The basic concepts in Class 10 Chemistry Chapter 2 include the definitions and properties of acids and bases, the concept of indicators, the pH scale and its importance, neutralisation reactions, and the chemical properties of acids and bases. It also covers the preparation, properties, and uses of common salts such as baking soda, washing soda, bleaching powder, and Plaster of Paris. These concepts link theory with practical applications in daily life and industry.
NCERT Class 10 Science Chapter 2 Notes Acids, Bases, and Salts- Download PDF Notes
Have you ever wondered why lemon juice is sour or why soap feels so slippery? It is all about acids, bases, and salts. Acids, such as those found in lemons, taste sour and cause blue litmus paper to become red. Bases, like baking soda, taste harsh and turn red litmus paper blue. A combination of acids and bases forms salts, and they include table salt and industrial chemicals. Learning about these chemicals allows us to understand their significance in academic and practical contexts.
This Story also Contains
- NCERT Class 10 Science Chapter 2 Notes: Download PDF
- NCERT Class 10 Science Chapter 2 Notes
- Previous Year Questions of Class 10 Science Chapter 2
- How to Master Class 10 Science Chapter 2 Acids, Bases, and Salts
- Advantages of Using Class 10 Science Chapter 2 Acids, Bases, and Salts Notes
- NCERT notes for Class 10 Science Chapter-wise
- NCERT Solutions for Class 10 Science Chapter-Wise

In ncert class 10 science chapter 2 acids, bases, and salts notes , topics like the behaviour of acids, bases and salts, their properties, and their reactions with different compounds are discussed in depth. The NCERT Notes for Class 10 Science will provide a precise and structured revision of the entire chapter that will eliminate the need for students to search for additional resources. These NCERT notes cover simple examples like how acids help our stomachs in digesting food and how bases are used in cleaning products. In this article, we have provided a step-by-step analysis of the topic by including diagrams and reactions that will help you get clarity on the topic and make the revision effective. For additional practice and conceptual clarity, students can also refer to NCERT Solutions.
NCERT Class 10 Science Chapter 2 Notes: Download PDF
Students can download the Acids, Bases, and Salts Class 10 Science notes PDF to access a clear explanation of all the important concepts from the Download PDF icon given below. These NCERT notes for Class 10 will help in quick revision and better preparation for exams.
Also read
NCERT Class 10 Science Chapter 2 Notes
The class 10 science chapter 2 acids, bases, and salts notes are a key guide for students preparing for boards. Students can use these notes for quick revision of the concepts that will, in turn, help them tackle the questions. Scroll down to know more!
2.1 Understanding the Chemical Properties of Acids and Bases
One hundred and fifteen different chemical elements are known to us at present. These elements combine to form a large number of compounds. Based on their chemical properties, all the compounds can be classified into three groups :
1. Acids
2. Bases
3. Salts
To know whether a substance is an acid or a base, we should first know the meaning of the term ‘acid-base indicator’ or just ‘indicator’. This is discussed below.
2.1.1 Acids and Bases in the Laboratory
Indicators for Testing Acids and Bases
An indicator is a ‘dye’ that changes colour when it is put into an acid or a base. An indicator gives different colours in acid and base. Thus, an indicator tells us whether the substance we are testing is an acid or a base by a change in its colour. In other words, an indicator tells us whether the substance we are testing is acidic or basic by a change in its colour. The three most common indicators to test for acids and bases are: Litmus, Methyl orange and Phenolphthalein. To strengthen your understanding and practice more problems, you can also refer to the NCERT Solutions for Class 10 Science Chapter 2
The most common indicator used for testing acids and bases in the laboratory is litmus. Litmus can be used in the form of a litmus solution or in the form of litmus paper. It is of two types: Blue litmus and Red litmus.
Concept of Acids
If we cut a lemon with a knife and taste it, the lemon appears to have a sour taste. The sour taste of lemon is due to the presence of an acid in it. The acid present in lemon, which gives it a sour taste, is citric acid. Thus, acids are those chemical substances that have a sour taste. Some of the common fruits, such as raw mango, raw grapes, lemon, orange, and tamarind, are sour in taste due to the presence of acids in them. Soured milk (or curd) also contains acid in it.
Change in colour: Acids change their colour from blue to red while performing a litmus test.
Concept of Bases
The solutions of substances like caustic soda, lime, and washing soda are bitter in taste, and soapy to the touch (slippery to touch). They are called bases. Thus, bases are those chemical substances that have a bitter taste.
Change in colour: Bases change their colour from red to blue while performing a litmus test.
Litmus: This can be extracted from lichen and is a purple dye solution.
It is the fact that if the litmus is neither acidic nor basic in nature, then it shows a purple colour.
Indication of acid and base inside any material:
Natural materials like:
-
Red cabbage leaves
-
Turmeric
-
Coloured petals of flowers such as Petunia, Geranium, etc.
Properties of Acids and Bases
Acids
- The word is derived from the Latin word called “acidus”.
- When dissolved in an aqueous medium, it gives hydrogen ions to the solution.
- Concentrated acids are mixtures of a larger amount of acid dissolved in a smaller amount of water.
- Dilute acids are mixtures of a larger amount of water dissolved in a smaller amount of acids.
- Examples of strong acids are $\mathrm{HCl}, \mathrm{H}_2 \mathrm{SO}_4, \mathrm{HNO}_3$, etc.
- Examples of weak acids are $\mathrm{CH}_3 \mathrm{COOH}$, Lactic acid, oxalic acid, etc.
Bases
- The substances which are basic in nature are soapy in touch.
- When dissolved in an aqueous medium, it gives hydroxide ions in the solution.
- Alkali is the base that is soluble in water. Examples of alkali are NaOH, KOH, etc.
- Examples of strong bases: $\mathrm{NaOH}, \mathrm{KOH}, \mathrm{Ca}(\mathrm{OH})_2$
- Example of weak bases: $\mathrm{NH}_4 \mathrm{OH}$
Indicators
Indicators are defined as changes in the colour or smell of a substance in different types of acids and bases.
Types of indicators are as follows:
-
Natural indicators
-
Synthetic Indicators
-
Olfactory indicators
2.1.2 How do Acids and Bases React with Metals
The reaction of Acids with Metals produces salt and hydrogen gas.
Acid + metal $\rightarrow$ salt + Hydrogen gas
Example of such reaction: $\mathrm{Zn}+2 \mathrm{HCl} \rightarrow \mathrm{ZnCl}_2+\mathrm{H}_2$
The reaction of Bases when reacting with Metals produces salt and hydrogen gas.
The base, which is reacting with metal, has to be more reactive than the base. But not all metals have the nature to react with bases.
Base + metal $\rightarrow$ salt + Hydrogen gas
Example of such reaction: $2 \mathrm{NaOH}+\mathrm{Zn} \rightarrow \mathrm{Na}_2 \mathrm{ZnO}_2+\mathrm{H}_2$
The evolved hydrogen gas burned with a pop sound when it came closer to the evolved gas.
2.1.3 How do Metal Carbonates and Metal Hydrogencarbonates React with Acids?
Reaction of acid with metal carbonates and hydrogen carbonates
Acid + Metal carbonates or hydrogen carbonates $\longrightarrow$ Salt +Water +Carbon dioxide
$\mathrm{HCl}+\mathrm{NaHCO}_3 \longrightarrow \mathrm{NaCl}+\mathrm{H}_2 \mathrm{O}+\mathrm{CO}_2$
The produced carbon dioxide will be further tested and passed through lime water solution, which turns the solution milky. But it should be noticeable that the amount of carbon dioxide is certain; if it passes in excess amount, then the milkiness will disappear.
Reaction when the amount of carbon dioxide is certain.
Milky lime water
$
\mathrm{Ca}(\mathrm{OH})_2+\mathrm{CO}_2 \longrightarrow \mathrm{CaCO}_3+\mathrm{H}_2 \mathrm{O}
$
Reaction when the amount of carbon dioxide is in excess.
$\mathrm{CaCO}_3+\mathrm{CO}_2+\mathrm{H}_2 \mathrm{O} \longrightarrow \mathrm{Ca}(\mathrm{HCO})_3$
The reaction of bases when reacting with carbonates and hydrogen carbonates
No such reaction will proceed when the base reacts with metal carbonate and metal hydrogen carbonates.
2.1.4 How do Acids and Bases React with each other?
When acids and bases react with each other, they produce salt and water such a reaction is termed a neutralisation reaction. You can also download acids, bases, and salts class 10 science chapter 2 CBSE notes for revision and exam preparation.
$\begin{aligned} & \text { Acid }+ \text { Base } \rightarrow \text { Salt }+ \text { Water } \\ & \mathrm{HCl}+\mathrm{NaOH} \rightarrow \mathrm{NaCl}+\mathrm{H}_2 \mathrm{O}\end{aligned}$
-
Strong acid + weak base gives an acidic salt and water.
-
Weak acid + strong base gives an acidic salt and water.
-
Strong acid + strong base gives neutral salt and water.
-
Weak acid + weak base gives neutral salt and water.
2.1.5 Reaction of Metallic Oxides with Acids
The nature of metallic oxides is basic.
Metallic oxide + acid $\longrightarrow$ salt + water
$
\mathrm{CaO}+2 \mathrm{HCl} \longrightarrow \mathrm{CaCl}_2+\mathrm{H}_2 \mathrm{O}
$
2.1.6 Reaction of a Non-metallic Oxide with Base
The nature of metallic oxides is acidic.
Non-metallic oxide + Base $\rightarrow$ Salt + Water
$
\mathrm{CO}_2+\mathrm{Ca}(\mathrm{OH})_2 \rightarrow \mathrm{CaCO}_3+\mathrm{H}_2 \mathrm{O}
$
2.2 What do all acids and all bases have in common?
Acids and bases have some similarities between them, and they are listed below:
-
Acids give hydrogen ions when dissolved in any solution, so the common ion is hydrogen ion,s which makes any mixture acidic in nature.
-
Bases give hydroxide ions when dissolved in any solution, so the common ion is hydroxide ion, which makes any mixture basic in nature.
Water solution with acids or bases-
When acids dissolve in water, they produce hydrogen ions, but hydrogen ions cannot exist alone; they exist in the form of hydronium ions. When bases dissolve in water, they give hydroxide ions.
Bases that are soluble in water are termed alkali.
Point to remember: When we are diluting any acid with water, it should be kept in mind that the acid must be added to the water, not the water to the acid, as the process of dissolving any acid or base is highly exothermic in nature.
2.2.1 What Happens to an Acid or a Base in a Water Solution?
This can be evaluated by an indicator known as a universal indicator.
Universal indicator: This indicator is a mixture of different indicators that shows colour when any solution concentration has hydrogen ions or hydroxide ions in it. Since the pH of a solution depends on the hydrogen ion concentration, so we can also say that the universal indicator shows different colours at different concentrations of hydrogen ions in the solution. When an acid or base solution is added to the universal indicator, the indicator produces a new color.
The colour produced by universal indicator is used to find the pH value of the acid or base solution by matching the colour with the colours on the pH colour chart. And knowing the pH value, we can make out whether the given solution is a strong acid, weak acid, strong base or a weak base.
2.3 How strong are acid or base solutions?
pH scale: For measuring the concentration of hydrogen ion in any solution, that how acidic or basic the solution, we must require a scale that scale is termed as pH scale. p in pH denotes potenz which in general means power.

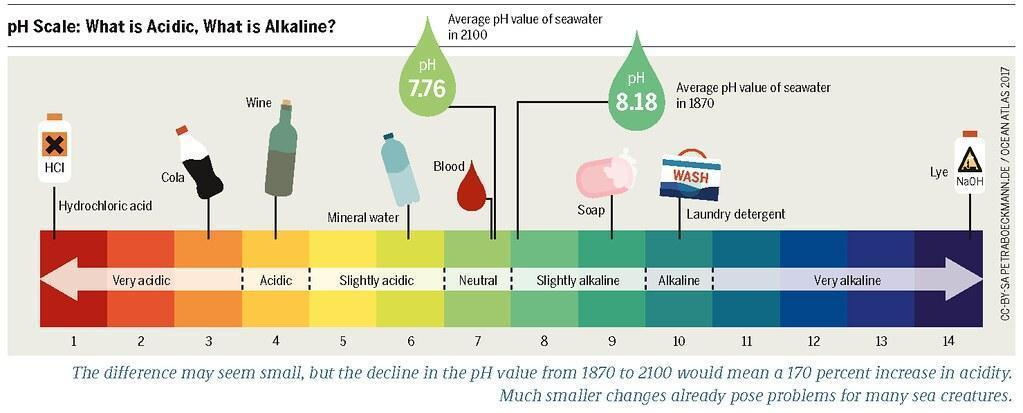
2.3.1 Importance of pH in everyday Life
pH plays a very important role in our everyday life.
In our digestive system- Hydrochloric acid produced in our stomach helps the digestion of food without causing any harm to the stomach. But when the amount of the acid goes beyond a certain limit due to indigestion, pain and irritation are created in the stomach. So, in order to neutralize the effect of excess acid, a mild base called antacid is usually taken. Magnesium hydroxide (milk of magnesia) is a mild base which is usually used as an antacid.
Acids cause tooth decay- When we eat sugary food, it gets degraded by bacteria present in the mouth and an acid is formed. When the pH becomes lower than 5.5, tooth enamel gets corroded. Saliva, which is slightly alkaline, produced in the mouth neutralizes some acid, but excess acid remains unaffected. The excess acid can be removed only by the use of toothpaste which is alkaline. Neem stick contains alkaline juice. So, the cleaning of tooth by Neem stick also helps to reduce tooth decay.
Acid is produced in fatigued muscle- As a result of physical exercise, stiffness and pain in the muscle starts due to the formation of lactic acid. The supply of oxygen in the muscle is reduced. This causes difficulty in the release of energy leading to an increase in the rate of anaerobic metabolism. As a result, lactic acid gets accumulated in the muscles.
Some animals and plants contain acids- Honey-bee injects an acid through its stings which causes pain and irritation. Hence, a mild base like baking soda is applied to treat the wound. Similarly, nettle leaves, which have stinging hairs, when touched, inject formic acid in our body. This causes a burning pain.
The brilliance of a tarnished copper vessel can be restored by using acid- You know, lemon juice contains an acid. In order to clean a copper vessel, we rub it with the piece of a lemon. The tarnish on the vessel is caused by the formation of a layer of basic copper oxide. Since lemon juice contains citric acid, it reacts with the copper oxide to form copper citrate and is washed away. The vessel then regains its shining appearance.
pH of soil- Soils are generally acidic. Plants require definite pH range for their proper growth. They do not grow in alkaline soil. Many plants do not grow properly in highly acidic or highly alkaline soil. So, highly acidic soil is treated by spreading quicklime, slaked lime or calcium carbonate to lower its acidity.
2.4 More about salts
A salt is a compound formed from an acid by the replacement of the hydrogen in the acid by a metal. Here is an example. Hydrochloric acid is HCl. Now, if we replace the hydrogen (H) of this acid by a metal atom, say a sodium atom (Na), then we will get a salt NaCl. This is called sodium chloride. It is a salt. In some salts, however, the hydrogen of acid is replaced by an ammonium group ($\mathrm{NH}_4$) as in the case of ammonium chloride, $\mathrm{NH}_4 \mathrm{Cl}$. The best known salt is sodium chloride (NaCl), which is usually known as common salt.
Salts are formed when acids react with bases. In a way, we can say that a salt has two parents : an acid and a base. So, the name of a salt consists of two parts : the first part of the name of salt is derived from the name of base, and the second part of the name of the salt comes from the name of acid. For example, the name of a salt called ‘sodium chloride’ comes from sodium hydroxide base and hydrochloric acid.
Properties of Salts
-
Salts are mostly solids.
-
They have high melting points and boiling points.
-
Salts are usually soluble in water. Just like acids and bases, solutions of salts in water conduct electricity. That is, salts are electrolytes.
-
Salt solutions conduct electricity due to the presence of ions in them. Salts are ionic compounds. Every salt consists of a positively charged ion (cation) and a negatively charged ion (called anion).
The salts that are produced by strong acids and strong bases are neutral in nature. The pH value of such a salt is 7.
Formation of Sodium Chloride
Seawater contains many salts dissolved in it. Sodium chloride is separated from these salts. Deposits of solid salt are also found in several parts of the world. These large crystals are often brown due to impurities. This is called rock salt.
Formation of Sodium Hydroxide
When electricity is passed through an aqueous solution of sodium chloride (called brine), it decomposes to form sodium hydroxide. The process is called the chlor-alkali process because of the products formed chlor for chlorine and alkali for sodium hydroxide. The chlorine gas is given off at the anode, and hydrogen gas at the cathode. Sodium hydroxide solution is formed near the cathode.
$2 \mathrm{NaCl}+2 \mathrm{H}_2 \mathrm{O} \longrightarrow 2 \mathrm{NaOH}+\mathrm{Cl}_2+\mathrm{H}_2$
Formation of Bleaching Powder
Bleaching powder is produced by the action of chlorine on dry slaked lime [Ca(OH)2]. Bleaching powder is represented as Ca(ClO)2, though the actual composition is quite complex.
$\underset{\substack{\mathrm{Ca}(\mathrm{OH})_2 \\ \text { slaked lime }}}{\mathrm{Cl}} \mathrm{Cl}_2 \longrightarrow \underset{\text { Bleaching powder }}{\mathrm{CaOCl}_2}+\mathrm{H}_2 \mathrm{O}$
Bleaching powder is used-
(i) for bleaching cotton and linen in the textile industry, for bleaching wood pulp in paper factories and for bleaching washed clothes in laundry.
(ii) as an oxidising agent in many chemical industries.
(iii) to make drinking water free from germs.
Formation of Baking Soda
The baking soda is commonly used in the kitchen for making tasty crispy pakoras, etc. Sometimes it is added for faster cooking. The chemical name of the compound is sodium hydrogencarbonate ( $\mathrm{NaHCO}_3$ ). It is produced using sodium chloride as one of the raw materials.
$
\mathrm{NaCl}+\mathrm{H}_2 \mathrm{O}+\mathrm{CO}_2+\mathrm{NH}_3 \rightarrow \underset{\substack{\text { (Ammonium } \\ \text { chloride) }}}{\mathrm{NH}_4 \mathrm{Cl}}+\underset{\text { (Sodium bicarbonate }}{\mathrm{NaHCO}_3}
$
Formation of Baking Powder
For making baking powder, which is a mixture of baking soda (sodium bicarbonate) and a mild edible acid such as tartaric acid. When baking powder is heated or mixed in water, the following reaction takes place -
$
\mathrm{NaHCO}_3+\mathrm{H}^{+} \rightarrow \mathrm{CO}_2+\mathrm{H}_2 \mathrm{O}+\text { Sodium salt of acid }
$
(From any acid)
$\underset{\text { Baking soda }}{\mathrm{NaHCO}_3}+\underset{\text { tartric acid }}{\mathrm{CHO}} \longrightarrow \mathrm{CO}_2+\mathrm{H}_2 \mathrm{O} {+} \mathrm{Na}_2 \mathrm{C}_4 \mathrm{H}_4 \mathrm{O}_6$
Washing soda
Washing soda is the common name for sodium carbonate decahydrate, used as a cleaning agent and water softener.
$
\mathrm{Na}_2 \mathrm{CO}_3+10 \mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{Na}_2 \mathrm{CO}_3 \cdot 10 \mathrm{H}_2 \mathrm{O}
$
2.4.4 Are the Crystals of Salts really Dry?
Copper sulphate crystals, which seem to be dry, contain water of crystallisation. When we heat the crystals, this water is removed and the salt turns white.
Water of crystallisation is the fixed number of water molecules present in one formula unit of a salt. Five water molecules are present in one formula unit of copper sulphate. The chemical formula for hydrated copper sulphate is CuSO4. 5H2O.
Plaster of Paris
Plaster of Paris is a white powdery substance made by heating gypsum ($\mathrm{CaSO}_4 \cdot 2 \mathrm{H}_2 \mathrm{O}$); it sets into a hard mass when mixed with water.
$
\mathrm{CaSO}_4 \cdot 2 \mathrm{H}_2 \mathrm{O} \xrightarrow{\text { Heat }} \mathrm{CaSO}_4 \cdot \frac{1}{2} \mathrm{H}_2 \mathrm{O}+\frac{3}{2} \mathrm{H}_2 \mathrm{O}
$
Previous Year Questions of Class 10 Science Chapter 2
Below are some previous year questions from chapter 2 to help students understand the exam pattern and focus on important topics. These solved questions strengthen concepts and improve problem-solving skills for CBSE Board exam preparation. These resources, along with Acids, Bases, and Salts Class 10 Science notes make revision easier and ensure students grasp the key concepts effectively.
Question 1: A white shirt has a yellow stain of curry. When soap is rubbed on this shirt during washing, the yellow stain turns reddish-brown. On rinsing the shirt with plenty of water, the reddish-brown stain turns yellow again. Name the natural indicator present in the curry stain.
(1) Baking soda
(2) Common salt
(3) Citric acid
(4) Turmeric
Answer: The yellow stain contains turmeric and as turmeric is a natural indicator, it turns red when soap is rubbed because soap is basic. When the shirt is rinsed off, the soap also gets rinsed off and hence again the colour turns to yellow.
Hence, the correct answer is option (4).
Question 2: Acids do not show acidic behaviour in the absence of water because
(1) Dissociation of hydrogen ions occurs in water only
(2) They release OH- ions in the absence of water
(3) Acids release heat after addition to water
(4) None of the above
Answer: Acids do not show acidic behaviour in the absence of water because the dissociation of hydrogen ions from an acid occurs in the presence of water only. It is the hydrogen ions that are responsible for the acidic behaviour.
Hence, the correct answer is option (1).
Question 3: Which of the given option represents a family of salts?
(1) $\mathrm{NaCl}, \mathrm{Na}_2 \mathrm{SO}_4, \mathrm{CaSO}_4$
(2) $\mathrm{K}_2 \mathrm{SO}_4, \mathrm{Na}_2 \mathrm{SO}_4, \mathrm{CaSO}_4$
(3) $\mathrm{NaNO}_3, \mathrm{CaCO}_3, \mathrm{Na}_2 \mathrm{CO}_3$
(4) $\mathrm{MgSO}_4, \mathrm{CuSO}_4, \mathrm{MgCl}_2$
Answer:
According to the given options, the second one represents the family of salts .
$\mathrm{K}_2 \mathrm{SO}_4$ : Potassium sulfate (salt of potassium and sulfate ion)
$\mathrm{Na}_2 \mathrm{SO}_4$ : Sodium sulfate (salt of sodium and sulfate ion)
$\mathrm{CaSO}_4$ : Calcium sulfate (salt of calcium and sulfate ion)
These salts have the same anion: sulfate ion $(\mathrm{SO}_4{ }^{2-})$, but different cations (potassium, sodium, calcium). This option represents a family of salts with a common anion ( $\mathrm{SO}_4{ }^{2-}$ ).
Hence, the correct answer is option (2).
Question 4: Which of the following is the correct use of sodium carbonate (washing soda)?
(1) To manufacture glass, soap, borax and caustic soda.
(2) It is used in water softening in laundry and cleaning.
(3) It is used in paper, paints and textile industries.
(4) All of the above
Answer:
All the above applications are important uses of washing soda.
Hence, the answer is the option (4).
Question 5: pH value of Sodium hydroxide is:
(1) Less than 2
(2) Equal to 7
(3) Less than 10
(4) Almost equal to 14
Answer:
pH value of Sodium hydroxide is almost equal to 14 as it is a very strong base.
Hence, the answer is the option (4).
How to Master Class 10 Science Chapter 2 Acids, Bases, and Salts
These Acids, Bases, and Salts Class 10 Science notes help to understand the behaviour of substances in chemistry and their applications in daily life. Given below are some points on how to master this chapter.
- Students must understand the definitions and properties of acids, bases, and salts, important theories like Arrhenius and Bronsted-Lowry.
- Then learn about pH scale and its significance in everyday life.
- Focus on Reactions, uses of common salts and importance of pH in agriculture, biology, and industry. Students can refer to class 10 science chapter 2 acids, bases, and salts notes to understand these concepts better.
- Lastly, students must solve questions.
Advantages of Using Class 10 Science Chapter 2 Acids, Bases, and Salts Notes
NCERT Class 10 Science Chapter 2 Acids, Bases, and Salts Notes help students understand important chemical reactions and properties. Given below some points on the advantages of these notes:
- Students can understand the topics like acids, bases, and salts, their properties, uses, and reactions.
- These notes provide clear explanations that help students understand how acids and bases react.
- The acids, bases, and salts class 10 science chapter 2 CBSE notes provide clear and concise summaries, reactions, and definitions.
- They are prepared by subject experts in a systematic manner that are helpful for both CBSE boards and competitive exams.
NCERT notes for Class 10 Science Chapter-wise
Apart from NCERT Class 10 Science Chapter 2 Notes Acids, Bases, and Salts, students can also go through the NCERT notes of other Class 10 chapters listed below.
NCERT Solutions for Class 10 Science Chapter-Wise
Besides acids, bases, and salts class 10 science chapter 2 CBSE notes, students can also follow Class 10 chapter-wise solutions of NCERT:
Frequently Asked Questions (FAQs)
Indicators are substances that change color in response to pH levels. For example, litmus paper turns red in acidic solutions and blue in basic solutions. Phenolphthalein is another indicator that turns pink in basic environments and remains colorless in acidic solutions, making them useful for identifying the nature of a solution.
The pH scale is a numerical scale that measures the acidity or basicity of a solution. It ranges from 0 to 14, where 7 is considered neutral. Values below 7 indicate acidic solutions, while values above 7 indicate basic solutions.
Acids and bases are naturally present in the human body and are vital for various biochemical processes. For instance, stomach acid HCl helps digest food. However, imbalances can lead to health issues, such as acid reflux.
Acids and bases play crucial roles in everyday life. For example, citric acid is found in fruits, vinegar is acetic acid, and baking soda is a common base. They are also important in cleaning products, food preservation, and even in our stomachs for digestion.
Salts are ionic compounds formed by the neutralization reaction between an acid and a base. For example, when hydrochloric acid reacts with sodium hydroxide, sodium chloride (table salt) and water are produced.
Salts are ionic compounds typically formed when an acid reacts with a base. They are composed of a cation from a base and an anion from an acid.
NCERT Class 10 Science Chapter 2 Notes Acids, Bases, and Salts are concise study materials that explain the properties, reactions, and uses of acids, bases, and salts. They include key concepts such as the pH scale, neutralisation reactions, preparation of important salts, and their applications in daily life, helping students revise effectively and prepare for CBSE board exams.
Neutralization is the reaction between an acid and a base, resulting in the formation of a salt and water. It is significant because it is used to neutralize harmful acids or bases in various industrial and laboratory settings.
The pH scale is a logarithmic scale used to measure the acidity or basicity of a solution. It ranges from 0 to 14, with 0 being the most acidic, 14 being the most basic, and 7 being neutral.
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters

