NCERT Class 10 Science Chapter 1 Notes Chemical Reactions and Equations- Download PDF Notes
Have you ever seen a plant grow from a tiny seed to a full-grown tree? Do you know what is happening during these transformations? It's all because of a chemical reaction. When substances change to form new products, a chemical reaction occurs. NCERT Class 10 Science Chapter 1 Notes Chemical Reactions and Equations give students a basic idea of this chapter. These NCERT notes cover important topics like definitions, examples, chemical equations, balancing them, types of chemical reactions, and the impact of oxidation reactions in everyday life. Students should go through each topic in these notes for a quick revision.
This Story also Contains
- NCERT Notes Class 10 Chapter 1: Download PDF
- NCERT Notes Class 10 Chapter 1
- Chemical Reactions and Equations: Previous Year Question and Answer
- How to Master Class 10 Science Chapter 1 Chemical Reactions and Equations
- Advantages of Using Class 10 Science Chapter 1 Chemical Reactions and Equations Notes
- CBSE Class 10 Science Chapter-wise Notes
- NCERT Solutions for Class 10 Science Chapter-Wise
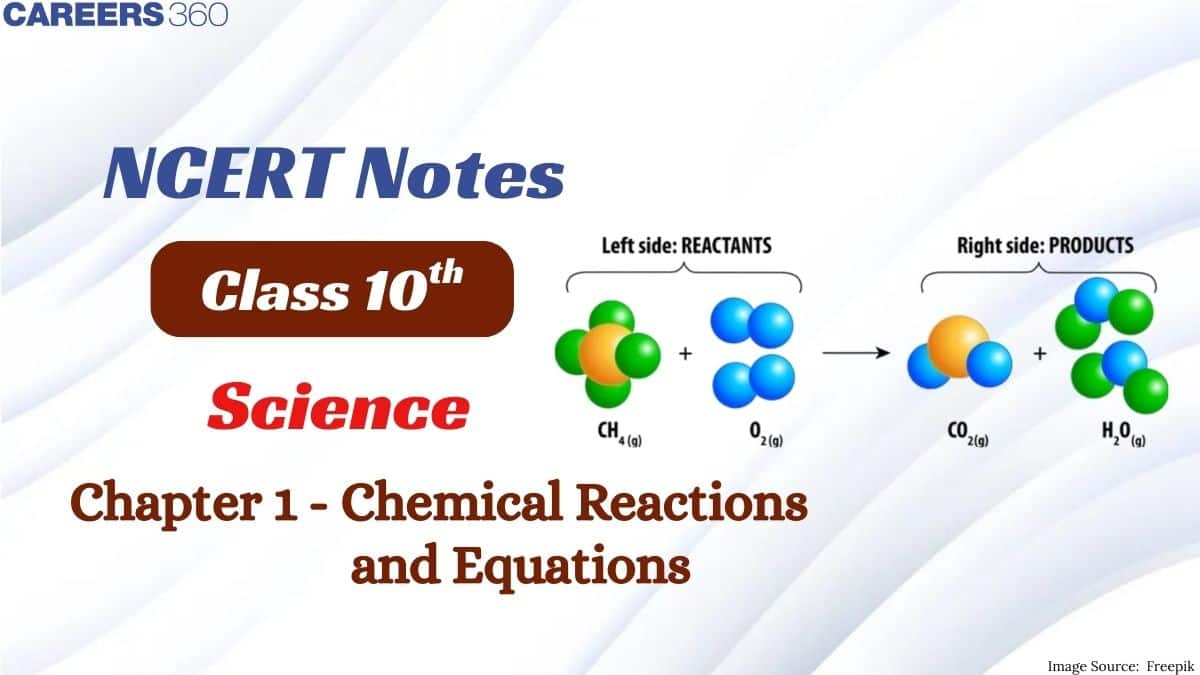
This article covers all the important concepts from the chapter, which are also significant for various competitive exams. NCERT Notes for Class 10 Science are prepared by subject experts in a systematic way that covers important reactions that occur in our surroundings. These notes include important examples that are frequently asked in exams. Students can also refer to the NCERT Solutions for understanding concepts better with the help of solved questions.
NCERT Notes Class 10 Chapter 1: Download PDF
You can download chemical reactions and equations class 10 science chapter 1 CBSE notes PDF to access a clear explanation of the reactions and important topics, from the Download PDF icon given below.
Also read
NCERT Notes Class 10 Chapter 1
Chemical Reactions and Equations Class 10 Science notes introduce the basics of chemical changes, how to represent them using balanced chemical equations, and the different types of chemical reactions, like combination, decomposition, displacement, and redox reactions. These NCERT notes for Class 10 also highlight real-life examples and applications of chemical reactions, making the concepts easier to understand and relate to daily life.
Chemical Reactions and Equations
This chapter discusses in detail the balanced and unbalanced chemical reactions, along with explaining the process of balancing these reactions. This chapter discusses the significance of chemical equations and their representation using various symbols. Understanding chemical reactions is important for grasping the fundamental concepts for science students.
Chemical Equations
The change of one chemical substance into another is known as a chemical reaction. Rusting of iron, curdling of milk, and respiration are examples of chemical reactions. The formed substance in a chemical reaction is completely different from its original substance; this process is an indication of a complete chemical reaction. Also, a chemical reaction is a process in which the rearrangement of atoms takes place, which leads to the formation of new compounds. To strengthen your understanding and practice more problems, you can also refer to the NCERT Solutions for Class 10 Science Chapter 1.
$\underset{\text { (Reactants) }}{\text { Magnesium }+ \text { Oxygen }} \rightarrow \underset{\text { (Product) }}{\text { Magnesium oxide }}$
Reactants-
The one that is taking part in a chemical reaction is a reactant.
Products-
The new substance that is formed after a chemical reaction is called a product.
Example-
Magnesium burns in the air to form magnesium oxide is a type of chemical reaction.
$\mathrm{Mg}+\mathrm{O}_2$ $\xrightarrow{\text { Heat }}$$ 2 \mathrm{MgO}(\mathrm{s})$
In this reaction, the reactants are magnesium and oxygen, while the new substance MgO is the product.
Writing a Chemical Equation
For representing a chemical reaction, some simple formulas can be used, and this representation is called a chemical equation. For example, when A and B are the reactants and C and D are the products formed after a reaction, the following representation can be used to represent the reaction.
$A+B \rightarrow C+D$
- When hydrogen undergoes a reaction with oxygen, water is formed. In which the hydrogen and oxygen are the reactants and the water obtained is the product, can be represented as
$\mathrm{H}_2+\mathrm{O}_2 \rightarrow \mathrm{H}_2 \mathrm{O}$
- So, with the help of a chemical equation, we can represent it in a concise and informative way. It can also be divided into two types of balance: the balanced chemical equation and the unbalanced chemical equation.
Characteristics of Chemical Reaction
Evolution of gas: In some reactions, there is an evolution of gas, for example, the reaction between zinc and dilute sulphuric acid is followed by the evolution of hydrogen gas. It can be represented as
$\mathrm{Zn}+\mathrm{H}_2 \mathrm{SO}_4 \rightarrow \mathrm{ZnSO}_4+\mathrm{H}_2$
Change in colour: In some other reactions, there may be a change in colour. For example, when citric acid and purple color potassium permanganate solution undergo a chemical reaction, there is a change in colour from purple to colourless. And also, the chemical reaction between sulfur dioxide and acidified potassium dichromate solution is also followed by a change in colour from orange to green.
State change: For some chemical reactions, there is a change in the state of the substance. For example, when candle wax undergoes a combustion reaction, there is a change of state from solid to liquid and gas. And the gas formed in this type of reaction is carbon dioxide.
Temperature change: For some reactions, there is a temperature change that is a rise in temperature or a fall in temperature. For example, the reaction between quicklime and water to form slaked lime is followed by a rise in temperature. And also, the reaction taking place between zinc granules and dilute sulphuric acid is also followed by a rise in temperature.
Formation of the precipitate: In the reaction between sulphuric acid and barium chloride solution, a white precipitate of barium sulfate.
$\mathrm{BaCl}_2+\mathrm{H}_2 \mathrm{SO}_4 \rightarrow \mathrm{BaSO}_4(\mathrm{~s})+2 \mathrm{HCl}$
Unbalanced Chemical Equation
When the number of atoms of each element in the reactant and product sides is not equal, it is called an unbalanced chemical equation.
$\mathrm{Fe}+\mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{Fe}_3 \mathrm{O}_4+\mathrm{H}_2$
In this reaction, the number of atoms of the element iron is not equal, and oxygen is also not equal. So we need to balance that unbalanced equation to its balanced form.
In this chemical equation, 3 iron is formed on the product side, so we need to multiply iron by 3 on the reactant side. And also the oxygen atom formed at the product is four, so the water is also multiplied by 4. So, to balance both sides, the hydrogen evolved should also be multiplied by 4.
$3 \mathrm{Fe}+4 \mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{Fe}_3 \mathrm{O}_4+4 \mathrm{H}_2$
So the unbalanced equation becomes a balanced equation. In the representation of the chemical equation, the state can also be mentioned, whether it is in the gaseous form, liquid form or solid form, can be given by descending with brackets.
Balanced Chemical Equation
When the number of atoms of each element in a chemical reaction is equal on both sides, it is called a balanced chemical equation.
$\mathrm{Zn}+\mathrm{H}_2 \mathrm{SO}_4 \rightarrow \mathrm{ZnSO}_4+\mathrm{H}_2$
Let's briefly discuss the steps of balancing a chemical reaction,
Step 1: Write the unbalanced chemical equation
Start with the correct chemical formulas of reactants and products.
Example:
Unbalanced equation:
$\mathrm{Fe}+\mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{Fe}_3 \mathrm{O}_4+\mathrm{H}_2$
Step 2: List the number of atoms of each element
Count atoms of each element on both the reactant and product sides.
Step 3: Start balancing with the element that appears least frequently
Start with elements that appear in fewer compounds, excluding hydrogen and oxygen initially.
In the example above, balance Fe first:
$3 \mathrm{Fe}+4 \mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{Fe}_3 \mathrm{O}_4+ \mathrm{H}_2$
Step 4: Balance the hydrogen and oxygen atoms at the end
Hydrogen and oxygen are often present in multiple compounds. Balance them after metals and other elements.
$\mathrm{Fe}+4 \mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{Fe}_3 \mathrm{O}_4+\mathrm{H}_2$
Step 5: Double-check atom count
Make sure the number of atoms of each element is equal on both sides.
Step 6: Confirm it's the simplest ratio
Coefficients must be the lowest possible whole numbers.
The final Balanced equation will be, $3 \mathrm{Fe}+4 \mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{Fe}_3 \mathrm{O}_4+4 \mathrm{H}_2$
Types of Chemical Reactions
Reactions are classified into different types based on the changes in reactants and products. Understanding these types helps in mastering important concepts for exams. You can also download chemical reactions and equations class 10 science notes for revision and exam preparation.
Combination Reaction
The chemical reaction in which two or more reactants are combined to form another product is a combination reaction. That is,
$A+B \rightarrow A B$
Magnesium reacts with oxygen to form magnesium oxide, which is a typical example of this reaction.
2Mg+$\mathrm{O}_2$ $\xrightarrow{\text { Heat }}$2MgO(s)
Decomposition reaction
When one compound decomposes into two or more compounds, it is called a decomposition reaction. That is,
$A B \rightarrow A+B$
A typical example of a decomposition reaction is the formation of calcium oxide and carbon dioxide by the decomposition of calcium carbonate.
$\mathrm{CaCO}_3(\mathrm{~s}) \xrightarrow{\text { Heat }} \mathrm{CaO}(\mathrm{s})+\mathrm{CO}_2(\mathrm{~g})$
Thermal decomposition is when a substance undergoes a decomposition reaction by heating it.
$\mathrm{CaCO}_3(\mathrm{~s}) \xrightarrow{\text { Heat }} \mathrm{CaO}(\mathrm{s})+\mathrm{CO}_2(\mathrm{~g})$
Electrolytic decomposition is the reaction signature of a compound that decomposes into another component by the supply of electricity. Electrolysis is a type of electrolytic decomposition.
For example, when electricity is passed through water, it decomposes into oxygen and hydrogen. That is,
$2 \mathrm{H}_2 \mathrm{O} \rightarrow 2 \mathrm{H}_2+\mathrm{O}_2$
White silver chloride turns grey in sunlight. This is due to the decomposition of silver chloride into silver and chlorine by light.
$2 \mathrm{AgCl}(\mathrm{s}) \xrightarrow{\text { Sunlight }} 2 \mathrm{Ag}(\mathrm{s})+\mathrm{Cl}_2(\mathrm{~g})$
Silver bromide also behaves in the same way.
$
2 \mathrm{AgBr}(\mathrm{~s}) \xrightarrow{\text { Sunlight }} 2 \mathrm{Ag}(\mathrm{~s})+\mathrm{Br}_0(\mathrm{~g})
$
Photolysis or Photodecomposition
The decomposition reaction performed in the presence of sunlight is known as photolysis. For example, when silver chloride is put in the sunlight, it will decompose to produce the metal silver and chlorine gas. The reaction is,
$2 \mathrm{AgCl} \rightarrow 2 \mathrm{Ag}+\mathrm{Cl}_2$
Displacement Reaction
A chemical reaction in which a reactive element is replaced with a less reactive element is a displacement reaction. It can be represented as,
$\mathrm{A}+\mathrm{BC} \rightarrow \mathrm{AC}+\mathrm{B}$
The reaction taking place between Zinc and hydrochloric acid is an example of a displacement reaction.
$\mathrm{Zn}+2 \mathrm{HCl} \rightarrow \mathrm{ZnCl}_2+\mathrm{H}_2$

Precipitation Reaction
The reaction in which a precipitate is formed after the chemical reaction is called a precipitation reaction. An example of it is,
$\mathrm{AgNO}_3+\mathrm{NaCl} \rightarrow \mathrm{AgCl}(\mathrm{s})+\mathrm{NaNO}_3$
AgCl is the precipitate.
Neutralisation Reaction
A chemical reaction in which an acid and a base react to form a neutral product, typically water, is called a neutralisation reaction.
$\mathrm{NaOH}+\mathrm{HCl} \rightarrow \mathrm{NaCl}+\mathrm{H}_2 \mathrm{O}$
Oxidation Reaction
The removal of a hydrogen or metallic element and the addition of oxygen or a nonmetallic element from a compound is known as oxidation. The element in which the nonmetallic element or oxygen is added and hydrogen or a metallic element is removed is said to be oxidised.
Example: Rusting iron.
Reduction Reaction
Removal of oxygen or nonmetallic elements and addition of hydrogen or metallic elements from any compound is called reduction. And the element in which it will undergo A reduction process is said to be reduced.
Redox Reaction
When the process of reduction and oxidation takes place simultaneously, it is said to be a redox reaction. And the substance which removes hydrogen or gives oxygen is said to be an oxidising agent. And the one which gives hydrogen or removes oxygen is said to be a reducing agent.
$\mathrm{CuO}+\mathrm{H}_2 \rightarrow \mathrm{Cu}+\mathrm{H}_2 \mathrm{O}$
In this reaction, oxygen is removed from CuO; therefore, it is said to be a reduction process. While oxygen is added to H2, it is an oxidation process.
Exothermic Reaction
The reaction in which energy is produced is called an exothermic reaction. And the energy is produced in the form of heat.
$\mathrm{CaO}+\mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{Ca}(\mathrm{OH})_2+$ Energy
Endothermic Reaction
When heat energy is absorbed or consumed to undergo a chemical reaction, it is an endothermic reaction.
$\mathrm{CaCO}_3$ $\xrightarrow{\text { Heat }}$ CaO+$\mathrm{CO}_2$
Corrosion
Have you noticed the colour of the coating formed on copper and silver? When a metal is attacked by substances surrounding it, such as moisture and acids, it is said to corrode, and this process is called corrosion. The black coating on silver and the green coating on copper are other examples of corrosion.
Rancidity
Have you ever tasted or smelled the fat/oil-containing food materials left for a long time? When fats and oils are oxidised, they become rancid and their smell and taste change. Usually, substances that prevent oxidation (antioxidants) are added to foods containing fats and oils. Keeping food in air-tight containers helps to slow down oxidation.
Chemical Reactions and Equations: Previous Year Question and Answer
Students can practice previous year questions from this chapter to help them understand the exam pattern and focus on important topics. These solved questions on chemical reactions and equations strengthen concepts and improve problem-solving skills. Consistent revision of chemical reactions and equations in the class 10 science chapter 1 CBSE notes ensures a strong conceptual foundation and enhances exam readiness.
Question: The white precipitates formed in a chemical reaction between sulphuric acid and barium chloride solution are of:
(1) Sulphur
(2) Barium sulphate
(3) Chlorine
(4) Silver chloride
Answer: White precipitates formed in a chemical reaction between sulphuric acid and barium chloride solution are of Barium sulphate.
Hence, the answer is option (2).
Question: In the standard notation for a voltaic cell, the double vertical line "||" represents:
(1) salt bridge
(2) gas electrode
(3) a wire (metal) connection
(4) a phase boundary
Answer: In the standard notation for a voltaic cell, the double vertical line "||" represents the salt bridge.
Hence, the answer is option (1).
Question: Dry HCl gas does not change the colour of dry litmus paper. Why?
Answer:
1. Dry HCl gas does not change the colour of dry litmus paper because it does not release hydrogen ions
$\left(\mathrm{H}^{+}\right)$in the absence of water.
2. Acids show their acidic properties only in aqueous (water) solution, where they ionise to give $\mathrm{H}^{+}$ions.
Since there is no water in dry HCl gas or on dry litmus paper, no $\mathrm{H}^{+}$ions are produced, and thus, no colour change is observed.
Question 4: Which of the following methods can be used to prevent the food from getting rancid:
i. Storing the food in the air-tight containers
ii. Storing the food in refrigerator
iii. Keeping food in open
iv. Keeping food in moist atmosphere
(1) (i) and (ii)
(2) (i) and (iii)
(3) (iii) and (iv)
(4) All of the above
Answer:
Rancidity can be prevented by storing food in airtight containers because in airtight containers there is little exposure to oxygen then oxidation of fats and oils present in food is slowed down and thus rancidity is retarded. Rancidity can be prevented by keeping food in a refrigerator because in a refrigerator when the food is kept the oxidation of fats and oils in it is slowed down due to low temperature and thus rancidity is retarded.
Hence, the answer is the option (1).
Question 5: Which type of reaction does the following equation represent?
$\mathrm{CH}_4+2 \mathrm{O}_2 \rightarrow \mathrm{CO}_2+2 \mathrm{H}_2 \mathrm{O}$
(1) Addition reaction
(2) Decomposition reaction
(3) Combustion reaction
(4) Reduction reaction
Answer:
The above reaction represents Combustion reaction and oxidation reaction.
Hence, the answer is the option (3).
How to Master Class 10 Science Chapter 1 Chemical Reactions and Equations
These class 10 science chapter 1 chemical reactions and equations notes introduce the basics of how substances interact and transform. Given below are some points on how to master this chapter.
- First, students must understand the difference between physical and chemical changes, characteristics of chemical reactions like evolution of gas, change in colour, formation of precipitate, temperature change, and state change.
- Then they must study the types of chemical reactions like combination, decomposition, displacement, double displacement, oxidation, and reduction.
- Learn how to write and balance chemical equations.
- Questions related to equations involving heat, gas evolution, and precipitate formation are often asked in exams. Students can understand these concepts better with the help of the NCERT Class 10 Science Chapter 1 Chemical Reactions and Equations notes.
- Lastly, students must solve questions.
Advantages of Using Class 10 Science Chapter 1 Chemical Reactions and Equations Notes
NCERT Class 10 Science Chapter 1 Chemical Reactions and Equations Notes helps students to understand the concepts of chemistry in Class 10. The advantages of using these notes are given below:
- These notes are the best source for understanding the topics like chemical reactions, balancing equations, types of reactions, oxidation, reduction, displacement, and double displacement reactions.
- Students can refer to these notes for understanding how to write and balance chemical equations.
- They are prepared by subject experts in a very clear and comprehensive manner that includes examples and reactions.
- The class 10 science chapter 1 chemical reactions and equations notes are helpful for both CBSE Boards and competitive exams.
CBSE Class 10 Science Chapter-wise Notes
In addition to the NCERT Class 10 Science Chapter 1 Notes Chemical Reactions and Equations, students can refer to the notes of other Class 10 chapters provided below.
NCERT Solutions for Class 10 Science Chapter-Wise
Besides class 10 science chapter 1 chemical reactions and equations notes, students can also follow Class 10 chapter-wise solutions of NCERT:
Frequently Asked Questions (FAQs)
Balancing a chemical equation involves ensuring that the number of atoms for each element is the same on both the reactant and product sides. This is based on the Law of Conservation of Mass, which states that mass is neither created nor destroyed in a chemical reaction.
Catalysts are substances that speed up a chemical reaction without being consumed in the process. They lower the activation energy required for the reaction to occur.
Balancing chemical equations is important because it reflects the conservation of mass. By ensuring that the same number of atoms exists before and after a reaction, it allows chemists to predict the amounts of reactants needed and the products formed.
A chemical equation is a symbolic representation of a chemical reaction. It shows the reactants on the left side and the products on the right side, separated by an arrow that indicates the direction of the reaction.
To prepare effectively, start by thoroughly reading the NCERT textbook and understanding the concepts rather than memorising them. Practise balancing chemical equations daily, revise the types of reactions with examples, and focus on oxidation and reduction. Prepare short notes or flowcharts for quick revision, and solve NCERT exercises, previous years’ papers, and sample questions to strengthen exam readiness.
Chapter 1 chemical reactions and equations notes are concise study materials that explain the fundamentals of chemical changes, their characteristics, and how to represent them using chemical equations. These notes cover important topics like types of reactions, balancing equations, oxidation and reduction, corrosion, and rancidity, helping students understand the chapter easily and prepare effectively for CBSE board exams.
The key concepts covered in this chapter are: Chemical Reactions, Chemical Equations, Balancing Chemical Equations, Types of Chemical Reactions (Combination, Decomposition, Displacement, Double Displacement, Redox), Corrosion, Rancidity
Physical Change: A change in the form or appearance of a substance, but not in its chemical composition. No new substance is formed. Examples: melting ice, boiling water.
Chemical Change: A change that results in the formation of a new substance (or substances) with different chemical properties. Examples: burning wood, rusting iron.
It is necessary to balance a chemical equation to satisfy the Law of Conservation of Mass. This law states that matter cannot be created or destroyed in a chemical reaction. Therefore, the number of atoms of each element must be the same on both the reactant and product sides of the equation.
A precipitation reaction is a type of double displacement reaction where two aqueous solutions react to form an insoluble solid called a precipitate.
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
