These notes summarise key concepts of carbon’s structure, bonding, and catenation, types of hydrocarbons, functional groups, and important reactions. They also cover alcohols, acids, esters, soaps, and detergents with their properties and uses, helping students revise quickly and prepare for exams.
NCERT Class 10 Science Chapter 4 Notes Carbon and its Compounds- Download PDF Notes
Have you ever wondered why fossil fuels are used to power vehicles, how plastics are made, or why plants need carbon dioxide to survive? The answer to all these questions lies in carbon and its compounds. Carbon is a fundamental element that can be seen everywhere in our surroundings. The food we eat, the clothes we wear, everything has some element of carbon in it.
This Story also Contains
- NCERT Notes for Class 10 Chapter 4: Download PDF
- NCERT Notes for Class 10 Chapter 4
- Carbon and Its Compounds: Previous Years' Questions and Answers
- How to Master Class 10 Science Chapter 4: Carbon and its Compounds
- Advantages of Using Class 10 Science Chapter 4 Carbon and Its Compounds Notes
- NCERT notes for Class 10 Science Chapter-wise
- NCERT Solutions for Class 10 Science Chapter-Wise
- NCERT Class 10 Exemplar Solutions
.jpg)
NCERT Notes for Class 10 are provided to revise all the important concepts given in this chapter. These notes include an explanation of each topic and formula. They are designed by our experienced subject matter experts, which ensures the credibility of the content provided. It becomes difficult and time-consuming for students to read the NCERT textbooks point-to-point. So, to solve this problem, we are providing these NCERT notes that cover all the topics and concepts provided in the NCERT textbook in a very clear and comprehensive way.
NCERT Notes for Class 10 Chapter 4: Download PDF
Students can download the carbon and its compounds class 10 science chapter 4 CBSE notes PDF to access a clear explanation of all the important concepts from the Download PDF icon given below. These notes will help in quick revision and better preparation for exams. For additional practice, students can also refer to the NCERT Solutions for this chapter.
Also Read
NCERT Notes for Class 10 Chapter 4
These notes give you a basic idea of the key features of carbon and its compounds. Some of the main topics covered in ncert class 10 science chapter 4 carbon and its compounds notes PDF are: definitions, bonding in carbon the covalent bond, versatile nature of carbon, saturated and unsaturated carbon compounds, homologous series, the nomenclature of carbon compounds, chemical properties of carbon compounds, some important carbon compounds, ethanol and ethanoic acid, soaps, and detergents. Students must go through each topic in carbon and its compounds, which are given in the NCERT Notes Class 10 Science.
Bonding in Carbon: The Covalent Bond
- We know that an ionic bond is formed when an atom loses an electron and another atom gains an electron.
- In the case of carbon, which has 4 electrons in its outermost shell, it is not feasible to lose all of the 4 electrons due to high energy requirements or even gain 4 electrons due to repulsion between the electrons.
- Therefore, carbon forms bonds by sharing its 4 electrons with another carbon atom, hence completing its octet. This type of bond is called a covalent bond.
- This type of bond is also formed between other compounds, such as in $\mathrm{H}_2, \mathrm{O}_2, \mathrm{~N}_2$, etc.
Allotropes of Carbon
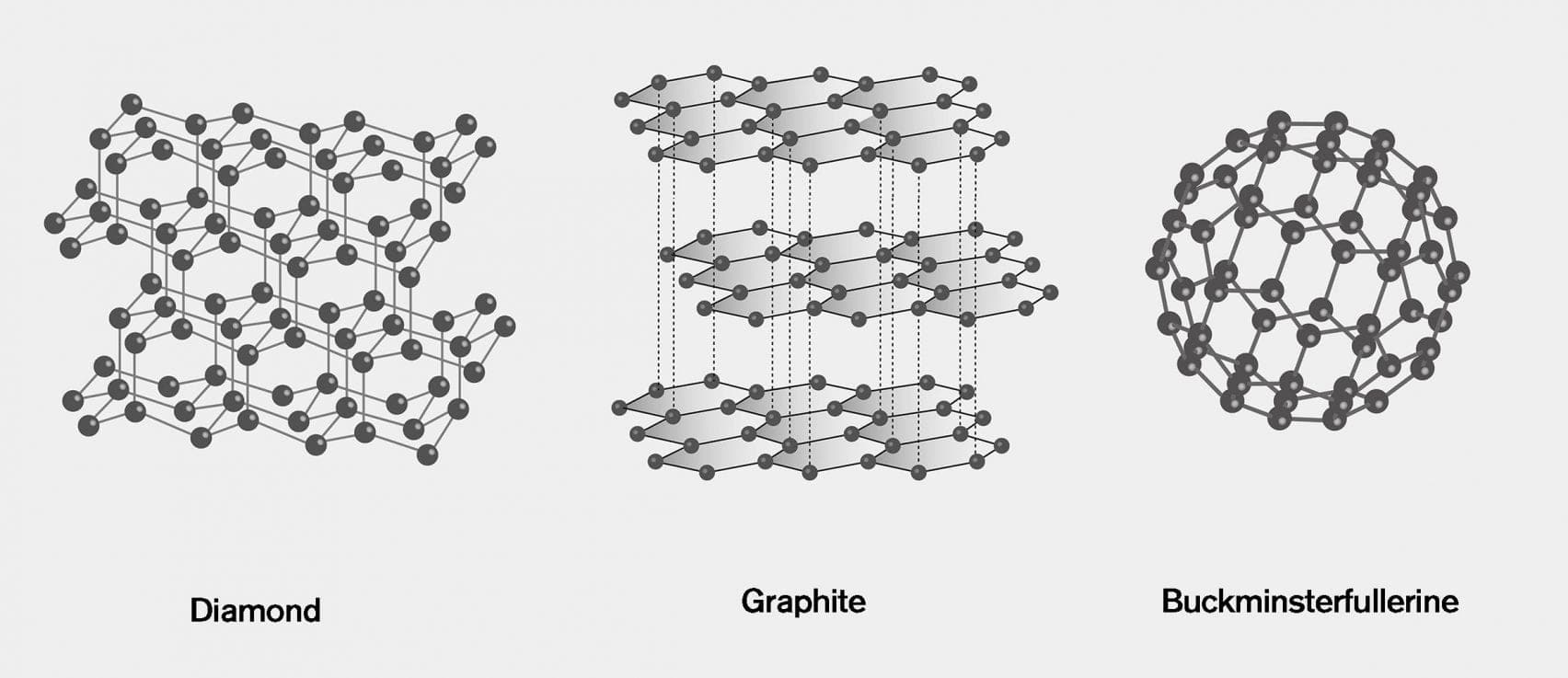
Diamond: In a diamond, every carbon atom is bonded to four other carbon atoms, giving rise to a rigid three-dimensional structure.
Graphite: In graphite, every carbon atom is bonded to three other carbon atoms in the same plane, giving rise to a hexagonal array.
C-60 Buckminsterfullerene: In C-60 Buckminsterfullerene, carbon atoms are arranged in the shape of a football.
Versatility Nature of Carbon
1. Carbon has the property of catenation, that is, it can bond with carbon atoms, giving rise to a large molecule.
Carbon can bond with a single bond, a double bond, or a triple bond.
2. Carbon forms strong bonds with elements, and because of its small size, the nucleus can hold the shared pair of electrons. Carbon forms bonds with many elements, such as oxygen, hydrogen, nitrogen, sulphur, chlorine, etc, thus forming compounds with specific properties.
Saturated and Unsaturated Carbon Compounds
This section explains the difference between saturated and unsaturated carbon compounds with the help of examples and structures. You can also download Class 10 Science Carbon and its compounds notes PDF for revision and exam preparation.
Saturated Carbon Compounds
Carbon compounds that involve single bonds are called saturated carbon compounds.
Unsaturated Carbon Compounds
Carbon compounds that involve double or triple bonds are called unsaturated carbon compounds.
Naming of Saturated Carbon Compounds based on the number of Carbon atoms
|
Number of Carbon Atoms |
Name |
|
1 |
Methane |
|
2 |
Ethane |
|
3 |
Propane |
|
4 |
Butane |
|
5 |
Pentane |
|
6 |
Hexane |
The functional group in Carbon Compounds
|
Hetero Atom |
Class of Compounds |
Formula |
|
Cl/Br |
Halo-(chloro, bromo)alkane |
-Cl, -Br |
|
Oxygen |
Alcohol |
-OH |
|
Aldehyde |
-CHO | |
|
Ketone |
-CO | |
|
Carboxylic acid |
-COOH |
Chains, Branches, and Rings
Carbon Chains: Carbon atoms can form long chains by bonding with other carbon atoms. These can be straight or branched chains.
Example: Straight chain: Butane $\left(\mathrm{C}_4 \mathrm{H}_{10}\right) \rightarrow \mathrm{CH}_3-\mathrm{CH}_2-\mathrm{CH}_2-\mathrm{CH}_3$
Branched Chains: Chains where one or more carbon atoms branch off from the main chain.
Example: Isobutane $\rightarrow \mathrm{CH}_3-\mathrm{CH}\left(\mathrm{CH}_3\right)-\mathrm{CH}_3$
Ring Compounds (Cyclic Compounds): Carbon atoms can also form closed rings. These can be saturated (single bonds) or unsaturated (double or triple bonds).
Example: Cyclohexane ($\mathrm{C}_6 \mathrm{H}_{12}$), Benzene ($\mathrm{C}_6 \mathrm{H}_6$)
Homologous Series
A series of compounds in which the same functional group substitutes for hydrogen in a carbon chain is called a homologous series.
Examples:
Alkanes: $\mathrm{CH}_4, \mathrm{C}_2 \mathrm{H}_6, \mathrm{C}_3 \mathrm{H}_8$, ......
Alcohols: $\mathrm{CH}_3 \mathrm{OH}, \mathrm{C}_2 \mathrm{H}_5 \mathrm{OH}, \mathrm{C}_3 \mathrm{H}_7 \mathrm{OH}$..
Nomenclature of Carbon Compounds
-
Find the number of carbon atoms in the compound, and based on the number of carbon atoms, name the compound as given above. For example, a compound with 4 carbon atoms would have the name butane.
-
If a functional group is present, the name of the compound is given based on the functional group with either a prefix or a suffix.
-
If the name of the functional group is to be given as a suffix, and if the suffix of the functional group starts with a vowel a, e, i, o, u, then the name of the carbon chain is modified by replacing the final ‘e’ with the appropriate suffix. For example, a 4-carbon chain with a ketone group would be named Butane – ‘e’ = Butan + ‘one’ = Butanone.
Chemical Properties of Carbon Compounds
This part discusses the important chemical properties of carbon compounds, such as combustion, oxidation, addition, and substitution reactions. To strengthen your understanding and practice problems, you can also refer to the NCERT Solutions for Class 10 Science Chapter 4.
Combustion
Carbon reacts with oxygen, giving out carbon dioxide, heat and light.
$\begin{aligned} & \mathrm{C}+\mathrm{O}_2 \rightarrow \mathrm{CO}_ 2+\text { heat and light } \\ & \mathrm{CH}_4+\mathrm{O}_2 \rightarrow \mathrm{CO}_2+\mathrm{H}_2 \mathrm{O}+\text { heat and light } \\ & \mathrm{CH}_3 \mathrm{CH}_2 \mathrm{OH}+\mathrm{O}_2 \rightarrow \mathrm{CO}_2+\mathrm{H}_2 \mathrm{O}+\text { heat and light }\end{aligned}$
Why do substances burn with a flame?
Substances burn with a flame because a flame is produced when gaseous substances burn and glow. For instance, on ignition of wood or charcoal, the volatile substances present in them vaporise and burn with a flame. Each element produces its own characteristic colour.
Formation of coal and petroleum
Coal has been formed from remains of trees and plants that were subjected to various biological and geological processes millions of years ago, wherein they were crushed and buried down the earth layer by layer by earthquakes or volcanoes.
Petroleum has been formed from remains of plants and animals in the sea, which have been attacked by bacteria under high pressure and converted into oil and gas, which are trapped between the rocks.
Oxidation
When oxygen is added to the compound, the reaction is called an oxidation reaction. It is done in the presence of an oxidising agent.
$\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{OH} \xrightarrow[\text { OrAcidified }_2 \mathrm{Cr}_2 \mathrm{O}_7]{\text { AlkalinKMn }_4+\mathrm{Heat}} \mathrm{CH}_3 \mathrm{COOH}$
Addition Reaction
When hydrogen is added to an unsaturated compound in the presence of a catalyst such as palladium or nickel, giving rise to saturated hydrocarbons, the reaction is called an addition reaction.
Substitution Reaction
When an atom or group of atoms is replaced by another atom, the reaction is called a substitution reaction.
Some Important Carbon Compounds - Ethanol And Ethanoic Acid
Ethanol
Ethanol is commonly called alcohol, is a good solvent that is soluble in water and is used as a medicine to treat cough.
Reactions of Ethanol:
(Reaction with sodium)
$2Na + 2CH_{3}CH_{2}OH\rightarrow 2CH_{3}CH_{2}O-Na^{+} +H_{2}$
(Reaction to give unsaturated hydrocarbon)
$\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{OH} \xrightarrow[Heat]{Conc.\mathrm{H}_2\mathrm{SO}_4} \mathrm{CH}_2=\mathrm{CH}_2+\mathrm{H}_2 \mathrm{O}$
Denatured Alcohol: To prevent the misuse or wrong use of ethanol produced for industrial use, it is made unfit for use that is for drinking by adding poisonous substances like methanol to it and also dyes to give it a fake colour. This alcohol is called denatured alcohol.
Ethanoic Acid
Ethanoic Acid is colloquially called acetic acid; its 5-8% solution is called vinegar and is used widely for cooking purposes. Pure ethanoic acid is called glacial acetic acid.
Reactions of ethanoic acid:
(Esterification reaction)
$\mathrm{CH}_3 \mathrm{COOH}+\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{OH} \rightarrow \mathrm{CH}_3 \mathrm{COOCH}_2 \mathrm{CH}_3+\mathrm{H}_2 \mathrm{O}$
(Reaction with a base)
$\mathrm{NaOH}+\mathrm{CH}_3 \mathrm{COOH} \rightarrow \mathrm{CH}_3 \mathrm{COONa}+\mathrm{H}_2 \mathrm{O}$
(Reaction with carbonates and hydrogen carbonates)
$\begin{aligned} & 2 \mathrm{CH}_3 \mathrm{COOH}+\mathrm{Na}_2 \mathrm{CO}_3 \rightarrow 2 \mathrm{CH}_3 \mathrm{COONa}+\mathrm{H}_2 \mathrm{O}+\mathrm{CO}_2 \\ & \mathrm{CH}_3 \mathrm{COOH}+\mathrm{NaHCO}_3 \rightarrow \mathrm{CH}_3 \mathrm{COONa}+\mathrm{H}_2 \mathrm{O}+\mathrm{CO}_2\end{aligned}$
Soaps and Detergents
Soaps are the long-chain carboxylic acids of sodium or potassium. The ionic end of soap, called the head, interacts with water, and the carbon chain, called the tail, interacts with oil.
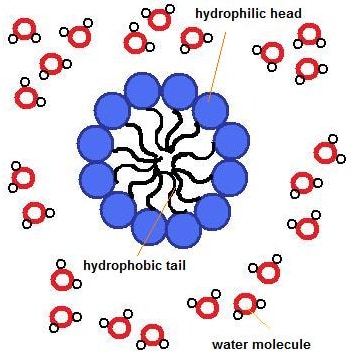
In hard water, soaps form scum with water. Hence, detergents are used to overcome this problem, as detergents are sodium salts of sulfonic acids or ammonium salts with chlorides or bromide ions. Their charged ends do not form scum and thus are used for cleansing purposes.
Carbon and Its Compounds: Previous Years' Questions and Answers
Below are a few previous year questions from carbon and its compounds to help students understand the important topics and the type of questions asked in exams. These solved questions strengthen conceptual understanding and improve problem-solving abilities, making them very helpful for CBSE Board exam preparation. Also refer to the carbon and its compounds class 10 science chapter 4 CBSE notes for understanding the concepts used to solve questions.
Question 1. $CH_{3}-CH_2-OH\xrightarrow[Heat]{Alkaline KMnO_{4}}CH_{3}-COOH$
In the above given reaction, alkaline $KMnO_4$ acts as
(1) reducing agent
(2) oxidizing agent
(3) catalyst
(4) dehydrating agent
Answer:
Here, ethanol is converted to ethanoic acid.
$KMnO_4$ acts as an oxidising agent as it removes hydrogen from $CH_3CH_2OH$ and adds one oxygen to it.
Hence, the correct answer is option (2).
Question 2. Chlorine reacts with saturated hydrocarbons at room temperature in the
(1) absence of sunlight
(2) presence of sunlight
(3) presence of water
(4) presence of hydrochloric acid
Answer:
Chlorine reacts with saturated hydrocarbons at room temperature in the presence of sunlight,
$CH_{4}+Cl_{2}\overset{sunlight}{\rightarrow}CH_{3}Cl+HCl$
$CH_3Cl+Cl_{2}\overset{sunlight}{\rightarrow}CH_{2}Cl_{2}+HCl$
$CH_2Cl_{2}+Cl_{2}\overset{sunlight}{\rightarrow}CHCl_{3}+HCl$
$CHCl_{3}+Cl_{2}\overset{sunlight}{\rightarrow}CCl_{4}+HCl$
Hence, the correct answer is option (2).
Question 3. Which member of the homologous series of alkynes corresponds to $\mathrm{C}_3 \mathrm{H}_4$?
(1) $\mathrm{C}_6 \mathrm{H}_6$
(2) $\mathrm{C}_2 \mathrm{H}_4$
(3) $\mathrm{C}_2 \mathrm{H}_6$
(4) $\mathrm{C}_3 \mathrm{H}_4$
Answer:
The general formula of alkynes is $\mathrm{C}_{\mathrm{n}} \mathrm{H}_{2 \mathrm{n}-2}$.
When n = 3, it gives $\mathrm{C}_3 \mathrm{H}_4$.
Hence, the correct answer is option (4).
Question 4: Which of the following is allotrope of carbon?
(1) Diamond
(2) Graphite
(3) Lonsdaleite
(4) All of them
Answer:
All of them are correct.
Allotropes of carbon:
a) Diamond
b) Graphite
c) Lonsdaleite
Hence, the answer is the option (4).
Question 5: Which of following prefix is used for ester ?
(1) Alkoxy
(2) Carboxylate
(3) Both (1) and (2)
(4) None of these
Answer:

Hence, the answer is the option (4).
How to Master Class 10 Science Chapter 4: Carbon and its Compounds
NCERT Class 10 Science Chapter 4 Notes Carbon and its Compounds help to understand the structure, bonding, properties, and reactions of carbon compounds. Given below are some points on how to master this chapter.
- First, understand the unique properties of carbons like tetravalency of carbon, catenation, and the ability of carbon to form single, double, and triple bonds.
- Then learn how compounds of carbon are classified as saturated and unsaturated hydrocarbons.
- Study functional groups and their significance in determining chemical behaviour, then read about alcohols, carboxylic acids, esters, and their general formulas, properties, and reactions.
- Questions related to combustion, oxidation, substitution, addition, and esterification reactions are often asked in exams. They can refer to Carbon and its Compounds Class 10 Science notes for understanding these concepts better.
- Also study soaps, detergents, and saponification and learn about esters and their use in perfumes and flavourings.
- Lastly, students must solve questions.
Advantages of Using Class 10 Science Chapter 4 Carbon and Its Compounds Notes
NCERT Class 10 Science Chapter 4 Carbon and its Compounds Notes helps students to understand the nature of carbon and its compounds. Given below some points on the advantages of these notes:
- Students can use these notes to understand the topics like covalent bonding, homologous series, functional groups, nomenclature, chemical properties of ethanol, ethanoic acid, soaps, and detergents.
- These notes are prepared by subject experts in a very clear and comprehensive manner that help students to understand how different organic compounds are formed and react.
- Every topic of NCERT book is covered in these Carbon and its Compounds Class 10 Science notes.
- They provide solved examples and chemical equations that are helpful in both CBSE board and competitive exams.
NCERT notes for Class 10 Science Chapter-wise
In addition to the class 10 science carbon and its compounds notes, students can refer to the NCERT notes of other Class 10 chapters provided below.
NCERT Solutions for Class 10 Science Chapter-Wise
Besides ncert class 10 science chapter 4 carbon and its compounds notes, students can also follow Class 10 chapter-wise solutions of NCERT:
NCERT Class 10 Exemplar Solutions
Class 10 NCERT exemplar subject-wise solutions are given below:
Frequently Asked Questions (FAQs)
Carbon compounds are essential to life and are found in many everyday materials. For example, hydrocarbons are the primary components of fuels like petrol and diesel. Organic compounds like sugars, proteins, and fats are crucial for biological processes.
Carbon compounds, primarily organic compounds, usually involve covalent bonding where electrons are shared between atoms. In contrast, ionic compounds involve the transfer of electrons between atoms, resulting in the formation of charged ions. This fundamental difference affects their properties; carbon compounds tend to have lower melting and boiling points, are generally not soluble in water.
Functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. They play a vital role in determining the properties and reactivity of organic compounds.
Hydrocarbons are organic compounds that consist solely of hydrogen and carbon atoms. They are classified into two main categories: aliphatic hydrocarbons and aromatic hydrocarbons.
Carbon is special because of its unique ability to form strong covalent bonds with other carbon atoms and with various elements, creating a vast array of compounds.
This chapter covers questions on the structure and bonding of carbon, catenation, and types of carbon compounds like alkanes, alkenes, alkynes, alcohols, carboxylic acids, and esters. It includes chemical reactions such as combustion, oxidation, substitution, addition, and esterification, as well as practical applications like soaps, detergents, and uses of carbon compounds. Students may also be asked to write chemical equations, draw structural formulas, and compare properties of different compounds.
In Class 10 Science, carbon is an element known for its tetravalency and ability to form long chains. Carbon compounds are organic compounds containing carbon atoms, including hydrocarbons, alcohols, acids, esters, soaps, and detergents, with distinct properties and chemical reactions. These compounds form the basis of organic chemistry and have wide practical applications.
Class 10 Science revision notes are concise summaries of important concepts, formulas, reactions, and diagrams from Physics, Chemistry, and Biology. They help students quickly revise key topics, understand essential points, and practice important questions before exams.
- Diamond: In diamond, every carbon atom is bonded to four other carbon atoms, giving rise to a rigid three-dimensional structure.
- Graphite: In graphite, every carbon atom is bonded to three other carbon atoms in the same plane, giving rise to a hexagonal array.
- C-60 Buckminsterfullerene: In C-60 Buckminsterfullerene, carbon atoms are arranged in the shape of a football.
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
