NCERT Class 10 Science Chapter 3 Notes Metals And Non-Metals: Download PDF Notes
Do you know how iron rusts, what makes Gold and silver perfect metals for jewellery, and why metals conduct electricity and Non-metals do not? The answer to all such interesting questions lies in Metals and Non-metals. From the air we breathe to the iron and steel used in bridges and buildings, they make up everything around us. Approximately 91 out of 118 known elements are metals, and the rest are nonmetals and metalloids.
This Story also Contains
- NCERT Notes for Class 10 Chapter 3: Download PDF
- NCERT Notes for Class 10 Chapter 3
- Metals and Non-metals: Previous Year Question and Answer
- How to Master Class 10 Science Chapter 3 Metals And Non-Metals
- Advantages of Using Class 10 Science Chapter 3 Metals and Non-Metals Notes
- NCERT Notes for Class 10 Science Chapter-wise
- NCERT Solutions for Class 10 Science Chapter-Wise
- NCERT Class 10 Exemplar Solutions

Metals are known for their strength, malleability and conductivity, while non-metals are known for their role in life processes and the environment. The NCERT Class 10 Science notes will provide a precise and structured revision of the entire chapter that will eliminate the need for students to search for additional resources. In the NCERT Class 10 notes, we have provided a step-by-step analysis of the topic by including diagrams and reactions that will help you get clarity on the topics and make the revision effective. Students can also refer to the NCERT Solutions for better practice and understanding.
NCERT Notes for Class 10 Chapter 3: Download PDF
You can download the Metals and Non-Metals Class 10 Science notes PDF to access a clear explanation of all the important concepts from the Download PDF icon given below. These notes will help in quick revision and better preparation for exams.
NCERT Notes for Class 10 Chapter 3
NCERT Class 10 Science Chapter 3 Notes Metals and Non-Metals covers the physical and chemical properties of metals and non-metals, their reactions and a wide range of applications. The NCERT notes are a key guide for students preparing for boards. Students can use these notes for quick revision of the concepts that will, in turn, help them to solve the questions effectively.
3.1 Physical Properties
Physical properties are those characteristics of a substance that can be observed or measured without a change in its chemical composition. These properties help identify and describe matter.
3.1.1 Metals
Metals are elements that are generally hard, shiny, malleable, ductile, and good conductors of heat and electricity. Metals tend to lose electrons to form positive ions in chemical reactions.
Lustre: Metals are shining in nature.
Hardness: hard in nature. But an exception exists for Na, Li, and K; they are soft and can be cut with a knife.
State: Metals exist in a solid state except in the case of mercury, which is liquid at room temperature.
Malleability: the property of metal through which we can make sheets of metal with the use of a hammer. The most malleable metals are gold and silver.
Ductility: The property of metal through which we can make wire.
Conductivity and heating: Metals are conductive in nature and have a good capacity for heating. For example, copper and silver are the best conductors, whereas lead and mercury are the worst conductors.
Density: The density of metals is high, as are their melting points.
Sonorous: Metals are sound-producing in nature when they strike a hard surface.
Oxides: The oxides of metals are basic in nature.
3.1.2 Non-metals
Non-metals are elements that are generally brittle, dull and poor conductors of heat and electricity. They tend to gain electrons during chemical reactions and are usually found in solid, liquid or gaseous states at room temperature.
Lustre: Non-metals are not shining in nature, except in the case of iodine.
Hardness: Soft in nature. But an exception exists for a diamond, which is the hardest among all
State: Metals exist in a solid or gaseous state, except in the case of bromine, which is liquid at room temperature.
Malleability: They are non-malleable in nature.
Ductility: They are also non-ductile in nature, as we won’t be able to make wire from non-metals.
Conductivity and heating: Non-metals are insulators, as they don’t have a good capacity for heating. Exception in case of Graphite.
Density: The densities of non-metals are low, as are their melting points.
Sonorous: They do not produce any sound when struck on the surface.
Oxides: The oxides of non-metals are acidic in nature.
3.2 Chemical Properties of Metals
Metals exhibit characteristic chemical behaviours such as reacting with air, water, acids, and other substances to form new compounds. These reactions often involve the loss of electrons and the formation of ions.
3.2.1 What happens when Metals are burnt in Air?
When metals are burnt in air, they react with oxygen to form metal oxides. These oxides are usually basic in nature and show different colours depending on the metal. To strengthen your understanding and practice more problems, you can also refer to the NCERT Solutions for Class 10 Science Chapter 3.
Reaction of metals with air:
Metal $+\mathrm{O}_2 \longrightarrow$ Metal oxide
$2 \mathrm{Cu}+3 \mathrm{O}_2 \longrightarrow 2 \mathrm{CuO}$
Points to remember:
-
The reaction of metals, especially with oxygen, depends on the reactivity of the metal, as mentioned in the list of reactivity orders.
-
Sodium and potassium are kept inside kerosene as they catch fire when exposed to the air and react vigorously with it.
-
For further oxidation of magnesium, aluminium, and zinc, they are covered with a thin layer of oxide so that they can be prevented from further oxidation.
-
Iron filings burn vigorously in nature, but Fe does not.
-
The coating is done on copper with black copper oxide, but still, it does not burn.
-
The reaction of oxygen can not proceed with silver and gold.
The reaction of metals with amphoteric oxides: These are the oxides that react with both acids and bases to produce salts and water, and are termed amphoteric oxides.
Amphoteric Oxides
$\mathrm{Al}_2 \mathrm{O}_3+6 \mathrm{HCl} \longrightarrow 2 \mathrm{AlCl}_3+\mathrm{H}_2 \mathrm{O}$
$\mathrm{Al}_2 \mathrm{O}_3+2 \mathrm{NaOH} \rightarrow 2 \mathrm{NaAlO}_2+\mathrm{H}_2 \mathrm{O}$
Most metal oxides are insoluble in water, but some of these dissolve in water to form alkalis. Sodium oxide and potassium oxide dissolve in water to produce alkalis as follows
$\mathrm{Na}_2 \mathrm{O}(\mathrm{s})+\mathrm{H}_2 \mathrm{O}(\mathrm{l}) \rightarrow 2 \mathrm{NaOH}(\mathrm{aq})$
$\mathrm{K}_2 \mathrm{O}(\mathrm{s})+\mathrm{H}_2 \mathrm{O}(\mathrm{l}) \rightarrow 2 \mathrm{KOH}(\mathrm{aq})$
3.2.2 What happens when Metals react with Water?
Metal and water react with each other to form metal oxide and hydrogen gas. Further, Metal oxide reacts with water to produce metal hydroxide.
Metal + Water $\rightarrow$ Metal oxide + Hydrogen
Metal oxide + Water $\rightarrow$ Metal hydroxide
Metals like potassium and sodium react violently with cold water. In the case of sodium and potassium, the reaction is so violent and exothermic that the evolved hydrogen immediately catches fire.
$\begin{aligned} & 2 \mathrm{~K}(\mathrm{~s})+2 \mathrm{H}_2 \mathrm{O}(\mathrm{l}) \rightarrow 2 \mathrm{KOH}(\mathrm{aq})+\mathrm{H}_2(\mathrm{~g})+\text { heat energy } \\ & 2 \mathrm{Na}(\mathrm{s})+2 \mathrm{H}_2 \mathrm{O}(\mathrm{l}) \rightarrow 2 \mathrm{NaOH}(\mathrm{aq})+\mathrm{H}_2(\mathrm{~g})+\text { heat energy }\end{aligned}$
The reaction of calcium with water is less violent. The heat evolved is not sufficient for the hydrogen to catch fire.
$\mathrm{Ca}(\mathrm{s})+2 \mathrm{H}_2 \mathrm{O}(\mathrm{l}) \rightarrow \mathrm{Ca}(\mathrm{OH})_2(\mathrm{aq})+\mathrm{H}_2(\mathrm{~g})$
Metals like aluminium, iron and zinc do not react with either cold or hot water. But they react with steam to form the metal oxide and hydrogen
$\begin{aligned} & 2 \mathrm{Al}(\mathrm{s})+3 \mathrm{H}_2 \mathrm{O}(\mathrm{g}) \rightarrow \mathrm{Al}_2 \mathrm{O}_3(\mathrm{~s})+3 \mathrm{H}_2(\mathrm{~g}) \\ & 3 \mathrm{Fe}(\mathrm{s})+4 \mathrm{H}_2 \mathrm{O}(\mathrm{g}) \rightarrow \mathrm{Fe}_3 \mathrm{O}_4(\mathrm{~s})+4 \mathrm{H}_2(\mathrm{~g})\end{aligned}$
Metals such as lead, copper, silver and gold do not react with water at all. You can also download Class 10 Science Metals and Non-Metals notes PDF for revision and exam preparation.
3.2.3 What happens when Metals react with Acids?
When metals react with acids, they produce a salt and hydrogen gas. The rate of reaction depends on the metal’s reactivity.
In the case of copper, silver, and mercury, they do not react with dilute acids.
$\mathrm{Fe}+2 \mathrm{HCl} \longrightarrow \mathrm{FeCl}_2+\mathrm{H}_2$
3.2.4 How do Metals react with Solutions of other Metal Salts?
Let us suppose that metal A reacts with another salt solution B to produce a salt solution of metal A and metal B.
The displacement of elements depends upon the reactivity series of metals; the more reactive the metal, the more it displaces the less reactive one.
$\mathrm{Fe}+\mathrm{CuSO}_4 \longrightarrow \mathrm{FeSO}_4+\mathrm{Cu}$
3.2.5 The Reactivity Series
The reactivity series is a list of metals arranged in the order of their decreasing reactivity. After performing displacement experiments, the reactivity or activity series has been developed.
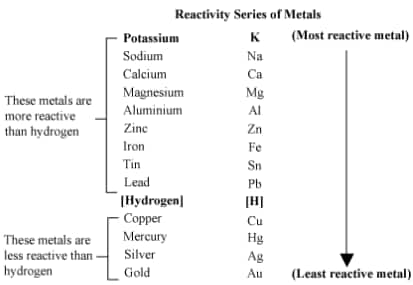
3.3 How do Metals and Non-metals React?
Metals and non-metals react through ionic bonding, where metals lose electrons to form positively charged ions and non-metals gain electrons to form negatively charged ions. These oppositely charged ions attract each other to form ionic compounds, such as sodium chloride. When an electron comes out of the shell, it forms the cation, which is a property of metal elements; on the other hand, when an electron is gained by a valence shell, it forms the anion, which is a property of the non-metal element.
Electronic configuration of some elements
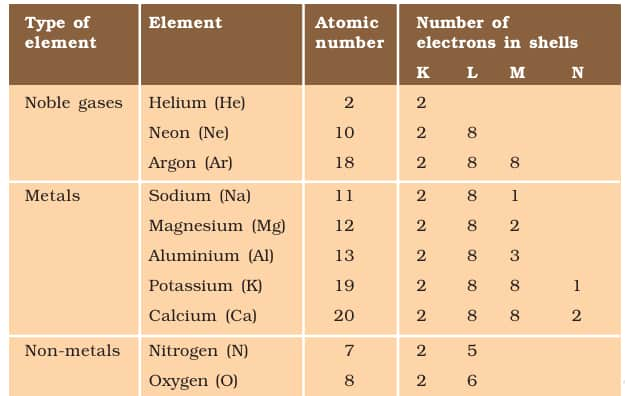
Let us see the formation of one ionic compound, magnesium chloride
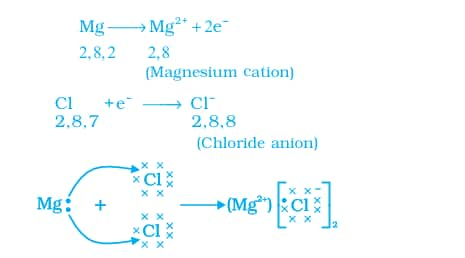
3.3.1 Properties of Ionic Compounds
The formation of an ionic compound is done by the transfer of electrons. Electrons from metals transfer to non-metals and are called ionic compounds, also known as electrovalent compounds.
Properties:
-
Physical nature of ionic compounds: They are brittle, solid, and hard compounds.
-
Melting and boiling points: Ionic compounds have high melting and boiling points.
-
Solubility: Ionic compounds are insoluble in a solution of kerosene, petrol, etc., whereas they are soluble in water.
-
The conductivity of ionic compounds: ionic compounds are conductive in the molten state but not in the solid state.
3.4 Occurrence of Metals
Metals occur in nature either in their free state or as compounds in ores. Their occurrence depends on their reactivity.
-
Minerals: Elements or compounds that are found in nature or in the earth’s crust naturally are termed minerals.
-
Ores: Those minerals that contain a very high percentage of a particular metal, and those metals that can also be extracted from that mineral, are called ores.
3.4.1 Extraction of Metals
A metal is extracted from its ore. Some metals are found in the Earth’s crust in the free state. Some are found in the form of their compounds. The metals at the bottom of the activity series are the least reactive. They are often found in a free state.
For example
- Gold, silver, platinum and copper are found in the free state.
- Copper and silver are also found in the combined state as their sulphide or oxide ores.
- The metals at the top of the activity series (K, Na, Ca, Mg and Al) are so reactive that they are never found in nature as free elements.
- The metals in the middle of the activity series (Zn, Fe, Pb, etc.) are moderately reactive.

Steps involved in the extraction of metals from ores:
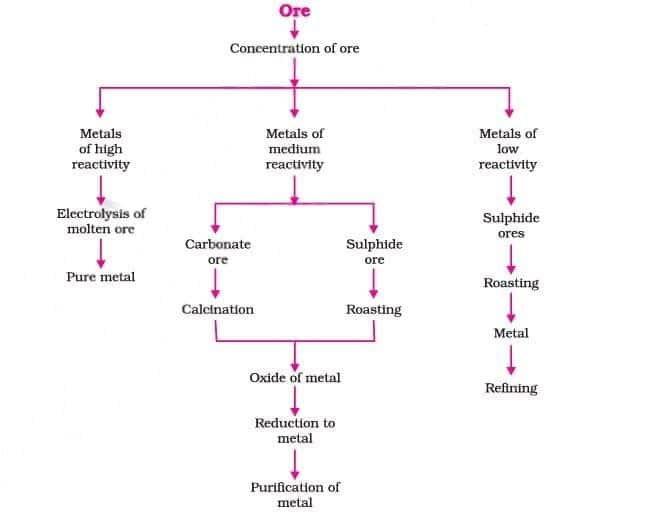
3.4.2 Enrichment of Ores
Ores mined from the earth are usually contaminated with large amounts of impurities such as soil, sand, etc., called gangue. The impurities must be removed from the ore prior to the extraction of the metal. The processes used for removing the gangue from the ore are based on the differences between the physical or chemical properties of the gangue and the ore.
Different separation techniques are accordingly employed.
3.4.3 Extracting Metals Low in the Activity Series
Metals low in the activity series are very unreactive. The oxides of these metals can be reduced to metals by heating alone.
For example, cinnabar (HgS) is an ore of mercury. When it is heated in air, it is first converted into mercuric oxide (HgO). Mercuric oxide is then reduced to mercury on further heating.
$2 \mathrm{HgS}(\mathrm{s})+3 \mathrm{O}_2(\mathrm{~g}) \xrightarrow{\text { Heat }} 2 \mathrm{HgO}(\mathrm{s})+2 \mathrm{SO}_2(\mathrm{~g})$
$2 \mathrm{HgO}(\mathrm{s}) \xrightarrow{\text { Heat }} 2 \mathrm{Hg}(\mathrm{l})+\mathrm{O}_2(\mathrm{~g})$
Copper, which is found as Cu2S in nature, can be obtained from its ore by just heating it in air.
$\begin{aligned} & 2 \mathrm{Cu}_2 \mathrm{~S}+3 \mathrm{O}_2(\mathrm{~g}) \xrightarrow{\text { Heat }} 2 \mathrm{Cu}_2 \mathrm{O}(\mathrm{s})+2 \mathrm{SO}_2(\mathrm{~g}) \\ & 2 \mathrm{Cu}_2 \mathrm{O}+\mathrm{Cu}_2 \mathrm{~S} \xrightarrow{\text { Heat }} 6 \mathrm{Cu}(\mathrm{s})+\mathrm{SO}_2(\mathrm{~g})\end{aligned}$
3.4.4 Extracting Metals in the Middle of the Activity Series
Metals like iron, zinc, lead, and copper are moderately reactive and usually found as sulfides or carbonates. Since it is easier to extract metals from their oxides, these ores are first converted into oxides.
The sulphide ores are converted into oxides by heating strongly in the presence of excess air. This process is known as roasting. The carbonate ores are changed into oxides by heating strongly in limited air. This process is known as calcination.
Roasting
$2 \mathrm{ZnS}(\mathrm{s})+3 \mathrm{O}_2(\mathrm{~g}) \xrightarrow{\text { Heat }} 2 \mathrm{ZnO}(\mathrm{s})+2 \mathrm{SO}_2(\mathrm{~g})$
Calcination
$\mathrm{ZnCO}_3(\mathrm{~s}) \xrightarrow{\text { Heat }} \mathrm{ZnO}(\mathrm{s})+\mathrm{CO}_2(\mathrm{~g})$
The metal oxides are then reduced to the corresponding metals by using suitable reducing agents such as carbon. For example, when zinc oxide is heated with carbon, it is reduced to metallic zinc.
$\mathrm{ZnO}(\mathrm{s})+\mathrm{C}(\mathrm{s}) \rightarrow \mathrm{Zn}(\mathrm{s})+\mathrm{CO}(\mathrm{g})$
Besides using carbon to reduce metal oxides to metals, sometimes displacement reactions can also be used. The highly reactive metals such as sodium, calcium, aluminium, etc., are used as reducing agents because they can displace metals of lower reactivity from their compounds. For example, when manganese dioxide is heated with aluminium powder, the following reaction takes place:
$3 \mathrm{MnO}_2(\mathrm{~s})+4 \mathrm{Al}(\mathrm{s}) \rightarrow 3 \mathrm{Mn}(\mathrm{l})+2 \mathrm{Al}_2 \mathrm{O}_3(\mathrm{~s})+$ Heat
3.4.5 Extracting Metals towards the Top of the Activity Series
Highly reactive metals like sodium, magnesium, calcium and aluminium cannot be extracted by carbon reduction due to their strong affinity for oxygen. They are obtained by electrolytic reduction of their molten chlorides.
At cathode $\mathrm{Na}^{+}+\mathrm{e}^{-} \rightarrow \mathrm{Na}$
At anode $\quad 2 \mathrm{Cl}^{-} \rightarrow \mathrm{Cl}_2+2 \mathrm{e}^{-}$
3.4.6 Refining of Metals
Electrolytic refining is the most extensively used method for refining.
This method is used for impure metals and refining metals.
On Anode: Impure Copper
On Cathode: Strip of pure Copper
On electrolyte: Solution of acidified Copper sulphate.
When current passes through the electrolyte, the metal that is impure on the anode will dissolve in the electrolyte.
At the cathode, the pure metal will be deposited in an equivalent amount.
The anode mud is settled down in the tank; these are insoluble impurities.
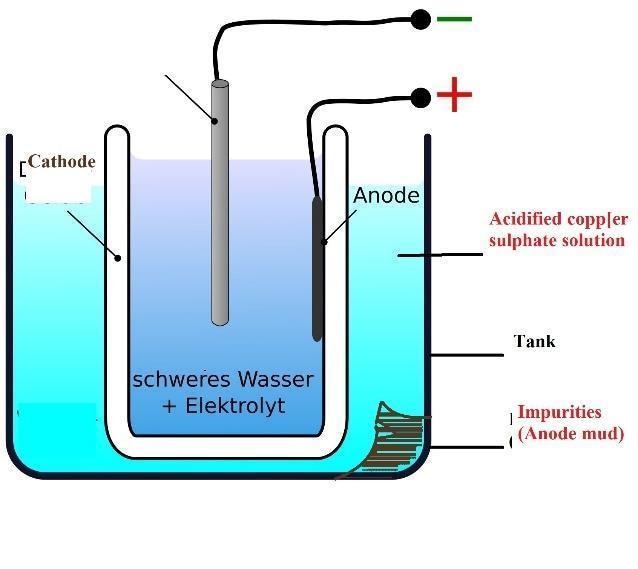
3.5 Corrosion
Corrosion can be defined as the process by which the surface of metals is exposed to moist air for a longer period of time, and the surface is corroded, and the phenomenon is termed corrosion.
Corrosion is usually an undesirable phenomenon since it negatively affects the desirable properties of the metal. For example, iron is known to have good tensile strength and rigidity. However, when subjected to rusting, iron objects become brittle, flaky, and structurally unsound. On the other hand, corrosion is a diffusion-controlled process, and it mostly occurs on exposed surfaces. Therefore, in some cases, attempts are made to reduce the activity of the exposed surface and increase a material’s corrosion resistance. Processes such as passivation and chromate conversion are used for this purpose. However, some corrosion mechanisms are not always visible, and they are even less predictable.
Examples: When silver is exposed to air, it reacts with the air to become black.
3.5.1 Prevention of Corrosion
The rusting of iron can be prevented by painting, oiling, greasing, galvanising, chrome plating, anodising or making alloys.
1. Painting - A coat of paint prevents direct contact of metal with air and moisture.
2. Oiling and Greasing - Lubricants form a protective layer that prevents moisture and air from reaching the metal surface.
3. Galvanisation - Galvanisation is a method of protecting steel and iron from rusting by coating them with a thin layer of zinc. The galvanised article is protected against rusting even if the zinc coating is broken.
4. Electroplating - A thin layer of a less reactive metal (like chromium or nickel) is deposited on a metal surface using electricity.
5. Alloying - Mixing metals with other elements to form an alloy that is more resistant to corrosion.
6. Anodising - Anodising aluminium forms a thick oxide layer that protects it from further oxidation.
7. Cathodic Protection - The metal to be protected is connected to a more reactive metal like magnesium, which corrodes instead.
Metals and Non-metals: Previous Year Question and Answer
Given below are some previous year questions from metals and non-metals that will help students get familiar with the exam pattern and key topics. These solved questions build conceptual clarity and enhance problem-solving skills. Also refer to the metals and non-metals class 10 science chapter 3 CBSE notes for understanding of concepts used to solve questions.
Question 1: Assertion (A): Brass is prepared by first melting copper and then dissolving tin into it in a definite proportion.
Reason (R): The primary metal of brass is copper.
(1) Both Assertion (A) and Reason (R) are true, and Reason (R) is the correct explanation of Assertion (A).
(2) Both Assertion (A) and Reason (R) are true, but Reason (R) is not the correct explanation of Assertion (A).
(3) Assertion (A) is true, but Reason (R) is false.
(4) Assertion (A) is false, but Reason (R) is true.
Answer :
According to the Assertion (A), copper is melted, and then tin is added. This turns metal into brass.
Reason (R) says that copper is the main metal in brass.
Brass is made of copper and zinc, not copper and tin. Copper and tin are mixed together to make bronze.
This shows that the assertion is false, and the reason that brass is mostly made of copper is true.
Hence, the correct answer is Option (4)
Question 2: Assertion (A): Alloys are commonly used in electrical heating devices like electric irons and heaters.
Reason (R): Resistivity of an alloy is generally higher than that of its constituent metals, but the alloys have lower melting points than their constituent metals.
(1) Both (A) and (R) are true, and (R) is a correct explanation of the assertion.
(2) Both (A) and (R) are true, but (R) is not the correct explanation of the assertion.
(3) (A) is true, but (R) is false.
(4) (A) is false but (R) is true.
Answer:
1. Alloys are commonly used in electrical heating devices like electric irons and heaters.
2. The resistivity of an alloy is generally higher than that of its constituent metals, but if the alloys have low melting points then their constituent metals are a false statement.
Therefore, A is true, but R is false.
Hence, the correct answer is option (3).
Question 3: Arrange the following elements as they are arranged in the reactivity series :
Aluminium, Calcium, Copper, Lead
Answer:
Reactivity Series in simplified order for the given elements:

Thus, the correct order is,
Calcium $(\mathrm{Ca})>$ Aluminum $(\mathrm{Al})>$ Lead $(\mathrm{Pb})>$ Copper $(\mathrm{Cu})$
Question 4: Which of the following is the correct arrange-ment of the given metals in descending order of their reactivity?
Zinc, Iron, Magnesium, Sodium
(1) Zinc > Iron > Magnesium > Sodium
(2) Sodium > Zinc > Magnesium > Iron
(3) Sodium > Magnesium > Iron > Zinc
(4) Sodium > Magnesium > Zinc > Iron
Answer:
The decreasing order of chemical reactivity is given below.
(most active) Sodium > Magnesium > Zinc > Iron (least active)
Hence, the answer is the option (4).
Question 5: Non-metals are not lustrous. Which of the following non-metal is lustrous?
(1) Nitrogen
(2) Sulphur
(3) Iodine
(4) Oxygen
Answer:
Shining metals are also called lustrous metal. For example, gold. Non-metals such as sulphur, oxygen, nitrogen are non-lustrous but iodine is a greyish black solid and crystals have a metallic lustre.
Hence , the answer is the option (3).
How to Master Class 10 Science Chapter 3 Metals And Non-Metals
To master this chapter, understand the physical and chemical properties, reactions, and uses. Students can refer to the class 10 science chapter 3 metals and non-metals notes for revision and understanding questions.
- First, learn key definitions, reactions, and important chemical equations such as reactions with oxygen, water, acids, and bases.
- Then understand the comparison between properties of metals and non-metals.
- Questions related to extraction of metals are often asked in exams, refer to metals and non-metals class 10 science chapter 3 CBSE notes for understanding these concepts better.
- Revise corrosion and its prevention methods like rusting of iron.
- Lastly, students must solve questions.
Advantages of Using Class 10 Science Chapter 3 Metals and Non-Metals Notes
NCERT Class 10 Science Chapter 3 Metals and Non-Metals Notes helps students to understand the physical and chemical properties of elements. Given below some points on the advantages of these notes:
- Students can use these notes to understand the topics like properties, reactions, uses of metals and non-metals, reactivity series, ionic bonding, corrosion, and metal extraction.
- These notes are prepared by subject experts in a very clear and comprehensive manner and they provide systematic explanations that help students understand how metals and non-metals react.
- The ncert class 10 science chapter 3 metals and non-metals notes contains examples and equations that help to understand chemical reactions and properties.
- These notes are beneficial for both CBSE boards and competitive exams as they help in revision of all NCERT topics.
NCERT Notes for Class 10 Science Chapter-wise
In addition to the ncert class 10 science chapter 3 metals and non-metals notes, students can refer to the NCERT notes of other Class 10 chapters provided below.
NCERT Solutions for Class 10 Science Chapter-Wise
Besides the class 10 science chapter 3 metals and non-metals notes, students can also follow the Class 10 chapter-wise solutions of NCERT:
NCERT Class 10 Exemplar Solutions
The links below will give you access to the NCERT exemplar solutions for other subjects. Follow them to ace your exam preparation.
Frequently Asked Questions (FAQs)
Metals typically possess properties such as luster , ductility, malleability, and high density. They also have high melting and boiling points and are generally good conductors of heat and electricity.
An alloy is a mixture of two or more elements, where at least one is a metal. Alloys possess enhanced properties compared to their constituent elements. Examples include steel, bronze , and brass . These alloys are often stronger, more durable, or resistant to corrosion.
Metallurgy is the science and technology concerned with the extraction of metals from their ores and the processing of metals to create useful materials. It involves techniques such as smelting, alloying, and refining.
Metals react with water in varying degrees, forming metal hydroxides and releasing hydrogen gas. For example, sodium and potassium react vigorously with water, while magnesium reacts with hot water.
To prepare for the Class 10 Science exam, study the NCERT textbook thoroughly, focus on understanding concepts, and make short notes for quick revision. Solve all NCERT exercises, exemplar problems, and previous year papers. Revise important formulas, definitions, and diagrams regularly, and practise sample papers to improve speed and accuracy.
Metals and Non-Metals Class 10 Science notes provide a concise summary of important concepts such as the physical and chemical properties of metals and non-metals, their reactions with oxygen, water, acids, and bases, the process of metallurgy, corrosion and its prevention, along with examples and key equations.
To prepare for Chapter 3 Metals and Non-Metals in Class 10, students should focus on learning the physical and chemical properties, important reactions with oxygen, water, acids, and bases, and the steps of metallurgy. Corrosion and its prevention methods, like galvanisation and alloying, must be revised with examples.
Metals are used in a wide variety of applications due to their strength and conductivity. Common uses include construction materials, electrical wiring, and manufacturing of machinery.
No, not all metals are solid at room temperature. While most metals, such as iron and gold, are solid, mercury is an exception it is a liquid at room temperature.
Metals are excellent conductors of heat and electricity due to the presence of free-moving electrons in their structure. At the same time, non-metals are poor conductors, often acting as insulators because they lack these free electrons.
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
