In NCERT Class 12 Chemistry, d-block elements are transition elements where the last electron enters the d-orbital. f-block elements are inner transition elements where the last electron enters the f-orbital, and they are placed separately at the bottom of the periodic table.
NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f block elements
Did you know the middle of the periodic table has special metals known as d and f-block elements that are used in industries, colorful fireworks and important chemical reactions? This chapter features transition metals known as d-block elements and the inner transition metals known as f-block elements, as well as the principles and theories that govern their behaviour.The important topics like electronegativity, ionic size and colour in transition metal complexes are all discussed in this chapter.
The Central Board Of Secondary Examination (CBSE) will not conduct any exam for CBSE Class 10 tomorrow. The board will now conduct exam for beauty and wellness, marketing and sales, multi skill foundation course, data science on February 20.
This Story also Contains
- NCERT Solutions for Class 12 Chemistry Chapter 4: Download PDF
- NCERT Solutions of Class 12 Chemistry Chapter 4 ( Intext Questions )
- NCERT Solutions for Class 12 Chemistry Chapter 4 (Exercise Questions )
- NCERT Class 12 Chemistry Chapter 4: Higher Order Thinking Skill (HOTS) Questions
- Approach to Solve Questions of Class 12 Chemistry Chapter 4
- Topics and subtopics covered in the NCERT Textbook Class 12 Chemistry Chapter 4
- What Extra Should Students Study Beyond the NCERT for JEE?
- What Students Learn from NCERT Solutions for Class 12 Chemistry Chapter 4 The d- and f-Block Elements
- NCERT Solutions Class 12 Chemistry Chapter-Wise

The NCERT Solutions for Class 12 Chemistry are designed to offer a systematic approach to help students develop a clear understanding of critical concepts through a series of solved examples and conceptual explanations. These solutions also provide a valuable resource to enhance performance in competitive exams like NEET, JEE Mains, etc. The higher-order thinking skills questions are added in this article to improve your analytical thinking. We have also added some important points that will help you build a good approach for NCERT Solutions.
NCERT Solutions for Class 12 Chemistry Chapter 4: Download PDF
Students can download the d and f block elements ncert solutions pdf for free from the download icon given below. These solutions of NCERT are designed to help you understand the fundamental concepts and solve textbook questions with ease.
Also Read :
NCERT Solutions of Class 12 Chemistry Chapter 4 ( Intext Questions )
These class 12 chemistry chapter 4 d and f block elements solutions provide step by step explanations for all the in text questions, helping students grasp the key concepts of NCERT with clarity and confidence.
Page 92
Answer:
Silver atom(atomic no. = 47) has completely filled d-orbital in its ground state(4$d^{10}$ ). However, in +2 oxidation state, the electron of d-orbitals gets removed. As a result, the d-orbital becomes incomplete( $d^{9}$ ). Hence it is a transition element.
Page 95
Answer:
The enthalpy of atomisation of zinc is lowest due to the absence of an unpaired electron, which is responsible for metallic bonding in the elements. Therefore, the inter-atomic bonding is weak in zinc( $Zn$ ). Hence it has a low enthalpy of atomisation.
Page 97
Question 4.3 Which of the 3d series of transition metals exhibits the largest number of oxidation states and why?
Answer:
In 3d series of transition metals Manganese shows largest number of oxidation states because it has highest number of unpaired electrons in its $d$ -orbitals.So, that by removing its all electrons we get different oxidation states.
Example- $MnO_{2}(+4),\:MnO_{4}^{-}(+7),\:MnO(+2)$ etc.
Page 98
Answer:
The $E^{\ominus }(M^{2+}/M)$ value for metal depends on-
- Sublimation energy
- Ionisation energy
- Hydration energy
Copper has a high value of atomisation enthalpy and low hydration energy. Thus, as a result, the overall effect is $E^{\ominus }(M^{2+}/M)$ for copper is positive.
Page 100
Answer:
The irregular variation in ionisation enthalpies is due to the extra stability of the configuration like $d^{0}\,d^{5}\,d^{10}$ because these states are extremely stable and have high ionisation enthalpies.
In the case of chromium ( $Cr$ ) has a low 1st IE because after losing one electron it attains stable configuration ( $d^{5}$ ). But in the case of Zinc ( $Zn$ ), the first IE is very high, because we remove an electron from a stable configuration(3 $d^{10},4s^{2}$ ).
The second IE is much higher than the 1st IE. This is because it becomes difficult to remove an electron when we already did that and it already has a stable configuration (such as $d^{0}\,d^{5}\,d^{10}$ ). For example elements such as $Cr^{+}$ and $Cu^{+}$ the second IE is extremely high because they are already in a stable state. We know that removal of an electron from a stable state requires a lot of energy.
Page 101
Question 4.6 Why is the highest oxidation state of a metal exhibited in its oxide or fluoride only?
Answer:
Oxygen and fluorine are strong oxidising agents and both of their oxides and fluorides are highly electronegative in nature and also small in size. Because of these properties, they can oxidise the metal to its highest oxidation states.
Page 101
Question 4.7 Which is a stronger reducing agent $Cr^{2+}$ or $Fe^{2+}$ and why?
Answer :
$\mathrm{Cr}^{+2}$ is a better reducing agent as compared to $\mathrm{Fe}^{+2}$, as this can be explained on the basis of standard electrode potential of $\mathrm{Cr}^{+2}$ (-0.41) and $\mathrm{Fe}^{+2}$ (+0.77).
It can also be explained on the basis of their electronic configuration achieved. $\mathrm{Cr}^{+2}$ obtained $d^3$ configuration, whereas $\mathrm{Fe}^{+2}$ gets $d^5$ configuration upon reduction. It is known that $d^3$ is more stable than $d^5$ So, $\mathrm{Cr}^{+2}$ is a better reducing agent as compared to $\mathrm{Fe}^{+2}$.
Page 103
Question 4.8 Calculate the ‘spin only’ magnetic moment of $M^{2+}\; _{(aq)}$ ion $(Z=27)$.
Answer :
Atomic number (Z)= 27
So the electronic configuration cobalt ( $Co$ ) is $3d^{7}, 4s^{2}$
$M^{2+}\; _{(aq)}$ ion means, it loses its two electrons and become $d^{7}$ configuration. It has 3 unpaired electrons
So, $\mu = \sqrt{n(n+2)}$ , where n = no. of unpaired electron
by putting the value of n= 3
we get, $\mu = \sqrt{15}$
$\approx 4\ BM$
Page 105
Question 4.9 Explain why $Cu^{+}$ ion is not stable in aqueous solutions?
Answer :
$Cu^{+}$ ion is unstable in aq. solution and disproportionate to give $Cu^{2+}$ and $Cu$
$2Cu^{+}(aq)\rightarrow Cu^{2+}(aq)+Cu(s)$
The hydration energy released during the formation of $Cu^{2+}$ compensates the energy required to remove an electron from $d^{10}$ -configuration.
Page 113
Question 4.10 Actinoid contraction is greater from element to element than lanthanoid contraction. Why?
Answer :
Actinoid contraction is greater from element to element than lanthanoid contraction. The reason behind it is the poor shielding effect of 5 f$ (in actinoids) orbitals than 4$f$ orbitals( in lanthanoids). As a result, the effective nuclear charge experienced by valence electrons is more in actinoids than in lanthanoid elements.
NCERT Solutions for Class 12 Chemistry Chapter 4 (Exercise Questions )
These d and f block elements class 12 question answer chemistry explain all exercise questions in a simple and clear way. They help students understand key concepts of d and f block elements and solve numerical problems effectively. Practising with these solutions improves accuracy and exam confidence.
Question 4.1(i) Write down the electronic configuration of:
Answer :
Chromium has an atomic number 24. So, the nearest noble gas element is Argon ( $Ar$ )
So electronic configuration of $(i)\; Cr^{3+}$ = $[Ar]^{18}3d^{3}4s^{0}$
Question 4.1(ii) Write down the electronic configuration of:
Answer :
The atomic number of promethium is 61 and the nearest noble gas is xenon( $Xe$ )
So, atomic configuration of $Pm^{3+}=[Xe]^{54}4f^{4}$
Question 4.1(iii) Write down the electronic configuration of:
Answer :
The atomic number of copper is 29 and the previous noble element is Argon ( $Ar$ )
the electronic configuration of $Cu^{+}=[Ar]^{18}3d^{10}$
Question 4.1(iv) Write down the electronic configuration of:
Answer :
The atomic number of cerium ( $Ce$ ) is 58, and the previous noble element is Xenon ( $Xe$ )
The electronic configuration of $Ce^{4+}=[Xe]^{54}4f^{0}$
Question 4.1(v) Write down the electronic configuration of:
Answer :
The atomic number of cobalt (Co) is 27 and the previous noble element is Argon ( $Ar$ )
Thus electronic configuration of $Co^{2+}=[Ar]^{18}3d^{7}4s^{0}$
Question 4.1(vi) Write down the electronic configuration of:
Answer :
The atomic number of lutetium is 71 and the previous noble element is Xe (xenon)
The electronic configuration of $Lu^{2+}=[Xe]^{54}4f^{14}5d^{1}6s^{0}$
Question 4.1(vii) Write down the electronic configuration of:
Answer :
The atomic number of Manganese is 25 and the previous noble element is Ar (argon)
So, the electronic configuration of $Mn^{2+}= [Ar]^{18}3d^{5}4s^{0}$
Question 4.1(viii) Write down the electronic configuration of:
Answer :
The atomic number of thorium (Th) is 90 and the previous noble gas element is Xenon (Xe)
So, the elelctronic configuration of $Th^{4+}= [Rn]^{86}5f^{0}$
Question 4.2 Why are $Mn^{2+}$ compounds more stable than $Fe^{2+}$ towards oxidation to their $+3$ state?
Answer :
$Mn^{2+}= 1s^2,2s^2p^6,3s^2p^6d^5\:\:\:(Half\:filled \:d-orbital)$
$Fe^{2+}= 1s^2,2s^2p^6,3s^2p^6d^6$
In +2 oxidation state of manganese has more stability than +2 oxidation state of iron, it is because half-filled and fully filled d-orbitals are more stable and $Mn^{2+}$ has half-filled electron stability Manganese ( $Mn^{2+}$ ) has $d^{5}$ configuration so it wants to remain in this configuration. On the other hand, $Fe^{2+}$ has $d^{6}$ configuration and after losing one electron it becomes $d^{5}$ configuration and attains its stability. That's why $Mn^{2+}$ compounds more stable than $Fe^{2+}$ towards oxidation to their $+3$ state.
Answer :
According to our observation, except scandium, all other elements of the first row show +2 oxidation state. On moving from Sc to Mn the atomic number increases from 21 to 25 and also the increasing number of electrons in 3d orbitals from $d^{1} - d^{5}$. When metals lose two electrons from their 4s orbital then they achieve +2 oxidation state. Since the number of d electrons in (+2) state increases from $Ti(+2) - Mn(+2)$, the stability of the +2 oxidation state increases as d-orbitals become more and more half-filled.
Mn(+2) has $d^{5}$ configuration, which is half filled (it makes it highly stable)
Answer :
Elements of the first half of the transition series exhibit many oxidation states. manganese shows the maximum number of oxidation states (+2 to +7). The stability of +2 oxidation states increases with the increase in atomic number (as more number of electrons are filled in d-orbital). However, the $Sc$ does not exhibit +2 oxidation states, its EC is $3d^{1}4s^{2}$. It loses all three electrons to attain stable $d^{0}$ -configuration (noble gas configuration). $Ti(IV)$ and $V(+5)$ are stable for the same reason. In the case of manganese, (+2) oxidation state is very stable because of the half-filled d-electron( $d^{5}$ -configuration).
Answer :
$3d^{3}$
Vanadium (atomic number- 23)
E.C = $[Ar]^{18}3d^{3}4s^{2}$ ,
So the stable oxidation states are (+2, +3, +4, +5)
$3d^{5}$
Manganese (atomic number = 25)
E.C = $[Ar]^{18}3d^{5}4s^{2}$ ,
So the stable oxidation states are (+2, +4, +6, +7)
$3d^{5}$
Chromium (atomic number = 24)
E.C = $[Ar]^{18}3d^{5}4s^{1}$ ,
So the stable oxidation states are (+3, +4, +6)
$3d^{4}$
No elements have $d^{4}$ electronic configuration in their ground state.
Answer :
Following oxometal anions of the first series that exhibit the oxidation state equal to its group number-
- Vanadate $(VO_{3}^{-})$
The group number of vanadium $(V)$ is 5 and here the oxidation state is also +5 - Chromate ion $(CrO_{4}^{2-})$
The group number of chromium is (VIB) and the oxidation state is +6 - Permanganate ion $(MnO_{4}^{-})$
The group number of $(Mn)$ is VIIB and here the oxidation number is also +7
Question 4.7 What is lanthanoid contraction? What are the consequences of lanthanoid contraction?
Answer :
On moving along the lanthanoid series, the atomic number is gradually increased by one. It means the no. of electrons and protons of the atom is also increases by one. And because of it the effective nuclear charge increases (electrons are added in the same shell, and the nuclear attraction overcomes the interelectronic repulsion due to the addition of a proton). Also, with the increase in atomic number, the number of electrons in orbital also increases. Due to the poor shielding effect of the electrons, the effective nuclear charge experienced by an outer electron is increased, and also the attraction of the nucleus for the outermost electron is increased. As a result, there is a gradual decrease in the atomic size as an increase in atomic number. This is known as lanthanoid contraction.
Consequences of Lanthanoid contraction-
- Similarities in the properties of the second and third transition series
- Separation of lanthanoids can be possible due to LC.
- Due to LC, there is variation in the basic strength of the hydroxide of lanthanoid. (basic strength decrease from $La(OH)_{3}-Lu(OH)_{3}$ ).
Answer :
Transition elements are those which have partially filled $d$ or $f$ orbitals. These elements lie in the $d-block$ and show transition properties between s block and p-block. Thus these are called transition elements.
$Zn, Hg, Cd$ are not considered transition elements due to the fully filled d-orbitals.
Question 4.9 In what way is the electronic configuration of the transition elements different from that of the non-transition elements?
Answer :
Transition elements have partially filled $d$ -orbitals. Thus general electronic configuration of transition elements is
$(n-1)d^{1-10}ns^{0-2}$
Non-transition elements either have fully filled d-orbitals or do not have d-orbitals. Therefore general electronic configuration is
$ns^{1-2}$ or $ns^{2}np^{1-6}$
Question 4.10 What are the different oxidation states exhibited by the lanthanoids?
Answer :
In lanthanoid +3 oxidation states are more common. $Ln(III)$ compounds are most predominant. However, +2 and +4 oxidation are also formed by them in the solution or solid compounds.
Question 4.11(i) Explain giving reasons:
(i) Transition metals and many of their compounds show paramagnetic behaviour.
Answer :
Paramagnetism is arising due to the presence of unpaired electrons. And we know that transition metals have unpaired electrons in their -orbitals. That's why they show paramagnetic behaviour.
Question 4.11(ii) Explain giving reasons:
(ii) The enthalpies of atomisation of the transition metals are high.
Answer :
Transition metals have high effective nuclear charge and also high outermost electrons. Thus they form a very strong metallic bond and due to these, transition elements have a very high enthalpy of atomisation.
Question 4.11(iii) Explain giving reasons:
(iii) The transition metals generally form coloured compounds.
Answer :
Most of the complex transition elements are coloured. This is due to the absorption of radiation from the visible light region to excite the electrons from its one position to another position in d-orbitals. In the presence of ligands, d-orbitals split into two sets of different orbital energies. Here transition of electrons takes place and emits radiation which falls on the visible light region.
Question 4.11(iv) Explain giving reasons:
(iv) Transition metals and their many compounds act as good catalyst.
Answer :
The catalytic activity of transition metals is because of two reasons-
- They provide a suitable surface for the reaction to occur.
- Ability to show variable oxidation states and form complexes, transition metals are also able to form intermediate compounds and thus they give the new path, which has lower activation energy for the reaction.
Question 4.12 What are interstitial compounds? Why are such compounds well-known for transition metals?
Answer :
Transition metals contain lots of interstitial sites. These elements trap the other elements which are small in sizes such as Carbon, Hydrogen and Nitrogen in their interstitial site of the crystal lattice as a result forms interstitial compounds.
Answer :
In transition metals, the variation of oxidation states id from +1 to the highest oxidation number, by removing all its valence electrons. Also in transition metals, the oxidation number is differed by one unit like ( $Fe^{3+}-Fe^{2+}$; $F e^{3+}-F e^{2+}$ ). But in non-transition elements, the oxidation states are differed by two (+2 and +4 or +3 and +5 etc.)
Answer :
potassium dichromate is obtained from the fusion of chromite ore $(FeCr_{2}O_{4})$ with sodium and potassium carbonate in the free supply of air.
$4FeCr_{2}O_{4}+8Na_{2}CO_{3}+7O_{2}\rightarrow 8Na_{2}CrO_{4}+2Fe_{2}O_{3}+8CO_{2}$
Sodium chromate is filtered and acidified with sulphuric acid ( $H_{2}SO_{4}$ ) to form sodium dichromate, $2 \mathrm{Na}_2 \mathrm{CrO}_4+2 \mathrm{H}^{+} \rightarrow \mathrm{Na}_2 \mathrm{Cr}_2 \mathrm{O}_7+2 \mathrm{Na}^{+}+\mathrm{H}_2 \mathrm{O}$ can be crystallized
$2Na_{2}CrO_{4}+2H^{+}\rightarrow Na_{2}Cr_{2}O_{7}+2Na^{+}+H_{2}O$
Sodium dichromate is more soluble than potassium dichromate. So, treat the solution of dichromate with the potassium chloride( $KCl$ )
$Na_{2}Cr_{2}O_{7}+KCl\rightarrow K_{2}Cr_{2}O_{7}+2NaCl$
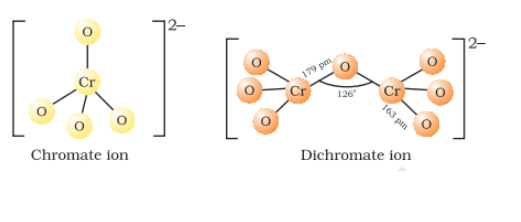
The chromate and dichromate are interconvertible in aqueous solution at pH 4
Structures of chromate and dichromate ion
Question 4.15(i) Describe the oxidising action of potassium dichromate and write the ionic equations for its reaction with:
(i) iodide
Answer :
Potassium dichromate $(K_{2}Cr_{2}O_{7})$ acts as a strong oxidising agent in an acidic medium. It takes the electron to get reduced.
$(K_{2}Cr_{2}O_{7})$ oxidises iodide to iodine
$\left.\mathrm{Cr}_2 \mathrm{O}_7^{2-}+14 \mathrm{H}^{+}+6 e^{-} \rightarrow 2 \mathrm{Cr}^{3+}+7 \mathrm{H}_2 \mathrm{O} 2 I^{-} \rightarrow \mathrm{I}_2+2 e^{-}\right] \times 3------$
$-------\mathrm{Cr}_2 \mathrm{O}_7^{2-}+14 \mathrm{H}^{+}+6 \mathrm{I}^{-} \rightarrow 2 \mathrm{Cr}^{3+}+3 \mathrm{I}_2+7 \mathrm{H}_2 \mathrm{O}$
In the first reaction oxidation state of chromium reduced from +6 to +3
Question 4.15(ii) Describe the oxidising action of potassium dichromate and write the ionic equations for its reaction with:
(ii) iron(II) solution
Answer :
Potassium dichromate react with $(Fe^{2+})$ ion to produce solution of $(Fe^{3+})$ ion and chromium reduced to +3 oxidation state.
$Cr_{2}O_{7}^{2-}+14H^{+}+6e^{-}\rightarrow 2Cr^{3+}+7H_{2}O$
$Fe^{2+}\rightarrow Fe^{3+}+e^{-}]\times 6$
----------------------------------------------------------------------------------
$\\Cr_{2}O_{7}^{2-}+14H^{+}+Fe^{2+}\rightarrow 2Cr^{3+}+7H_{2}O+ 6Fe^{3+}$
Question 4.15(iii) Describe the oxidising action of potassium dichromate and write the ionic equations for its reaction with:
Answer :
Potassium dichromate oxidises $H_{2}S$ (hydrogen sulphide ) to sulphur (zero oxidation state)
The oxidizing action of dichromate ion is -
$Cr_{2}O_{7}^{2-}+14H^{+}+6e^{-}\rightarrow 2Cr^{3+}+7H_{2}O$
$H_{2}S\rightarrow S + 2H^{+}+2e^{-}]\times 3$
$Cr_{2}O_{7}^{2-}+14H^{+}+3H_{2}S\rightarrow 2Cr^{3+}+3S+7H_{2}O$
Question 4.16(i) Describe the preparation of potassium permanganate. How does the acidified permanganate solution react with
(i) iron
Write the ionic equations for the reactions.
Answer :
Potassium permanganate can be prepared from the fusion of pyrolusite ore( $MnO_{2}$ ) with an alkali metal hydroxide and an oxidising agent (like $KNO_{3}$ ). This gives dark green $K_{2}MnO_{4}$ . It disproportionates in an acidic or neutral medium to give permanganate.
$2 \mathrm{MnO}_2+4 \mathrm{KOH}+\mathrm{O}_2 \rightarrow 2 \mathrm{~K}_2 \mathrm{MnO}_4+2 \mathrm{H}_2 \mathrm{O}$
$3 \mathrm{MnO}_4^{2-}+4 \mathrm{H}^{+} \rightarrow 2 \mathrm{MnO}_4^{-}+\mathrm{MnO}_2+\mathrm{H}_2 \mathrm{O}$
(i)Acidified permanganate ion reacts with iron-
$MnO_{4}^{-}+8H^{+}+5e^{-}\rightarrow Mn^{2+}+4H_{2}O$
$Fe^{2+}\rightarrow Fe^{3+}+e^{-}]\times 5$
-------------------------------------------------------------------------------
$MnO_{4}^{-}+5Fe^{2+}+8H^{+}\rightarrow Mn^{2+}+ 5Fe^{3+}+4H_{2}O\\$
Question 4.16(ii) Describe the preparation of potassium permanganate. How does the acidified permanganate solution react with
Write the ionic equations for the reaction.
Answer :
Reaction of acidified permanganate solution with sulphur dioxide ( $SO_{2}$ ). It oxidizes the $SO_{2}$ to sulphuric acid ( $H_{2}SO_{4}$ )
Here are the reactions-
$MnO_{4}^{2-}+6H^{+}+5e^{-}\rightarrow Mn^{2+}+3H_{2}O]\times 2$
$SO_{2}+2H_{2}O+O_{2}\rightarrow 4H^{+}+2SO_{4}^{2-}+2e^{-}]\times 5$
------------------------------------------------------------------------------------------------
$2MnO_{4}^{2-}+10SO_{2}+4H_{2}O+5O_{2}\rightarrow 2Mn^{2+}+10SO_{4}^{2-}+8H^{+}$
Question 4.16(iii) Describe the preparation of potassium permanganate. How does the acidified permanganate solution react with
(iii) oxalic acid
Write the ionic equations for the reactions.
Answer :
When acidified permanganate solution react with oxalic acid ( $H_{2}C_{2}O_{4}$ ) it converts oxalic acid into carbon dioxide ( $CO_{2}$ )
Here are the reactions-
$MnO_{4}^{-}+8H^{+}+5e^{-}\rightarrow Mn^{2+}+4H_{2}O]\times 2$
$C_{2}O_{4}^{2-}\rightarrow 2CO_{2}+2e^{-}]\times 5$
--------------------------------------------------------------------------------------------------
$2MnO_{4}^{2-}+5C_{2}O_{4}^{2-}+16H^{+}\rightarrow 2Mn^{2+}+10CO_{2}+8H_{2}O$ overall reaction
Question 4.17(i) For $M^{2+}/M$ and $M^{3+}/M^{2+}$ systems the $E^{\ominus }$ values for some metals are as follows:

Use this data to comment upon:
(i) the stability of $Fe^{3+}$ in acid solution as compared to that of $Cr^{3+}$ or $Mn^{3+}$
Answer :
The $E^{\Theta }$ value of $Fe^{3+}/Fe^{2+}$ is higher than that of $Cr^{3+}/Cr^{2+}$ but less than that of $Mn^{3+}/Mn^{2+}$ . So, the reduction of ferric ion ( $Fe^{3+}$ ) to ferrous ion( $Fe^{2+}$ ) is easier than $Mn^{3+}/Mn^{2+}$ but as not easy as $Cr^{3+}/Cr^{2+}$ . Hence ferric ion is more stable than manganese ion( $Mn^{3+}$ ), but less stable than chromium ion( $Cr^{3+}$ ).
The order of relative stabilities of different ions is-
$Mn^{3+}<Fe^{3+}<Cr^{3+}$
Question 4.17(ii) For $M^{2+}/M$ and $M^{3+}/M^{2+}$ systems the $E^{\ominus }$ values for some metals are as follows:

Use this data to comment upon:
(ii) the ease with which iron can be oxidised as compared to a similar process for either chromium or manganese metal.
Answer :
From the values of $E^{o}$, the order of oxidation of the given metal to the divalent cation is-
$Mn>Cr>Fe$
Question 4.18(i) Predict which of the following, will be coloured in aqueous solution?
$Ti^{3+}, \:V^{3+}\:,\:Cu^+,\:Sc^{3+},\:Mn^{3+},\:Fe^{3+}\: and \:Co^{2+}$
Answer :
Ions which have incomplete d-orbital, they are able to do $d-d$ transition, which is responsible for colour. And those which has vacant d-orbitals or complete $d$ -orbitals are colourless
|
$Ti^{3+}$ $=[Ar]3d^{1}$
|
Purple
|
|
$V^{3+}$ $=[Ar]3d^{1}$
|
green
|
|
$Sc^{3+}$ $=[Ar]3d^{0}$
|
colourless
|
|
$Mn^{2+}$ $=[Ar]^{18}d^{5}4s^{0}$
|
pink
|
|
$Fe^{3+}$ $=[Ar]^{18}3d^{5}4s^{0}$
|
Yellow
|
|
$Co^{2+}$ $=[Ar]^{18}d^{7}4s^{0}$
|
blue pink
|
|
$Cu^{+}$ $=[Ar]^{18}3d^{10}4s^{0}$
|
colourless
|
From the table, we notice that $Sc^{3+}$ and $Cu^{+}$ have $3d^{0}$ and $3d^{10}$ configuration, so their aqueous solutions are colourless. All others are coloured in an aqueous medium.
Question 4.18(ii) Predict for the following, will be coloured in aqueous solution? Give reason.
Answer:
Yes, $V^{3+}$ (vanadium) ions have coloured aqueous solution because vanadium has two electrons in its $d$ -orbitals, as a result d-d transition will occur and which is responsible for the colour of the solution.
Question 4.18(iii) Predict for the following, will be coloured in aqueous solution? Give reason.
Answer :
No, $Cu^{+}$ aqueous solution has no colour because it has fully filled d-orbitals. So, that d-d transition will not happen, which is responsible for colour.
electronic configuration of $Cu^{+}$ = $[Ar]^{18}3d^{10}4s^{0}$
Question 4.18(iv) Predict for the following, will be coloured in aqueous solution? Give reason.
Answer :
No, the aqueous solution of $Sc^{3+}$ ion will have no colour because it has empty d-orbitals. Thus the d-d transition will ot happen (due absence of electrons), which is responsible for colour.
The electronic configuration of $Sc^{3+}$ $=[Ar]^{18}3d^{0}4s^{0}$
Question 4.18(v) Predict for the following, will be coloured in aqueous solution? Give reason.
$(v)\; Mn^{2+}$
Answer :
Yes, the aqueous solution of $Mn^{2+}$ (manganese ion) will be coloured due to half-filled electron in its $d$ -orbitals( $d^{5}$ ) and because of that d-d transition will occurs, which is responsible for colour.
The electronic configuration of $Mn^{2+}$ $=[Ar]^{18}d^{5}4s^{0}$
Question 4.18(vi) Predict for the following, will be coloured in aqueous solution? Give reason.
$(vi)\; Fe^{3+}$
Answer :
Yes, the aqueous solution of $Fe^{3+}$ (ferric ion) will be coloured due to half-filled electron in it $d$ -orbitals( $d^{5}$ ) and because of that d-d transition will occurs, which is responsible for the colour
electronic configuration of $Fe^{3+}$ $=[Ar]^{18}3d^{5}4s^{0}$
Question 4.18(vii) Predict for the following, will be coloured in aqueous solution? Give reason.
$(vii)\; Co^{2+}$
Answer :
Yes, the aqueous solution of $Co^{2+}$ (ferric ion) will be coloured due to the presence of an electron in it $d$ -orbitals( $d^{7}$ ) and because of that d-d transition will occurs, which is responsible for colour
electronic configuration of $Co^{2+}$ $=[Ar]^{18}d^{7}4s^{0}$
Question 4.19 Compare the stability of +2 oxidation state for the elements of the first transition series.
Answer :
According to our observation, except scandium, all other elements of the first row shows +2 oxidation state. On moving from $Sc$ to $Mn$ the number of oxidation states increases but from $Mn$ to $Zn$ number of oxidation states decreases due to a decrease in unpaired electrons. The stability of +2 oxidation state increases on moving from $Sc$ to $Zn$ due to the increase in the difficulty level of removal of the third electron from d -orbital.
|
Sc
|
Ti
|
V
|
Cr
|
Mn
|
Fe
|
Co
|
Ni
|
Cu
|
Zn
|
|
+3
|
+2
+3
+4
|
+2
+3
+4
+5
|
+2
+3
+4
+5
+6
|
+2
+3
+4
+5
+6
+7
|
+2
+3
+4
+6
|
+2
+3
+4
|
+2
+3
+4
|
+1
+2
|
+2
|
Question 4.20(i) Compare the chemistry of actinoids with that of the lanthanoids with special reference to:
(i) electronic configuration
Answer :
The general electronic configuration of actinoids series is $[Rn]^{86}5f^{1-14}6d^{0-1}7s^{2}$ and that for lanthanoids are $[Xe]^{54}4f^{1-14}5d^{0-1}6s^{2}$ . 5 $f$ orbitals do not deeply participate in bonding to a large extent.
Question 4.20(ii) Compare the chemistry of actinoids with that of the lanthanoids with special reference to:
(ii) atomic and ionic sizes
Answer :
Similar to lanthanoids, actinoids also show actinoid contraction. But the contraction is greater in actinoids because of poor shielding effects of 5f orbitals
Question 4.20(iii) Compare the chemistry of actinoids with that of the lanthanoids with special reference to:
(iii) oxidation state
Answer :
The principal oxidation state of lanthanoids is +3, but sometimes it also shows +2 and +4 oxidation states. This is due to the extra stability of fully-filled and half-filled orbitals.
Actinoids have a greater range of oxidation states due to comparable energies of and it also have a principal oxidation state is +3 but have more compounds in +3 oxidation states than lanthanoids.
Question 4.20(iv) Compare the chemistry of actinoids with that of the lanthanoids with special reference to:
(iv) chemical reactivity.
Answer :
In the lanthanoid series, an earlier member of the series is more reactive, and that is comparable to. With an increase in atomic number, lanthanoids start behaving similarly to aluminium.
Actinoids are highly reactive metals, especially when they are finally divided. When we add them into the water, they give a mixture of oxide and hydride. Actinoids combine with most of the non-metals at moderate temperatures. Alkalies have no action on these actinoid metals
Question 4.21(i) How would you account for the following:
(i) Of the $d^{4}$ species, $Cr^{2+}$ is strongly reducing while manganese(III) is strongly oxidising.
Answer :
$Cr^{2+}$ is strongly reducing in nature. It has $d^{4}$ configuration. By losing one electron it gets oxidised to $Cr^{3+}$ (electronic configuration $d^{3}$ ) which can be written as $t^{3}_{2g}$ and it is a more stable configuration. On the other hand, $Mn^{3+}$ has also $d^{4}$ configuration by accepting one electron it gets reduced and acts as a strongly oxidising agent(electronic configuration $d^{5}$ ). Thus it is extra stable due to half-filled with d-orbital.
Question 4.21(ii) How would you account for the following:
(ii) Cobalt(II) is stable in aqueous solution but in the presence of complexing reagents, it is easily oxidised.
Answer :
Cobalt (II) is more stable in aq. solution but in the presence of strong field ligand complexing agents, it gets oxidised to Cobalt (III). Though the third ionisation energy of $Co$ is high but the CFSE ( crystal field stabilisation energy ) is very high in the presence of a strong field ligand which overcomes the ionisation energy.
Question 4.21(iii) How would you account for the following:
(iii) The $d^{1}$ configuration is very unstable in ions.
Answer :
The $d^{1}$ configuration is very unstable in ions because after losing one more electron it attains stable $d^{0}$ configuration.
Question 4.22 What is meant by ‘disproportionation’? Give two examples of disproportionation reactions in aqueous solution.
Answer :
In a chemical reaction a substance gets oxidised as well as reduced simultaneously is called a disproportionation reaction. For example-
- $3CrO_{4}^{3-}(V)+8H^{+}\rightarrow 2CrO_{4}^{2-}(VI)+Cr^{3+}(III)+4H_{2}O$
- $3MnO_{4}^{2-}(VI)+4H^{+}\rightarrow 2MnO_{4}^{-}(VII)+MnO_{2}(IV)+2H_{2}O$
Question 4.23 Which metal in the first series of transition metals exhibits +1 oxidation state most frequently and why?
Answer :
In the first transition series, Cu (copper) exhibits +1 oxidation states most frequently. This is because $Cu^{+}$ has stable electronic configuration of $[Ar]3d^{10}$ . The fully filled d-orbital makes it highly stable.
Question 4.24(i) Calculate the number of unpaired electrons in the following gaseous ions:
$(i) Mn^{3+}$
Answer :
The number of unpaired electron in $Mn^{3+}$ is 4
$Mn^{3+}(Z=25) =\left [ Ar \right ] 3d^{4}$
After losing 3 electrons, Mn has 4 electrons left.
Question 4.24(ii) Calculate the number of unpaired electrons in the following gaseous ions:
Answer :
Electronic configuration of chromium is $Cr = 3d^{5}4s^{1}$ . The number of unpaired electron in $Cr^{3+}$ is 3
$Cr^{3+}(Z=24) =\left [ Ar \right ] 3d^{3}$
After losing 3 electrons, Cr has 3 electrons left d-orbital
Question 4.24(iii) Calculate the number of unpaired electrons in the following gaseous ions:
Answer :
Electronic configuration of $V = 3d^{3}4s^{2}$ . The number of unpaired electron in $V^{3+}$ is 2
$V^{3+}(Z=23) =\left [ Ar \right ] 3d^{2}$
After losing 3 electrons, V has 2 electrons left d-orbital
Question 4.24(iv) Calculate the number of unpaired electrons in the following gaseous ions:
Answer :
Electronic configuration of $Ti= 3d^{2}4s^{2}$ . The number of unpaired electron in $Ti^{3+}$ is 1
$Ti^{3+}(Z=22) =\left [ Ar \right ] 3d^{1}$
After losing 3 electrons, Ti has 1 electron left d-orbital
Question 4.24 Which one of these is the most stable in aqueous solution?
$\\(i)Mn^{3+}\\(ii)Cr^{3+}\\(iii)V^{3+}\\(iv)Ti^{3+}$
Answer :
$Cr^{3+}$ is the most stable in the aqueous solution because it attains the $t^{3}_{2g}$ configuration, which is a stable $d-$ configuration.
Electronic configuration of $Cr^{3+}$ = $[Ar]3d^{3}4s^{0}$
Question 4.25(i) Give examples and suggest reasons for the following feature of the transition metal chemistry:
(i) The lowest oxide of transition metal is basic, and the highest is amphoteric/acidic.
Answer :
The lowest oxidation states of transition metals are basic because some of their valence electrons do not participate in bonding. Thus they have free electrons, which they can donate and act as a base. In the higher oxide of transition metals, valence electrons of their participate in bonding, so they are unavailable. But they can accept electrons and behave as an acid. For example $MnO$ (+2) behave as a base and $Mn_{2}O_{7}$ (+7)behave as an acid.
Question 4.25(ii) Give examples and suggest reasons for the following feature of the transition metal chemistry:
(ii) A transition metal exhibits the highest oxidation state in oxides and fluorides.
Answer :
Oxygen and fluorine are a strong oxidizing agent because of their small in size and high electronegativity. So, they help transition metals to exhibit the highest oxidation states. Examples of oxides and fluorides of transition metals are $OsF_{6}(+6)$ and $V_{2}O_{5}(+5)$
Question 4.25(iii) Give examples and suggest reasons for the following feature of the transition metal chemistry:
(iii) The highest oxidation state is exhibited in oxoanions of a metal.
Answer :
Oxygen is a strong oxidizing agent because of its small in size and high electronegativity. Thus oxo-anions of metals show the highest oxidation state.
For example- $KMnO_{4}$, here manganese shows +4 oxidation state.
Question 4.26(i) Indicate the steps in the preparation of:
(i) $K_{2}Cr_{2}O_{7}$ from chromite ore .
Answer :
(i) Potassium dichromate is obtained from the fusion of chromite ore $(FeCr_{2}O_{4})$ with sodium and potassium carbonate in the free supply of air.
$4FeCr_{2}O_{4}+8Na_{2}CO_{3}+7O_{2}\rightarrow 8Na_{2}CrO_{4}+2Fe_{2}O_{3}+8CO_{2}$
(ii) Sodium chromate is filtered and acidified with sulphuric acid ( $H_{2}SO_{4}$ ) to form sodium dichromate, $(Na_{2}Cr_{2}O_{7}.2H_{2}O)$ can be crystallized
$2Na_{2}CrO_{4}+2H^{+}\rightarrow Na_{2}Cr_{2}O_{7}+2Na^{+}+H_{2}O$
(iii) Sodium dichromate is more soluble than potassium dichromate. So, treat the solution of dichromate with the potassium chloride( $KCl$ )
$Na_{2}Cr_{2}O_{7}+KCl\rightarrow K_{2}Cr_{2}O_{7}+2NaCl$
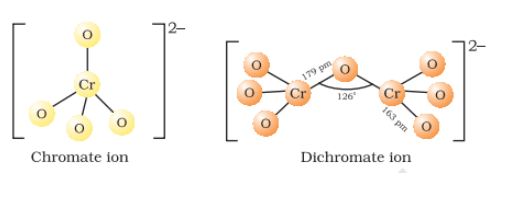
The chromate and dichromate are interconvertible in aqueous solution at pH 4
Structures of chromate and dichromate ion
Question 4.26(ii) Indicate the steps in the preparation of:
(ii) $KMnO_{4}$ from pyrolusite ore.
Answer :
Potassium permanganate can be prepared from the fusion of pyrolusite ore( $MnO_{2}$ ) with an alkali metal hydroxide and an oxidising agent (like $KNO_{3}$ ).
This gives dark green $K_{2}MnO_{4}$ . It disproportionates in an acidic or neutral medium to give permanganate.
$2 \mathrm{MnO}_2+4 \mathrm{KOH}+\mathrm{O}_2 \rightarrow 2 \mathrm{~K}_2 \mathrm{MnO}_4+2 \mathrm{H}_2 \mathrm{O}$
$3 \mathrm{MnO}_4^{2-}+4 \mathrm{H}^{+} \rightarrow 2 \mathrm{MnO}_4^{-}+\mathrm{MnO}_2+\mathrm{H}_2 \mathrm{O}$
Question 4.27 What are alloys? Name an important alloy which contains some of the lanthanoid metals. Mention its uses.
Answer :
It is a solid solution of two or more elements in a metallic matrix. Alloys possess different physical properties than component materials.
An important alloy of lanthanoids is mischmetal.
uses-
- mischmetal is used in cigarettes and gas lighters
- Used in flame-throwing tanks
- It is used in tracer bullets and shells
Answer :
Inner transition metals are those in which the last electrons are filled in f-orbitals. The elements in which 4f and 5f are filled are called f-block elements. 59, 95 and 102 are the inner transition elements.
Answer :
Lanthanoid primarily shows three oxidation states +2, +3, and +4 and out of these +3 is most common in lanthanoids. they show a limited no. of oxidation states due to the large difference in energies of 4$f$, 5$d$ and 6$s$ orbitals. But, actinoids shows large no. of oxidation state because they have comparable energy difference in 5$f$ ,6$d$ and 7$s$ orbitals. For example $U$ and $Pu$ exhibits +3, +4, +5 and +6 oxidation states.
Answer :
The last element of the actinoid series is Lawrencium ( $Lr$ ). Its atomic number is 103. The electronic configuration of $Lr$ is $[Rn]^{86}5f^{14}6d^{1}7s^{2}$ .
The possible oxidation state of lawrencium is +3 because after losing 3 electrons, it becomes a stable molecule.
Answer :
Electronic configuration of $Ce= 1s^{2}2s^{2}2p^{6}3s^{2}3p^{6}3d^{10}4s^{2}4p^{6}4d^{10}5s^{2}5p^{6}4f^{1}5d^{1}6s^{2}$
Magnetic moment can be calculated as $\mu = \sqrt{n(n+2)}$ , where n= no. of unpaired electrons
in Cerium n = 2
So, by putting the value of n we get $\mu = \sqrt{2(2+2)}= \sqrt{8}=2.828BM$
Answer :
Members of the lanthanoids which exhibit +4 oxidation states are- $Ce, Pr, Nd, Tb, D y$
Members who exhibit +2 oxidation states = $Nd,Sm,Eu,Tm,Yb$
After losing 4 electrons $Ce^{4+}$ attains stable configuration $[Xe]$ and also the same thing happen to $Tb = [Xe]4f^{7}$
In the case of $Eu$ and $Yb$, after losing two electrons they also get their stable electronic configuration.
$\\Eu^{2+}=[Xe]4f^{7}\\ Yb^{2+}= [Xe]4f^{14}$
Question 4.34 Write the electronic configurations of the elements with the atomic numbers 61, 91, 101, and 109.
Answer :
Atomic number = 61, Promethium
The electronic configuration is $[Xe]^{54}4f^{5}5d^{0}6s^{2}$
atomic number = 91, protactinium
The electronic configuration is $[Rn]^{86}5f^{2}6d^{1}7s^{2}$
Atomic number = 101, Mendelevium
The electronic configuration is $[Rn]^{86}5f^{13}6d^{0}7s^{2}$
Atomic number = 109, Meitnerium
The electronic configuration is $[Rn]^{86}5f^{14}6d^{7}7s^{2}$
(i) Electronic configurations
Answer :
Electronic configurations-
In 1st, 2nd and 3rd transition metal series 3 $d$ , 4 $d$ and 5 $d$ orbitals are used respectively. In the first series copper and zinc show unusual electronic configurations.
$\\Cr = 3d^54s^1\\ Cu = 3d^{10}4s^9$
In the second transition series, different electron configurations are shown by the following metals,
$Mo$ (42) = 4d 5 5s 1 , $Tc$ (43) = 4d 6 5s 1 , $Ru$ (44) = 4d 7 5s 1 , $Rh$ (45) = 4d 8 5s 1, $Pd$ (46) = 4d 10 5s 0 , $Ag$ (47) = 4d 10 5s 1
In the 3rd series there are also some metals which show this type of behaviour such as;
$W$ (74) = 5d 4 6s 2 , $Pt$ (78) = 5d 9 6s 1
(ii) oxidation states
Answer :
In each of the three transition series, the no. of oxidation state is minimum at the extremes and the highest at the middle of the row. In the first transition series, the +2 and +3 oxidation states are quite stable. Elements of first transition series metals form stable compounds of +2 and +3 oxidation states. But the stability of +2 and +3 oxidation state decreases in the second and third series.
Second and third transition series metals formed complexes in which their oxidation state is high ( $WCl_{6}, ReF_{7}$ ) and in the first transition series ( $[Co(NH_{3})_{6}]^{3+}, [Ti(H_{2}O)_{6}]^{3+}$ ) are stable complexes.
(iii) ionisation enthalpies
Answer :
In all of the three transition series, the 1st ionisation energy increases from the left side to the right side. But, there are some exceptions like the first ionisation enthalpies of the third transition series are more significant than those of the first and second transition series. This is happening due to the weak shielding effect of 4 electrons in the third series.
Some elements in the second series have higher first IE than elements of the same column in the first transition series. There are also elements in the 2nd transition series whose first IE are lower than those of the elements corresponding to the same vertical column in the 1st transition series.
(iv) atomic sizes.
Answer : Generally, atomic sizes decrease from left to right across the period. In among the three transition series, the size of the second series element is bigger than that of the first transition element of the same vertical group. But the atomic size of the third transition element is nearly the same as the element of the second transition series element. This is because of Lanthanoid contraction.
Answer : For $Ti^{2+}$ d-orbitals has two electrons. So, filling of d-orbitals can be $t^{2}_{2g}$
In $V^{2+}$ d-orbital has three electrons. So, the filling of d-orbital can be $t^{3}_{2g}$
Similarly
|
$Cr^{3+}$ (Ions)
|
$d^{3}$ (No. of d electrons)
|
$t^{3}_{2g}$ (Filling of d-orbitals)
|
|
$Mn^{2+}$
|
$d^{5}$
|
$t^{3}_{2g}, e^{2}_{g}$
|
|
$Fe^{2+}$
|
$d^{6}$
|
$t^{4}_{2g}, e^{2}_{g}$
|
|
$Fe^{3+}$
|
$d^{5}$
|
$t^{3}_{2g}, e^{2}_{g}$
|
|
$Co^{2+}$
|
$d^{7}$
|
$t^{5}_{2g}, e^{2}_{g}$
|
|
$Ni^{2+}$
|
$d^{8}$
|
$t^{6}_{2g}, e^{2}_{g}$
|
|
$Cu^{2+}$
|
$d^{9}$
|
$t^{6}_{2g}, e^{3}_{g}$
|
Question 4.37 Comment on the statement that elements of the first transition series possess many properties different from those of heavier transition elements.
Answer : Elements of the first transition series possess many properties different from those of heavier transition elements in the following ways-
- The atomic size of the 1st transition series is smaller than those of the 2nd and 3rd series elements. But due to lanthanoid contraction, the atomic size of the 2nd series elements is nearly the same as 3rd series element of the corresponding same vertical group.
- In 1st transition series +2 and +3 oxidation states are more common but in the 2nd and 3rd series higher oxidation states are more common.
- The enthalpy of atomisation of first series elements is lower than 2nd and 3rd series elements.
- The melting and boiling point of the 1st transition series is less than that of heavier metals. This is because of strong metallic bonding in heavier metals.
Question 4.38 What can be inferred from the magnetic moment values of the following complex species ?
Example Magnetic Moment (BM)
$K_{4}[Mn(CN)_{6})$ 2.2
$[Fe(H_{2}O)_{6}]^{2+}$ 5.3
$K_{2}[MnCl_{4}]$ 5.9
Answer :
Magnetic moment is given as - $\mu = \sqrt{n(n+2)}$
Putting the value on n = 1, 2, 3, 4, 5 (number of unpaired electrons in d-orbital)
We get the value of $\mu$ are 1.732, 2.83, 3.87, 4.899, 5.92 respectively.
$K_{4}[Mn(CN)_{6})$
By comparing with our calculation we get the values n nearest to 1. It means, in the above compound d-orbital has one unpaired electron( $Mn^{2+} = [d^{5}]$ ), which means $CN$ is a strong field ligand that cause force pairing of the electron.
$[Fe(H_{2}O)_{6}]^{2+}$
After comparing with our calculation the nearest value of n = 4. Here iron is in +2 oxidation state ( $d^{6}$ configuration). So, we can say that $H_{2}O$ is a weak field ligand, which does not cause any force pairing.
$K_{2}[MnCl_{4}]$
By observing we get the nearest value of n is 5. So, in this complex Manganese has $d^{5}$ configuration. So, we conclude that the $Cl$ ligand does not cause any force pairing and hence it is a weak ligand.
NCERT Class 12 Chemistry Chapter 4: Higher Order Thinking Skill (HOTS) Questions
These HOTS questions are designed to help students understand class 12 chemistry chapter 4 d and f block elements solutions beyond the basics. Solving them helps develop analytical skills, critical thinking, and problem-solving abilities required for board exams and competitive tests.
Question 1. The number of unpaired electrons responsible for the paramagnetic nature of the following complex species is respectively :
$\left[\mathrm{Fe}(\mathrm{CN})_6\right]^{3-},\left[\mathrm{FeF}_6\right]^{3-},\left[\mathrm{CoF}_6\right]^{3-},\left[\mathrm{Mn}(\mathrm{CN})_6\right]^{3-}$
1) $1,5,4,2$
2) $1,5,5,2$
3) $1,1,4,2$
4) $1,4,4,2$
Answer:
$\left[\mathrm{Fe}(\mathrm{CN})_6\right]^{3-} \Rightarrow \mathrm{Fe}^{3+}, \mathrm{d}^5, \mathrm{t}_{2 \mathrm{~g}}^5 \mathrm{e}_{\mathrm{g}}^0$
$\Rightarrow 1$ unpaired electron
$\left[\mathrm{FeF}_6\right]^{3-} \Rightarrow \mathrm{Fe}^{3+}, \mathrm{d}^5, \mathrm{t}_2^3 \mathrm{~g}_{\mathrm{g}}^2$
$\Rightarrow 5$ unpaired electrons
$\left[\mathrm{CoF}_6\right]^{3-} \Rightarrow \mathrm{Co}^{3+}, \mathrm{d}^6, \mathrm{t}_2^4 \mathrm{ge}_{\mathrm{g}}^2$
$\Rightarrow 4$ unpaired electrons
$\begin{aligned} {\left[\mathrm{Mn}(\mathrm{CN})_6\right]^{3-} \Rightarrow } & \mathrm{Mn}^{3+}, \mathrm{d}^4,\end{aligned} \mathrm{t}_{2 \mathrm{~g}}^4 \mathrm{e}_{\mathrm{g}}^0 \mathrm{C}$.
$\Rightarrow 2$ unpaired electron
Hence, the correct answer is option (1).
Question 2. Among, $\mathrm{Sc}, \mathrm{Mn}, \mathrm{Co}$ and Cu , identify the element with highest enthalpy of atomisation. The spin only magnetic moment value of that element in its +2 oxidation state is ___________ BM (in nearest integer).
Answer:
Enthalpy of atomisation of : Sc = $326 \mathrm{~kJ} / \mathrm{mol}, \mathrm{Mn}=$ $281 \mathrm{~kJ} / \mathrm{mol}, \mathrm{Co}=425 \mathrm{~kJ} / \mathrm{mol}, \mathrm{Cu}=338 \mathrm{~kJ} / \mathrm{mol}$
$ \mathrm{Co}^{2+}:[\mathrm{Ar}] 4 \mathrm{~s}_0 3 \mathrm{~d}^7: $
since the number of unpaired electrons are 3. Therefore, the magnetic moment will be,
$\mu=\sqrt{3(3+2)}=\sqrt{15}=3.87$
Hence, the answer is 3.87.
Question 3. The correct decreasing order of spin only magnetic moment values $(\mathrm{BM})$ of $\mathrm{Cu}^{+}, \mathrm{Cu}^{2+}, \mathrm{Cr}^{2+}$ and $\mathrm{Cr}^{3+}$ ions is:
(1) $\mathrm{Cu}^{+}>\mathrm{Cu}^{2+}>\mathrm{Cr}^{3+}>\mathrm{Cr}^{2+}$
(2) $\mathrm{Cu}^{2+}>\mathrm{Cu}^{+}>\mathrm{Cr}^{2+}>\mathrm{Cr}^{3+}$
(3) $\mathrm{Cr}^{2+}>\mathrm{Cr}^{3+}>\mathrm{Cu}^{2+}>\mathrm{Cu}^{+}$
(4) $\mathrm{Cr}^{3+}>\mathrm{Cr}^{2+}>\mathrm{Cu}^{+}>\mathrm{Cu}^{2+}$
Answer:
$\mathrm{Cu}^{+} \Rightarrow 3 \mathrm{~d}^{10} \Rightarrow$ 
$\mathrm{Cu}^{2+} \Rightarrow 3 \mathrm{~d}^9 \Rightarrow$ 
$\mathrm{Cr}^{2+} \Rightarrow 3 \mathrm{~d}^4 \Rightarrow$ 
$\mathrm{Cr}^{3+} \Rightarrow 3 \mathrm{~d}^3 \Rightarrow$ 
So order :
$\mathrm{Cr}^{2+}>\mathrm{Cr}^{3+}>\mathrm{Cu}^{2+}>\mathrm{Cu}^{+}$
Hence, the correct answer is option (3).
Question 4. The first transition series metal ' M ' has the highest enthalpy of atomisation in its series. One of its aquated ion $\left(\mathrm{M}^{\mathrm{n}}\right)$ exists in green colour. The nature of the oxide formed by the above $\mathrm{M}^{\mathrm{n}-}$ ion is :
(1) neutral
(2) acidic
(3) basic
(4) amphoteric
Answer:
In 3d series Vanadium has highest enthalpy of atomization and colour of $\mathrm{V}^{+3}$ is green.
Oxide form by $\mathrm{V}^{+3}$ is $\mathrm{V}_2 \mathrm{O}_3$ (Basic oxide)
Hence, the correct answer is option (3).
Question 5: The incorrect relationship in the following pairs in relation to ionisation enthalpies is :
(1) $\mathrm{Mn}^{+}<\mathrm{Cr}^{+}$
(2) $\mathrm{Mn}^{+}<\mathrm{Mn}^{2+}$
(3) $\mathrm{Fe}^{2+}<\mathrm{Fe}^{3+}$
(4) $\mathrm{Mn}^{2+}<\mathrm{Fe}^{2+}$
Answer:
$\mathrm{Mn}^{2+}:[\mathrm{Ar}] 3 \mathrm{~d}^5$
Half filled stability
Therefore, more I.E. than $\mathrm{Fe}^{2+}$
$\mathrm{Fe}^{2+}:[\mathrm{Ar}] 3 \mathrm{~d}^6$
Hence, the correct answer is option (4).
Question 6: Which of the following Lanthanoids does not have any electrons in the 5d subshell, while writing the electronic configuration in atomic form?
(1) Cerium $\left(_{58} \mathrm{Ce}\right)$
(2) Europium ( ${ }_{63}$ Eu)
(3) Gadolinium $\left({ }_{64} \mathrm{Cd}\right)$
(4) Lutetium ($_{71} \mathrm{Lu}$ )
Answer:
Electronic configuration.
$
E u: 4 f^7 6 s^2 \text {. }
$
Hence, the correct answer is option (2).
Question 7: The metal ions that have the calculated spin only magnetic moment value of 4.9 B.M. are
A. $\mathrm{Cr}^{2+}$
B. $\mathrm{Fe}^{2+}$
C. $\mathrm{Fe}^{3+}$
D. $\mathrm{Co}^{2+}$
E. $\mathrm{Mn}^{3+}$
Choose the correct answer from the options given below
(1) A, C and E only
(2) A, D and E only
(3) B and E only
(4) A, B and E only
Answer:
Given magnetic moment $=4.9 \mathrm{~B} . \mathrm{M}$.
We know M.M $=\sqrt{\mathrm{n}(\mathrm{n}+2)}$ B.M.
Where, $\mathrm{n} \rightarrow$ No. of unpaired $\mathrm{e}^{-}$
$4.9=\sqrt{n(n+2)}$
Solving this equation, we find that $n=4$.
(A) ${ }_{24} \mathrm{Cr}^{2+} \Rightarrow[\mathrm{Ar}] 3 \mathrm{~d}^4 \quad$ (4 unpaired $\mathrm{e}^{-}$)
(B) ${ }_{26} \mathrm{Fe}^{2+} \Rightarrow[\mathrm{Ar}] 3 \mathrm{~d}^6 \quad$ (4 unpaired $\mathrm{e}^{-}$)
(C) ${ }_{26} \mathrm{Fe}^{3+} \Rightarrow[\mathrm{Ar}] 3 \mathrm{~d}^5 \quad$ (5 unpaired $\mathrm{e}^{-}$)
(D) ${ }_{27} \mathrm{Co}^{2+} \Rightarrow[\mathrm{Ar}] 3 \mathrm{~d}^7 \quad\left(3\right.$ unpaired $\left.\mathrm{e}^{-}\right)$
(E) ${ }_{25} \mathrm{Mn}^{3+} \Rightarrow[\mathrm{Ar}] 3 \mathrm{~d}^4 \quad(4$ unpaired e $)$
The metal ions with a spin-only magnetic moment of approximately 4.9 B.M. are $\mathrm{Cr}^{2+}, \mathrm{Fe}^{2+}$, and $\mathrm{Mn}^{3+}$. Because, the value of n=4 for these ions.
Hence, the correct answer is option (4).
NCERT Exemplar Solutions Class 12 Subject-Wise
- NCERT Exemplar Class 12 Solutions
- NCERT Exemplar Class 12th Maths Solutions
- NCERT Exemplar Class 12th Physics Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Class 12th Biology Solutions
Approach to Solve Questions of Class 12 Chemistry Chapter 4
The elements present in the middle of the periodic table from Group 3 to 12 are called d-block elements. The name d-blocks because the last electron enters the d-orbital of the penultimate shell. This chapter includes both theory-based understanding and application-based questions. The step by step approach for class 12 chemistry chapter 4 d and f block elements solutions are given below:
1. Start by clearly understanding the electronic configuration, oxidation states and general properties of d- and f-block elements. These concepts form the foundation for most class 12 chemistry chapter 4 d and f block elements question answer. These question answer will help you understand these concepts better.
2. Make your focus on periodic trends such as atomic size, ionization enthalpy, melting/boiling points and magnetic properties within the transition and inner transition elements.
3. Try to remember common colored compounds and their oxidation states, especially for transition metals. You can make flowcharts or flashcards to revise it. You can learn these concepts in d and f block elements ncert notes.
4. Give proper attention to the important reactions like the preparation and properties of potassium dichromate and potassium permanganate, and their oxidizing behaviour in acidic, basic, and neutral media.
5. You can refer to the NCERT solved examples and try solving in-text questions for a better understanding of the concept. Also, solve the textbook exercise questions as they are often directly asked in board exams. You can also refer to the NCERT exemplar for better learning. Practice previous year questions and solve mock tests.
NCERT Books and NCERT Syllabus
Topics and subtopics covered in the NCERT Textbook Class 12 Chemistry Chapter 4
These class 12 chemistry d and f block elements question answer focuses on fundamental concepts, reaction rates, and factors affecting reactions. The chapter is divided into several topics and subtopics to help students understand the subject systematically
4.1 Position in the Periodic Table
4.2 Electronic Configurations of the d-Block Elements
4.3 General Properties of the Transition Elements
4.3.1. Physical Properties
4.3.2. Variation in Atomic and Ionic Sizes of Transition Metals
4.3.3. Ionisation Enthalpies
4.3.4. Oxidation States
4.3.5. Trends in the M2+/M Standard ElectrodePotentials
4.3.6. Trends in the M3+/M2+ Standard Electrode Potentials
4.3.7. Trends in Stability of Higher Oxidation States
4.3.8. Chemical Reactivity and Eo Values
4.3.9. Magnetic Properties
4.3.10. Formation of Coloured Ions
4.3.11 Formation of Complex Compounds
4.3.12 Catalytic Properties
4.3.13 Formation of Interstitial Compounds
4.3.14 Alloy Formation
4.4 Some Important Compounds of Transition Elements
4.4.1 Oxides and Oxoanions of Metals
4.5 The Lanthanoids
4.5.1 Electronic Configurations
4.5.2 Atomic and Ionic Sizes
4.5.3 Oxidation States
4.5.4 General Characteristics
4.6 The Actinoids
4.6.1 Electronic Configurations
4.6.2 Ionic Sizes
4.6.3 Oxidation States
4.6.4 General Characteristics and Comparison with Lanthanoids
4.7 Some Applications of d- and f-Block Elements
NCERT Solutions for Class 12 Subject-wise
What Extra Should Students Study Beyond the NCERT for JEE?
Along with class 12 chemistry chapter 4 d and f block elements question answer, students preparing for JEE should explore advanced problem solving books and practice higher level numerical questions. Here's a comparison table highlighting what to study beyond the NCERT for JEE:
What Students Learn from NCERT Solutions for Class 12 Chemistry Chapter 4 The d- and f-Block Elements
These class 12 chemistry chapter 4 d and f block elements solutions help students understand the unique properties and trends of transition and inner transition elements. Given key some points on key learning of this chapter:
- Using these solutions students can understand where the d-block and f-block elements are placed and their general characteristics.
- NCERT Class 12 solution help in learning the electron filling order, variable oxidation states, and reasons for electronic anomalies.
- Trends in metallic character, atomic and ionic sizes, ionisation enthalpies, and catalytic properties are well explained in these d and f block elements ncert solutions.
- Here students will learn about crystal field theory and how unpaired electrons cause colour.
- These d and f block elements class 12 question answers cover topics like how transition metals form coordination complexes, decrease in ionic radii of lanthanides, similarities and differences between actinides and lanthanides, and their variable oxidation states.
NCERT Solutions Class 12 Chemistry Chapter-Wise
Along with class 12 chemistry chapter 4 d and f block elements question answer , here are the links to chapter-wise NCERT Class 12 solutions:
Frequently Asked Questions (FAQs)
Class 12 Chemistry Chapter 4 The d and f block elements solutions are step by step answers to all the textbook questions from this chapter. They help students understand the concepts, properties, trends, and reactions of transition d-block and inner transition f-block elements clearly, making it easier to revise.
The colour of d-block elements is due to the d-d transitions. When white light falls on a transition metal ion, electrons in the partially filled d-orbitals absorb specific wavelengths of light to jump to higher energy d-orbitals. The remaining unabsorbed wavelengths constitute the observed colour.
D-block elements are those in which the last electron enters the d-orbital of the penultimate (n-1) shell. They are located in the middle of the periodic table, between the s-block and p-block elements, in Groups 3 to 12. They are also known as transition elements or transition metals.
f-block elements are those in which the last electron enters the f-orbital of the antepenultimate (n-2) shell. They are located at the bottom of the periodic table as two separate series: the lanthanides and the actinides. They are also known as inner transition elements.
d block elements have electrons in both their outermost shell and in the d subshell. This configuration allows them to lose different numbers of electrons when forming compounds. The presence of multiple oxidation states is a characteristic feature of transition metals due to the relatively close energy levels of the s and d orbitals, enabling them to participate in various chemical reactions.
Transition metals generally exhibit high melting and boiling points, strength, and are good conductors of heat and electricity. They are also known for their ability to form colored compounds, paramagnetism, and catalytic properties. These characteristics arise from their d orbitals, which allow for various bonding and interaction with light.
Lanthanides primarily consist of elements that fill the 4f subshell and are typically characterized by their high magnetic susceptibility and similar chemical properties. Actinides on the other hand, fill the 5f subshell and include radioactive elements, with many having significant applications in nuclear energy and medicine.
D and f block elements play vital roles in biological systems as trace elements or primary components of essential biomolecules. For instance, iron is crucial for hemoglobin function, while cobalt is vital for vitamin B12. Other elements like manganese, zinc, and copper are essential co-factors in various enzymatic reactions.
The solubility of transition metal compounds can be explained by several factors, including the charge on the metal ion, the size of the ions, and the nature of the ligands. Some metal ions can form complex ions with ligands which can enhance their solubility.
Questions related to CBSE Class 12th
On Question asked by student community
Hello
You will be able to download the CBSE Previous Year Board Question Papers from our official website, careers360, by using the link given below.
https://school.careers360.com/boards/cbse/cbse-previous-year-question-papers
I hope this information helps you.
Thank you.
Hello
You will be able to download the CBSE Pre-Board Class 12 Question Paper 2025-26 from our official website by using the link which is given below.
https://school.careers360.com/boards/cbse/cbse-pre-board-class-12-question-paper-2025-26
I hope this information helps you.
Thank you.
Hello,
Yes, it's completely fine to skip this year's 12th board exams and give them next year as a reporter or private candidate, allowing you to prepare better; the process involves contacting your current school or board to register as a private candidate or for improvement exams during the specified
HELLO,
Yes i am giving you the link below through which you will be able to download the Class 12th Maths Book PDF
Here is the link :- https://school.careers360.com/ncert/ncert-book-for-class-12-maths
Hope this will help you!
Hello,
Here is your Final Date Sheet Class 12 CBSE Board 2026 . I am providing you the link. Kindly open and check it out.
https://school.careers360.com/boards/cbse/cbse-class-12-date-sheet-2026
I hope it will help you. For any further query please let me know.
Thank you.
Popular CBSE Class 12th Questions
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
