oxide
(3) basic copper carbonate
(4) copper nitrate
Answer:
The Greening of copper is attributed to the formation of basic copper carbonate. The reaction is
$2 \mathrm{Cu}+\mathrm{CO}_2+\mathrm{O}_2+\mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{CuCO}_3 \cdot \mathrm{Cu}(\mathrm{OH})_2$
Hence, the answer is the option (3).
Approaches To Solve Questions of Chapter 3 Class 10 Science
Students can follow the strategy given below to solve metals and non-metals class 10 question answer. So that they can solve exercise questions effectively, which can help them score good marks in their exams.
1) Before solving questions properly read the chapter and focus more on reactivity series, properties, and chemical reactions involving metals and non-metals.
2) While solving questions related to metals and non-metals, it's important to learn basic reactions like
- Reactions of metals with acids, bases, and water.
- Reactions involving corrosion and prevention of corrosion.
- Displacement reaction.
3) To predict the reactions, students must know about the reactivity series. Students can follow the metals and non-metals ncert solutions to understand these concepts better through a series of solved questions.
4) Proper practice of writing a balanced equation is must to solve questions related to balancing chemical equations.
5) Practice in text and end of chapter exercise questions from class 10 science metals and non-metals question answer and students can also refer to NCERT exemplar questions for practice.
Topics and Sub-Topics Covered in NCERT Class 10 Science Chapter 3
Topics and subtopics covered in class 10 science chapter 3 metals and non-metals solutions are given below. A basic understanding of these topics helps students to solve complex problems easily.
3.1 Physical Properties
- 3.1.1 Metals
- 3.1.2 Non-Metals
3.2 Chemical Properties Of Metals
- 3.2.1 What happens when metals are burnt in air?
- 3.2 .2 What happens when metals react with water?
- 3.2.3 What happens when metals react with acids?
- 3.2.4 How do metals react with solutions of other Metal Salts
- 3.2.5 The Reactivity Series
3.3 How do Metals and Non-Metals react?
- 3.3.1 Properties of Ionic Compounds
3.4 Occurrence of metals
- 3.4.1 Extraction of metals
- 3.4.2 Enrichment of Ores
- 3.4.3 Extracting Metals Low in the Activity Series
- 3.4.4 Extracting Metals in the Middle of the activities Series
- 3.4.5 Extracting Metals towards the top of the Activity Series
- 3.4.6 Refining of Metals
3.5 Corrosion
- 3.5.1 Prevention of Corrosion
NCERT Class 10 Science Chapter 3: Important Reactions and E-book
Important reactions and tables from the class 10 science chapter 3 metals and non-metals question answer are given below. You can use these reactions and tables as a quick revision source.
Important Reactions
Metal + Oxygen$\longrightarrow$ Metal oxide
For example: 2 Cu (Copper) $+\mathrm{O}_2 \rightarrow 2 \mathrm{CuO}$ (Copper(II) oxide)
Aluminium oxide reacts in the following manner with acids and bases:
$\mathrm{Al}_2 \mathrm{O}_3+6 \mathrm{HCl} \rightarrow 2 \mathrm{AlCl}_3+3 \mathrm{H}_2 \mathrm{O}$
$\mathrm{Al}_2 \mathrm{O}_3+2 \mathrm{NaOH} \rightarrow 2 \mathrm{NaAlO}_2+\mathrm{H}_2 \mathrm{O}$
Sodium oxide and potassium oxide dissolve in water to produce alkalis as follows:
$\mathrm{Na}_2 \mathrm{O}(\mathrm{s})+\mathrm{H}_2 \mathrm{O}(\mathrm{l}) \rightarrow 2 \mathrm{NaOH}(\mathrm{aq})$
$\mathrm{K}_2 \mathrm{O}(\mathrm{s})+\mathrm{H}_2 \mathrm{O}(\mathrm{l}) \rightarrow 2 \mathrm{KOH}(\mathrm{aq})$
Metal + Water $\longrightarrow$ Metal oxide + Hydrogen
Example: $2 \mathrm{Al}(\mathrm{s})+3 \mathrm{H}_2 \mathrm{O}(\mathrm{g}) \rightarrow \mathrm{Al}_2 \mathrm{O}_3(\mathrm{~s})+3 \mathrm{H}_2(\mathrm{~g})$
Metal oxide + Water $\longrightarrow$ Metal hydroxide
Example: $2 \mathrm{~K}(\mathrm{~s})+2 \mathrm{H}_2 \mathrm{O}(\mathrm{l}) \rightarrow 2 \mathrm{KOH}(\mathrm{aq})+\mathrm{H}_2(\mathrm{~g})+$ heat energy
Metal + Dilute acid$\longrightarrow$ Salt + Hydrogen
Metal A + Salt solution of B $\longrightarrow$ Salt solution of A + Metal B
Reactivity series

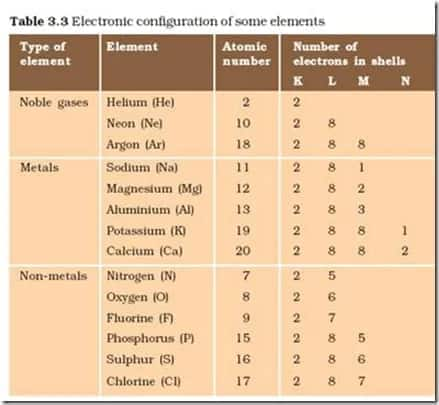
To download the E-Book click on the link given below:
What Students Learn from NCERT Solutions for Class 10 Science Chapter 3
These class 10 science chapter 3 metals and non-metals solutions helps students to learn about metals and non-metals, their properties, and their uses in daily life. These solutions also help students practice questions and prepare well for exams.
-
The physical and chemical properties of metals and non-metals.
-
They will learn about the reactivity series and how metals react with air, water, acids, and bases using class 10 science metals and non-metals question answer.
-
Processes like extraction of metals with the help of various techniques like roasting, calcination, and refining are explained in these solutions through a series of solved questions.
-
Here students will learn about the process of corrosion, its prevention methods, and the importance of alloy formation.
NCERT Solutions for Class 10th Science - Chapter-wise
Besides NCERT Solutions For Class 10 Science Chapter 3 Metals and Non-Metals, chapter-wise solutions for class 10 are given below:
Frequently Asked Questions (FAQs)
They are detailed, step by step answers to all textbook questions from Chapter 3, helping students understand concepts of metals and non-metals and prepare effectively for exams.
NCERT Solutions for Class 10 Science are comprehensive answers to all questions given in the NCERT textbook, designed to help students understand concepts clearly and improve their exam performance.
Corrosion is the gradual destruction of metals due to chemical reactions, usually with oxygen and moisture. For example, when iron is exposed to water and air, it forms rust. This process weakens the metal structure, which is why anti-corrosive measures are important in construction and manufacturing.
Metals react with acids to produce hydrogen gas and a salt. For example, when zinc reacts with hydrochloric acid, zinc chloride and hydrogen gas are produced. The reactivity varies among different metals, with some metals, like sodium, reacting vigorously, while others, like gold, do not react at all.
Metals have free electrons that allow them to easily conduct electricity. When voltage is applied, these free electrons move through the metal, enabling the flow of electric current. For instance, copper is extensively used in electrical wiring because of its excellent conductivity.
Corrosion is the gradual destruction of metals due to chemical reactions with environmental elements, such as water and oxygen. An example is rusting, where iron reacts with moisture and oxygen to form iron oxide.
Common non-metals include oxygen, nitrogen, sulfur, and carbon. Oxygen is essential for respiration and combustion, nitrogen is used in fertilizers, sulfur is used in the production of sulfuric acid.
The reactivity series is a list that ranks metals according to their reactivity from highest to lowest. It is important because it helps predict how metals will react with other substances, such as acids and water.
Non-metals generally gain or share electrons in chemical reactions, whereas metals tend to lose electrons. Non-metals can react with metals to form ionic compounds or with each other to form covalent compounds.
Popular Questions
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
