NCERT Class 10th Science Chapter 15 Our Environment Notes- Download PDF Notes
Did you know that the waste we throw away can harm our environment for years? The NCERT Class 10 Science Chapter 13 Our Environment Notes are prepared by subject experts in clear language, which are easy to read and a great resource for quick revision before exams. Our Environment chapter includes topics like food chains, food webs, energy flow, and types of waste. It also explains how human actions affect nature and the ozone layer. The NCERT notes make all topics simple and useful for board and competitive exam preparation.
This Story also Contains
- NCERT Class 10 Science Chapter 13 Our Environment Notes: Download PDF
- Class 10 Science Chapter 13 Our Environment Notes
- Chapter 13 Science Our Environment: Previous Year Question and Answers
- How to Use Our Environment Class 10 Notes Effectively?
- Advantages of Class 10 Science Chapter 13 Our Environment Notes
- Chapter-Wise NCERT Class 10 Notes Science

Our Environment Class 10 Notes introduces students to biotic (living) components and abiotic (non-living) components, and deals with the ozone layer and its vital role in protecting life on Earth from ultraviolet radiation. It also explains the importance of maintaining ecological balance and the role of humans in conserving natural resources. Questions from this chapter have been frequently asked in various exams. The NCERT Notes Class 10 Science cover all concepts in a less time, making it a valuable resource for students.
NCERT Class 10 Science Chapter 13 Our Environment Notes: Download PDF
Students can download the PDF from the link given below to revise all the concepts easily. The NCERT Class 10 Science Chapter 13 Notes PDF can be used both online and offline for quick revision. It covers topics like ecosystems, waste management, and how humans affect nature. The NCERT Notes for Class 10 provide clear explanations of important topics, making learning easier and effective for students.
Also Read:
Class 10 Science Chapter 13 Our Environment Notes
To help students with their studies, these notes are prepared in simple language. Our Environment chapter explains important topics like food chains, food webs, energy flow, biodegradable and non-biodegradable waste, and the impact of human activities. Our Environment Class 10 Notes explain all the important concepts easily included in the NCERT.
Biodegradable and Non-biodegradable Substances
Provided below are some of the key points to know the differences between the two different type of substances and their impact on the environment.
Biodegradable substances
- The substances that can be broken down by natural processes, such as the action of microorganisms, bacteria, and fungi.
- Food waste, paper products, wood, cotton fabric, and animal waste are some common examples of biodegradable substances.
- These materials are less harmful to the environment as they break down naturally.
Non-biodegradable Substances
- These substances are those that do not break down naturally or take an extremely long time to decompose.
- Plastic bags, bottles, metals, nylon, pesticides, and chemicals are some examples of non-biodegradable substances.
- These materials contribute to pollution and the degradation of ecosystems. They also harm wildlife and pollute the land and water bodies.
- Enzymes used in the degradation of substances have a unique effect, and certain enzymes are required for the breakdown of a given substrate. Different microorganisms, such as bacteria, fungi, and actinomycetes, are responsible for biodegradation.
- This waste requires recycling or proper waste management systems to prevent environmental damage.
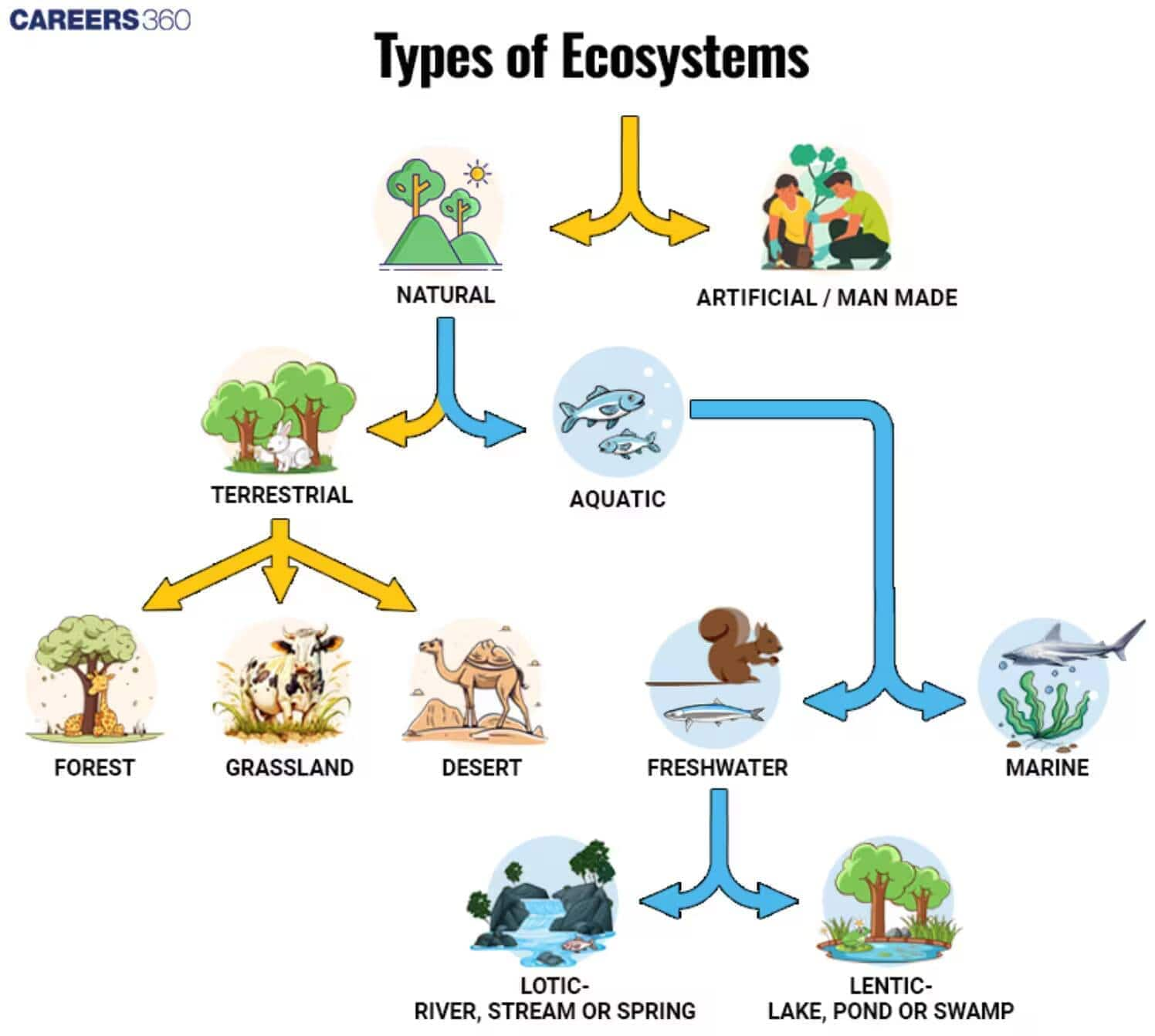
Ecosystem
- G. Tansley coined the term ecosystem, which, according to him, is the total of interactions between biotic and abiotic components capable of independent existence.
- Biotic components include plants, animals, and bacteria, and abiotic components include light, temperature, rainfall, wind, and soil.
- An ecosystem is a self-sufficient unit.
- It may be a very small example, a freshwater pond, or a very large example, the Sahara Desert.
- In an ecosystem, there is a cyclic exchange of materials between living beings and the environment. The only requirement is a constant input of energy.
- Desert grassland, forest, crop fields, etc., represent terrestrial ecosystems, whereas ponds, lakes, rivers, sea, etc., are aquatic ecosystems.
- Most of the ecosystems in the world are natural ones, but some of them are man-made ecosystems, such as aquariums, parks, etc.
Trophic Level
- Trophic means feeding, and hence, trophic levels are the levels or positions at which species feed.
- In an ecosystem, producers represent the first trophic level; herbivores, the second trophic level; primary carnivores, the third trophic level; and so on.
- Mainly, the organisms are classified as producers, consumers, or decomposers depending on how they receive the food from the environment.
Producers-
- Those organisms that produce food are called producers.
- The green plants are the ultimate producers in any ecosystem.
- They can prepare their food by using substances like carbon dioxide, water, and sunlight through the process of photosynthesis.
Consumers-
- Those organisms that consume the food prepared by producers are called consumers.
- All animals are consumers as they cannot prepare their food from simple inorganic substances.
- There are mainly three types of consumers:
Primary consumers- Animals that feed on plants are called primary consumers. Example - cow, buffalo, goat, etc.
Secondary consumers- Animals that eat the primary consumers are said to be secondary consumers. Example - Sparrow, crow, etc.
Tertiary consumers- Animals that feed on other carnivores are known as tertiary consumers. A wolf eating a fox is an example of a tertiary consumer.
Decomposers-
- The materials within the bodies of dead plants and animals are thus acted on by organisms of decay, which are known for the purpose of decomposition.
- Bacteria and fungi are well-known examples of decomposers.
Food Chains and Food Webs
Some key points of the food chain and food web are explained below, along with clear diagrams.
Food Chain:
- The sequence of living organisms in an ecosystem in which one organism consumes another organism to transfer food energy is called a food chain.
- A food chain is made up of a sequence of organisms that participate at distinct biotic levels.
- A trophic level is formed at each level of the food chain.
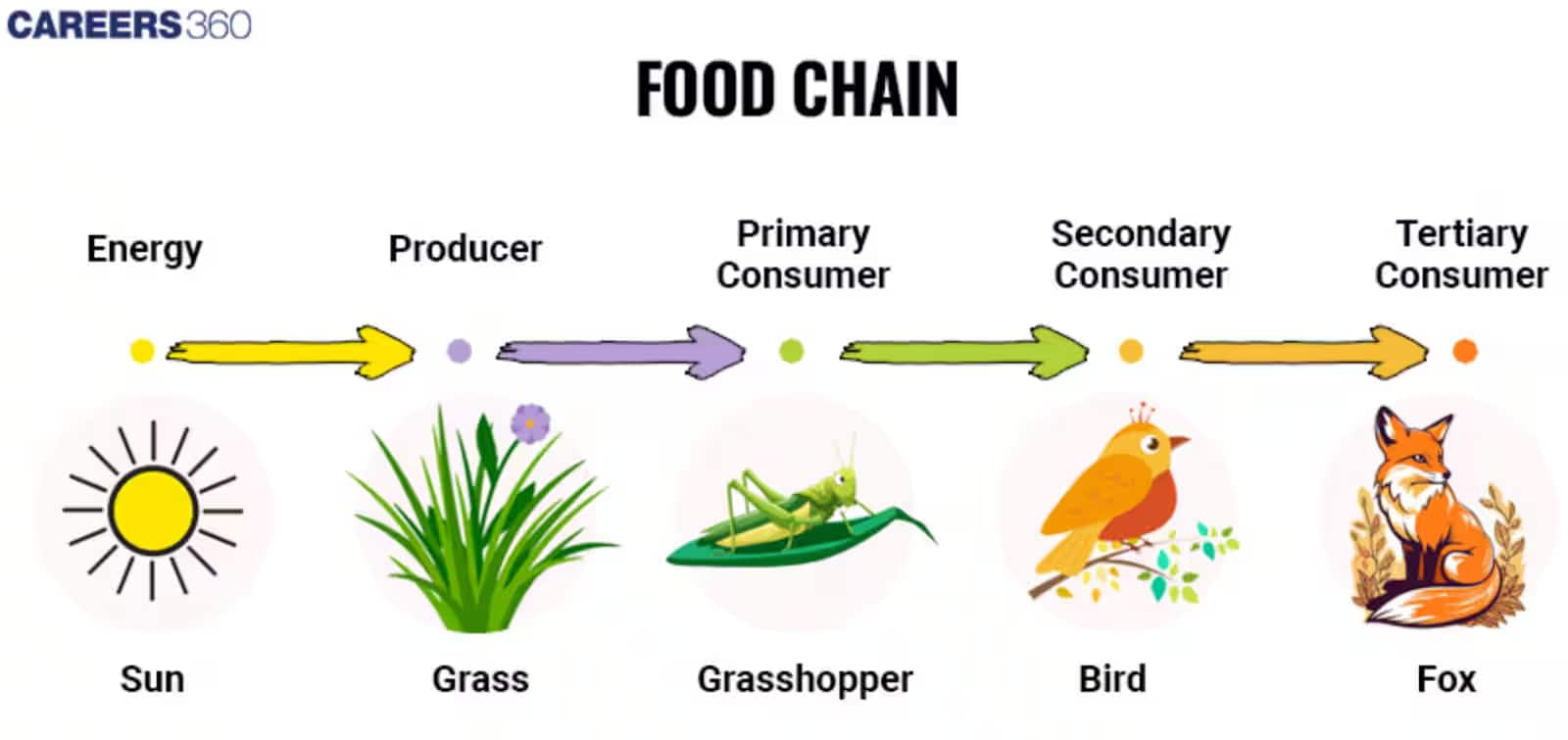
- In the above diagram, the first trophic level is occupied by the producers (green plants).
- The second trophic level is occupied by primary consumers, which are herbivores. In this diagram, the grasshopper occupies the second trophic level.
- The third trophic level is made up of secondary consumers that are small carnivores, such as birds.
- The fourth trophic level is made up of tertiary consumers known as larger carnivores, such as the fox.
- In a food chain, producers form the largest biomass, and energy decreases at higher trophic levels.
- Example: Grass insects, frogs, snakes, and eagles
- Grasshoppers eat grass, and birds eat the grasshoppers, which are then eaten by foxes, who are then devoured by eagles.
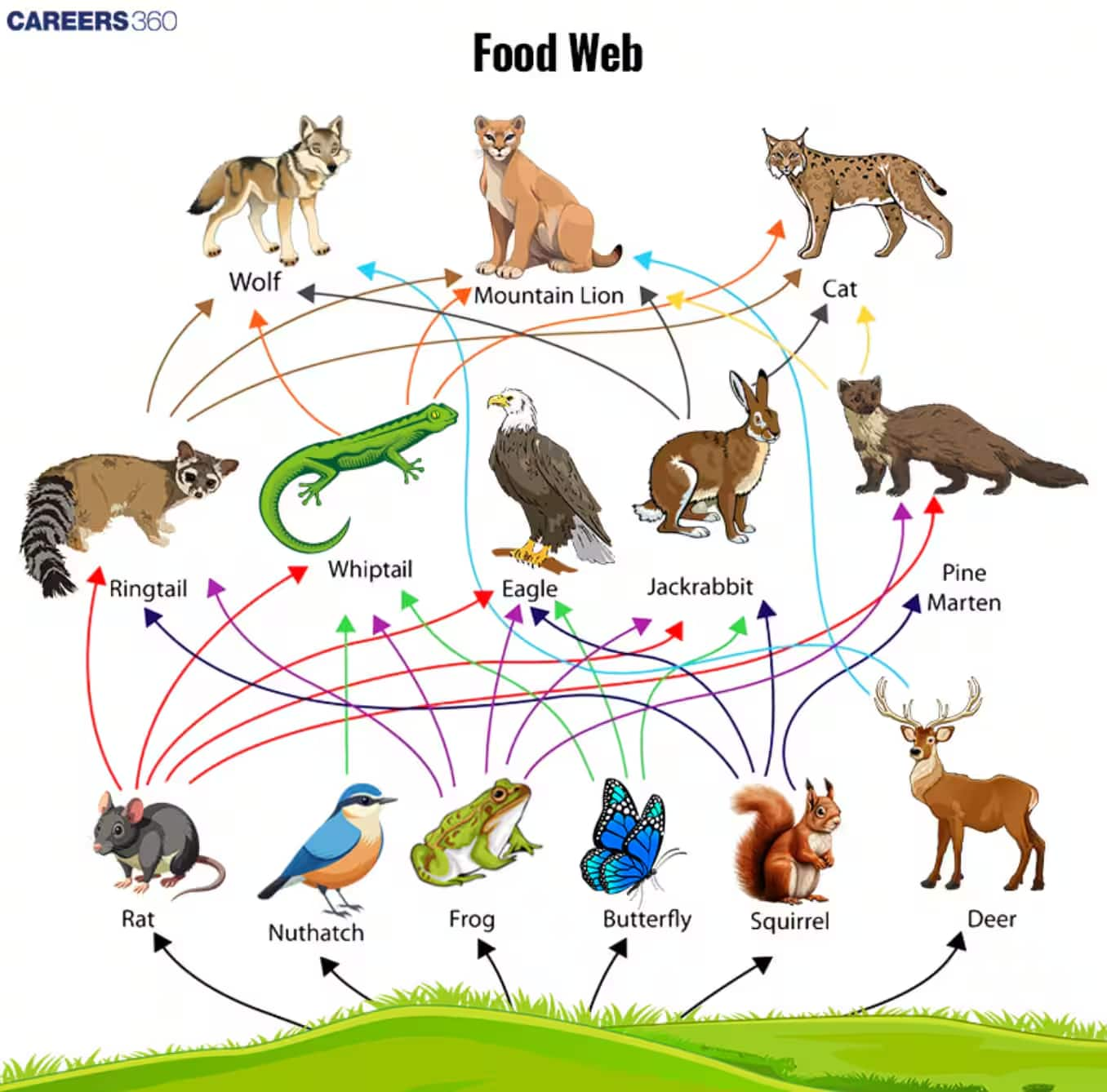
Food web:
- The interconnected food chains operating in an ecosystem that establish a network of relationships between various species are called a food web.
- It shows the pattern of energy or nutrient flow throughout an ecosystem.
- The energy flow in a food chain is one-way; once it reaches the next trophic level, it does not return.
- The complexity of the food web depends upon the diversity of organisms in the system.
Ozone Layer and How It Is Getting Depleted
Ozone is a highly reactive molecule containing three oxygen atoms(O3).
- A naturally occurring high altitude layer of ozone is important to life as it absorbs potentially damaging Ultraviolet radiation from the sun.
UV rays break up molecular oxygen (O2) into free oxygen (O) atoms, which mix with the molecular oxygen (O2) to generate ozone (O3).
O2 →O + O
O+O2 →O3
Ozone layer depletion:
- The ozone layer in the stratosphere protects us from the harmful UV radiation from the sun.
- The depletion of this ozone layer is due to human activities that have serious effects, and this has become a subject of concern over the last few years.
- The ozone layer depletion takes place due to the reaction between ozone and chlorofluorocarbons(CFCs), which are released from sprays, air conditioners, and refrigerators.
- One molecule of chlorofluorocarbons can damage 10,000 molecules of ozone.
- Although ozone depletion is occurring widely in the stratosphere, the depletion is particularly marked over the Antarctic region. This has resulted in the formation of a large area of the thinned ozone layer, commonly known as the ozone hole.
Effects of Ozone Depletion:
- Increased UV radiation reaching the Earth's surface due to ozone depletion in the stratosphere is harmful to human health, crops, forests, and animals.
- It causes sunburn and skin cancer in humans. It also increased the acid production.
Also Read, NCERT Books and Syllabus
Chapter 13 Science Our Environment: Previous Year Question and Answers
The previous years’ questions given below help students understand what kind of questions are asked in the exam. They can also check how much they have studied and where they need to improve. For quick and effective revision, students can refer to the NCERT Class 10 Science Chapter 13 Notes.
Question 1: _________ is a non-biodegradable substance.
Option 1. Paper
Option 2. Aluminum
Option 3. Plant products
Option 4. None of the above
Answer:
Paper, clipboards, metal containers like aluminium containers and human waste, and several plant products are classified under biodegradable substances. However, an example of a non-biodegradable substance could be plastic products.
Hence, the correct answer is option (4), None of the above
Question 2: Primary producers capture about __ of the energy of sunlight that falls on their leaves.
Option 1. 0.1%
Option 2. 1%
Option 3. 10%
Option 4. 0.01%
Answer:
Primary producers that are green plants, also known as autotrophs, capture about 1% of the energy of sunlight that falls on their leaves and convert it into food energy.
Hence, the correct answer is option (2), 1%
Question 3: Every food chain in the ecosystem begins with ____ , which is the primary source of food.
Option 1. Saprophytes
Option 2. Parasites
Option 3. Producers
Option 4. Consumers
Answer:
Primarily, all green plants, which are considered primary producers, capture the energy present in sunlight and convert it into chemical energy.
Hence, the correct answer is option (3), Producers
Question 4: Which of the following represents the correct flow of energy in an ecosystem?
Option 1. Consumers → Producers → Decomposers
Option 2. Decomposers → Producers → Consumers
Option 3. Producers → Consumers → Decomposers
Option 4. Consumers → Decomposers → Producers
Answer:
Energy in an ecosystem flows in one direction. It is first captured by producers, then transferred to consumers, and finally to decomposers.
Hence, the correct answer is option (3), Producers → Consumers → Decomposers
Question 5: Which group of organisms helps in recycling nutrients in the ecosystem?
Option 1. Producers
Option 2. Consumers
Option 3. Herbivores
Option 4. Decomposers
Answer:
Decomposers break down dead plants and animals into simpler substances. This process returns nutrients to the soil, maintaining ecosystem balance.
Hence, the correct answer is option (4), Decomposers
NCERT Solutions Subject Wise
How to Use Our Environment Class 10 Notes Effectively?
Knowing how humans interact with nature is important to protect the planet and maintain balance in ecosystems. Studying the chapter carefully helps students understand the causes and effects of environmental changes.
Start by studying the Class 10 Science Chapter 13 Our Environment Notes PDF to understand ecosystems, food chains, and the flow of energy in nature.
Focus on the causes and effects of pollution, deforestation, and climate change to understand human impact on the environment.
Revise definitions of biodiversity, conservation, sustainable development, and ecological balance to score well in exams.
Practice solving questions related to environmental issues, waste management, and natural resource conservation is important to improve accuracy.
Use the Class 10 Science Chapter 13 Our Environment Notes for quick revision before exams to remember important points, diagrams, and examples effectively.
Advantages of Class 10 Science Chapter 13 Our Environment Notes
Our Environment is an important chapter that helps students understand the interaction between living organisms and their surroundings. Well-organised notes make it easier to learn about the ecosystems, food chains, and environmental issues in a particular sequence.
- Class 10 Science Chapter 13 Our Environment Notes PDF explains concepts like trophic levels, food chain, and food web, in a clear manner, along with a well-labeled diagram.
- Important topics and terms such as energy flow, magnification, and waste management are highlighted for quick revision.
- Diagrams and flow charts are included in the notes to help students know how energy flows take place in an ecosystem.
- Regular revision of the notes boosts confidence to perform well in board exams and other entrance exams like NEET.
Chapter-Wise NCERT Class 10 Notes Science
Given below are the notes for each chapter that class 10 students have to study in the science subject. These are prepared to make the concepts easy and help students in doing effective exam preparation.
Frequently Asked Questions (FAQs)
No, the NCERT notes for Class 10 Science chapter 13 do not include all of the important derivations. This NCERT note provides a summary of the chapter's main concepts and equations and can be used to review the chapter.
From the notes for Class 10 Science chapter 13, students should expect 4 to 6 marks.
Producers are all organisms that can prepare their own food through photosynthesis using the sun's radiant energy received by the chlorophyll in the leaves, as per the NCERT Class 10 Science Chapter 13 Our Environment Notes.
Consumers are organisms that consume the food produced, either directly or indirectly.
Based on their manner of feeding, consumers can be classed as primary, secondary, or tertiary.
- Primary consumers include all herbivores and some parasites - Rabbits, for example, consume grass.
- Secondary consumers include many tiny carnivores and parasites - A snake that eats rabbits is an example.
- Tertiary consumers include larger carnivores and omnivores - An owl, for example, eats snakes.
Non-biodegradable compounds are those that are not broken down by bacteria or saprophytes. Plastics are a good example. Students can use the NCERT Class 10 Science Chapter 13 Our Environment Notes as a guide for a clear explanation.
Decomposers are creatures that break down the dead remnants and waste products of other organisms. Decomposers in the soil break down complex organic chemicals into simple inorganic substances that plants can utilise.
Our waste materials are discharged into the environment as a result of our daily activities. Biodegradable compounds are those that can be broken down by biological processes. For example - Plant and animal organic materials.
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
