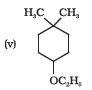
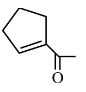
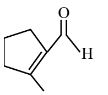
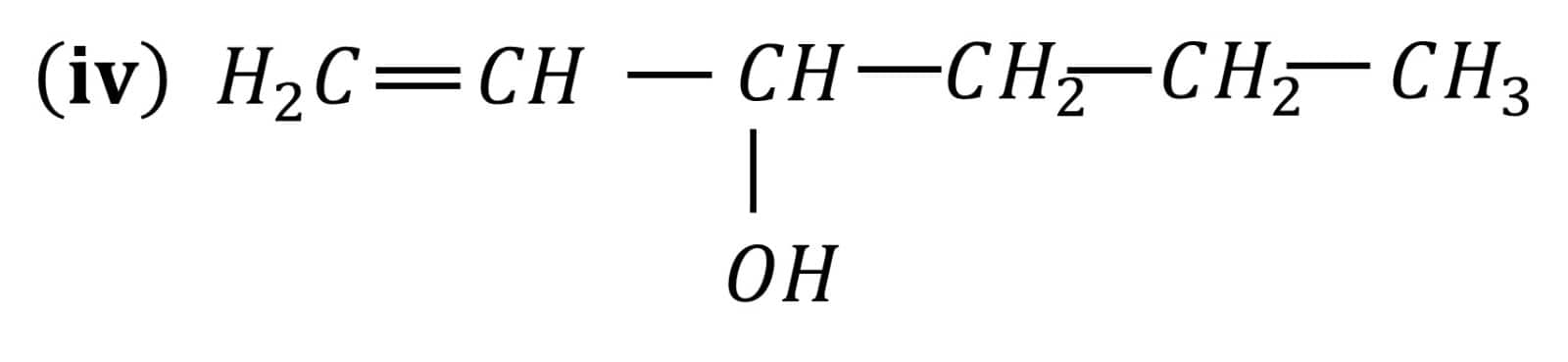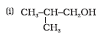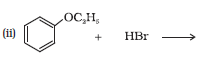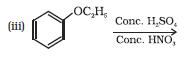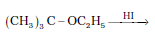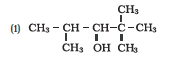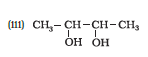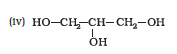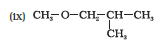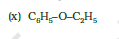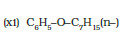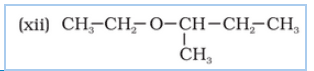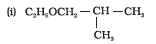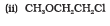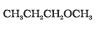NCERT solutions for Class 12 Chemistry Chapter 7 Alcohols, Phenols and Ethers
Have you ever wondered the things like soft drinks, perfumes, antiseptics, and disinfectants that we use in our daily lives come from? So, all of these come from oxygen-containing organic compounds like alcohol, such as ethanol used in soft drinks, beverages, and sanitizers, while Phenols are used in antiseptics and disinfectants, and Ether is used in the manufacturing of perfumes. These alcohols, phenols and ethers ncert solutions discusses three important classes of compounds that feature oxygen-containing organic compounds which are widely used in the medical industry and in daily life.
Candidates can check the CBSE class 10 marking scheme 2026 in the table below:
| Subjects | Theory marks | Internal assessment |
| English | 80 | 20 |
| Hindi | 80 | 20 |
| Maths | 80 | 20 |
| Science | 80 | 20 |
| Social Science | 80 | 20 |
This Story also Contains
- NCERT Solutions for Class 12 Chemistry Chapter 7: Download PDF
- NCERT Solutions for Class 12 Chemistry Chapter 7 (Intext Questions Exercise)
- NCERT Solutions for Class 12 Chemistry Chapter 7 (Exercise Questions with Answers)
- Class 12 Chemistry NCERT Chapter 7: Higher Order Thinking Skills (HOTS) Questions
- Approach to Solve Questions of Class 12 Chemistry Chapter 7
- Topics and Subtopics Covered in the NCERT Textbook
- What Extra Should Students Study Beyond the NCERT for JEE?
- What Students Learn from NCERT Solutions for Class 12 Chemistry Chapter 7 Alcohols, Phenols and Ethers
- Importance of Class 12 Chemistry Chapter 7 Alcohols, Phenols and Ethers Solutions
- NCERT Solutions Class 12 Chemistry Chapter-Wise
- NCERT Solutions for Class 12 Subject-wise
- NCERT Books and NCERT Syllabus

The NCERT Solution for Class 12 Chemistry explains various concepts that we observe in our daily lives. When the OH group replaces the hydrogen atom in aliphatic hydrocarbons, Alcohols are formed same goes for phenols when the OH group replaces the hydrogen of aromatic hydrocarbons, then Phenols are formed while Ethers are formed when alkoxy (R-O) or aryloxy (Ar-O) group substitutes the hydrogen atom in hydrocarbons. These NCERT Solutions provide a structured approach to understanding the classification, properties, preparation, and reactions. Students will learn about important concepts like nomenclature, structure, and uses of organic compounds containing oxygen. This article includes some higher-order thinking skills (HOTS) questions and approach to solve questions.
NCERT Solutions for Class 12 Chemistry Chapter 7: Download PDF
Students can download the class 12 chemistry chapter 7 alcohols, phenols and ethers solutions pdf for free. These NCERT Solution for Class 12 are designed to help you understand the fundamental concepts and solve textbook questions with ease.
Also Read:
NCERT Solutions for Class 12 Chemistry Chapter 7 (Intext Questions Exercise)
These class 12 chemistry chapter 7 alcohols, phenols and ethers question answer provide clear and detailed answers to all intext questions, helping students understand the key concepts of alcohols, phenols, and ethers.
Question 7.1 (1) Classify the following as primary, secondary and tertiary alcohols:

Answer :
To classify we look at the OH bonded carbon.
Here, only 1 carbon is attached to it, hence it is primary alcohol .
Question 7.1 (2) Classify the following as primary, secondary and tertiary alcohols:

Answer :
To classify we look at the OH bonded carbon.
Here, only 1 carbon is attached to it, hence it is primary alcohol.
Question 7.1 (3) Classify the following as primary, secondary and tertiary alcohols:
Answer :
To classify we look at the OH bonded carbon.
Here, only 1 carbon is attached to it, hence it is primary alcohol.
Question 7.1 (4) Classify the following as primary, secondary and tertiary alcohols:

Answer :
To classify we look at the OH bonded carbon.
Here, 2 carbons are attached to it, hence it is secondary alcohol.
Question 7.1 (5) Classify the following as primary, secondary and tertiary alcohols:

Answer :
To classify we look at the OH bonded carbon.
Here, 2 carbons are attached to it, hence it is secondary alcohol.
Question 7.1 (6) Classify the following as primary, secondary and tertiary alcohols :
Answer:
To classify we look at the OH bonded carbon.
Here, 3 carbons are attached to it, hence it is tertiary alcohol.
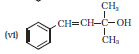
Question 7.2 Identify allylic alcohols in the above examples.
Answer :
The alcohols (ii) and (vi) are allylic alcohols. Because -C=C-C-OH is the skeleton of allylic alcohol.
Question 7.3 (1) Name the following compounds according to IUPAC system.
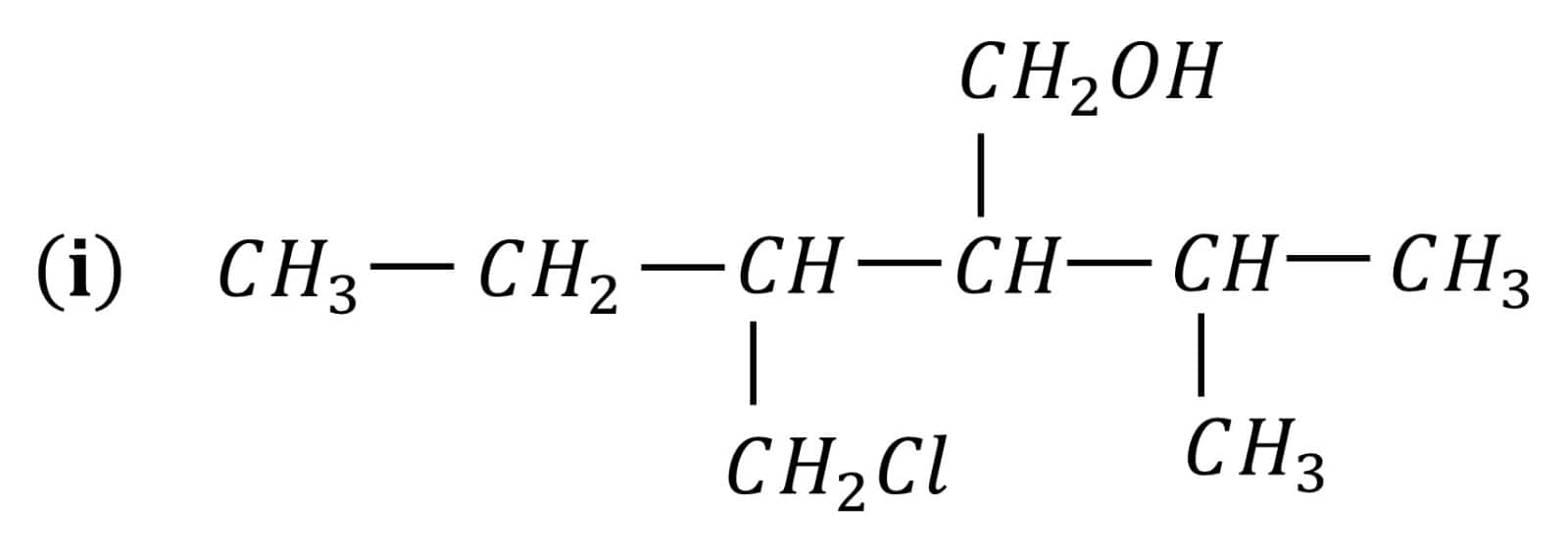
Answer :
3-Chloromethyl-2-isopropylpentan-1-ol
Question 7.3 (2) Name the following compounds according to IUPAC system.
.jpeg)
Answer :
2, 5-Dimethylhexane-1, 3-diol
Question 7.3 (3) Name the following compounds according to IUPAC system.
Answer :
3-Bromocyclohexanol
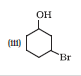
Question 7.3 (5) Name the following compounds according to IUPAC system.
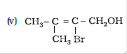
Answer :
2-Bromo-3-methylbut-2-en-1-ol
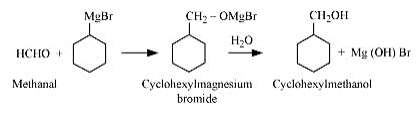
Question 7.4 (1) Show how are the following alcohols prepared by the reaction of a suitable Grignard reagent on methanal ?
Answer :
The reaction of a suitable Grignard reagent on the methanal is mentioned below:
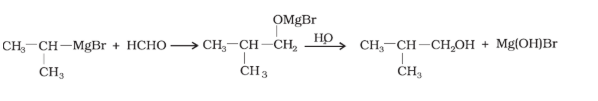
Question 7.4 (2) Show how are the following alcohols prepared by the reaction of a suitable
Grignard reagent on methanal ?
Answer :
Question 7.5 (2) Write structures of the products of the following reactions:
Answer :
The product of the given reaction is-
Question 7.5 (3) Write structures of the products of the following reactions:
Answer : 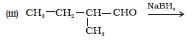
Product of the given reaction is
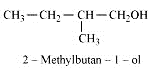
Question 7.6 (1) Give structures of the products you would expect when each of the following alcohol reacts with
(a) $HCL- ZnCl_2$ with Butan-1-ol
Answer :
Primary alcohols do no react with Lucas’ reagent.
Hence no reaction.
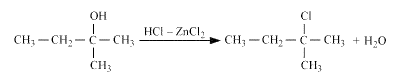
Question 7.6 (2) Give structures of the products you would expect when each of the following alcohol reacts with $HCl - ZnCl_2$ with 2-Methylbutan-2-ol
Answer :
Reaction of $HCl - ZnCl_2$ with 2-Methylbutan-2-ol
Question7.6 (3) Give structures of the products you would expect when each of the following alcohol reacts with HBr with Butan-1-ol
Answer :
Reaction of HBr with Butan-1-ol
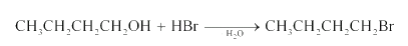
Question 7.6 (4) Give structures of the products you would expect when each of the following alcohol reacts with HBr with 2-Methylbutan-2-ol
Answer :
Reaction of HBr with 2-Methylbutan-2-ol
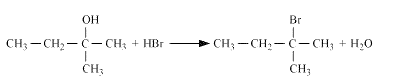
Question 7.6 (5) Give structures of the products you would expect when each of the following alcohol reacts with $SOCl_2$ with Butan-1-ol
Answer :
Reaction of $SOCl_2$ with Butan-1-ol
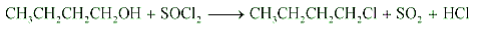
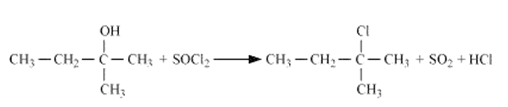
Question 7.6 (6) Give structures of the products you would expect when each of the following alcohol reacts with $SoCl_2$ with 2-Methylbutan-2-ol
Answer :
Reaction of $SoCl_2$ with 2-Methylbutan-2-ol
Question 7.7(1) Predict the major product of acid catalysed dehydration of 1-methylcyclohexanol
Answer :
Dehydration of 1-methylcyclohexanol
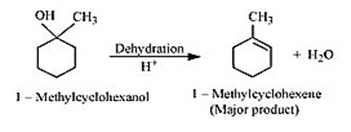
1-Methylcyclohexene is the major product.
Question 7.7 (2) Predict the major product of acid catalysed dehydration of butan-1-ol
Answer :
Dehydration of butan-1-ol
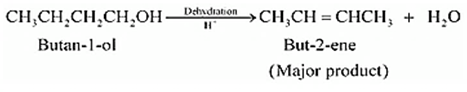
But-2-ene is the major product.
Answer :
Resonance structure of ortho-nitrophenol
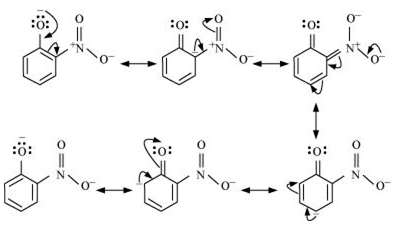
Resonance structure of para-nitrophenol
Question 7.9 (1) Write the equations involved in the following reactions: Reimer - Tiemann reaction
Answer :
Reimer - Tiemann reaction
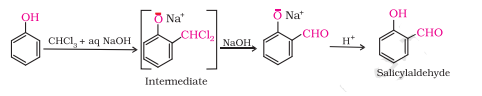
Question 7.9 (2) Write the equations involved in the following reactions: Kolbe’s reaction
Answer :
Kolbe’s reaction
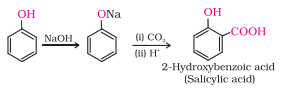
Question 7.10 Write the reactions of Williamson synthesis of 2-ethoxy-3-methylpentane starting from ethanol and 3-methylpentan-2-ol.
Answer :
1.Reaction of ethanol with hydrogen bromide.
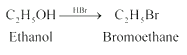
2. Reaction of 3-methylpentan-2-ol with sodium
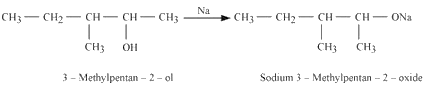
3. Reaction of product formed in 1st and reaction with the product formed in the 2nd reaction.
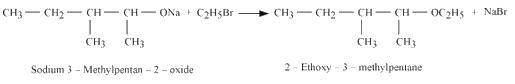
Question 7.11 Which of the following is an appropriate set of reactants for the preparation of 1-methoxy-4-nitrobenzene and why?
.jpeg)
Answer :
(ii) is appropriate because $CH_3Br$ is nucleophile whereas $CH_3ONa$ is also a strong base. So elimination will be dominating in (i).
Question 7.12 (1) Predict the products of the following reactions:
$CH_3 -CH_2 -CH_2 -O - CH_3 +HBr$
Answer :
Reaction is
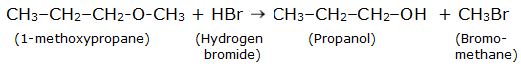
Question 7.12 (2) Predict the products of the following reactions:
Answer :
The reaction between ethoxybenzene and HBr is
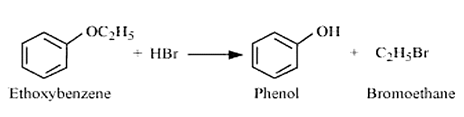
Question 7.12 (3) Predict the products of the following reactions:
Answer :
Reaction between ethoxybenzene and $Conc. \:H_{2}SO_{4} \:+\:conc. HNO_{3}$
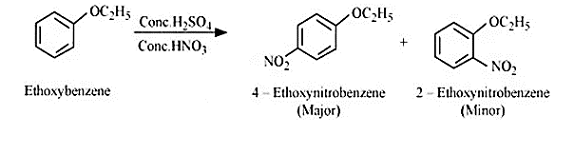
Question 7.12 (4) Predict the products of the following reactions:
Answer :
Reaction between ter - butyl ethyl ether and HI
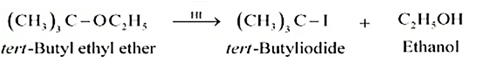
NCERT Solutions for Class 12 Chemistry Chapter 7 (Exercise Questions with Answers)
These class 12 chemistry chapter 7 alcohols, phenols and ethers solutions offer step by step answers to all exercise questions. They help students strengthen their understanding, practise reaction mechanisms, and prepare effectively for both board and competitive exams.
Question 7.1 (1) Write IUPAC names of the following compounds:
Answer :
IUPAC name of the given compound is 2,2,4-Trimethylpentan-3-ol
Question 7.1 (2) Write IUPAC names of the following compounds:
Answer :
IUPAC name of the given compound is 5-Ethylheptane-2,4-diol
Question 7.1 (3) Write IUPAC names of the following compounds:
Answer :
IUPAC name of the given compound is Butane-2,3-diol
Question 7.1 (4) Write IUPAC names of the following compounds:
Answer :
IUPAC name of the given compound is Propane-1,2,3-triol
Question 7.1 (5) Write IUPAC names of the following compounds:
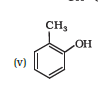
Answer :
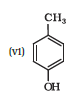
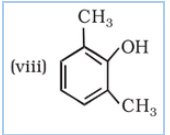
IUPAC name of the given compound is 2-Methylphenol
Question 7.1 (6) Write IUPAC names of the following compounds:
Answer :
IUPAC name of the given compound is 4-Methylphenol
Note : Also called p-cresol
Question 7.1 (7) Write IUPAC names of the following compounds:
Answer :
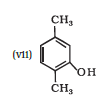
IUPAC name of the given compound is 2,5-Dimethylphenol
Question 7.1 (8) Write IUPAC names of the following compounds:
Answer :
IUPAC name of the given compound is 2,6-Dimethylphenol
Question 7.1 (9) Write IUPAC names of the following compounds:
Answer :
IUPAC name of the given compound is 1-Methoxy-2-methylpropane
Question 7.1 (10) Write IUPAC names of the following compounds:
Answer :
IUPAC name of the given compound is Ethoxybenzene
Question 7.1 (11) Write IUPAC names of the following compounds:
Answer :
IUPAC name of the given compound is 1-Phenoxyheptane
Question 7.1 (12) Write IUPAC names of the following compounds:
Answer :
IUPAC name of the given compound is 2-Ethoxybutane
Question 7.2 Write structures of the compounds whose IUPAC names are as follows:
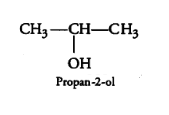
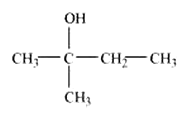
(i) 2-Methylbutan-2-ol
Answer :
Structure of 2-Methylbutan-2-ol
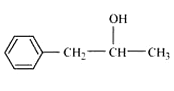
Question 7.2 Write structures of the compounds whose IUPAC names are as follows: (ii) 1-Phenylpropan-2-ol
Answer :
Structure of 1-Phenylpropan-2-ol
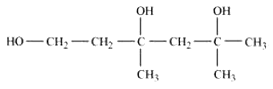
Question 7.2(iii) Write structures of the compounds whose IUPAC names are as follows: (iii) 3,5-Dimethylhexane –1, 3, 5-triol
Answer :
structure of 3,5-Dimethylhexane –1, 3, 5-triol
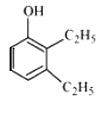
Question 7.2(iv) Write structures of the compounds whose IUPAC names are as follows: (iv) 2,3 – Diethylphenol
Answer :
structure of 2,3 – Diethylphenol
Question 7.2(v) Write structures of the compounds whose IUPAC names are as follows:
(v) 1 – Ethoxypropane
Answer :
structure of 1 – Ethoxypropane
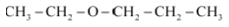
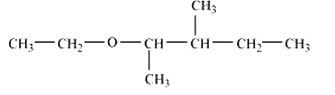
Question 7.2(vi) Write structures of the compounds whose IUPAC names are as follows:
(vi) 2-Ethoxy-3-methylpentane
Answer :
structure of 2-Ethoxy-3-methylpentane
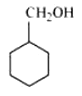
Question 7.2(vii) Write structures of the compounds whose IUPAC names are as follows:
(vii) Cyclohexylmethanol
Answer :
Structure of Cyclohexylmethanol
Question 7.2(viii) Write structures of the compounds whose IUPAC names are as follows:
(viii) 3-Cyclohexylpentan-3-ol
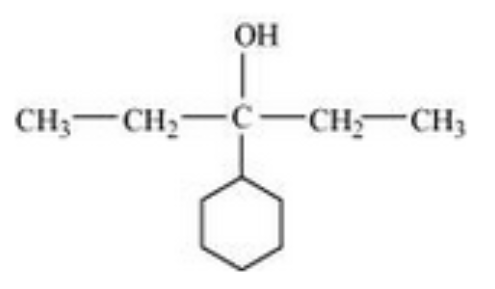
Answer :
The structure of 3-Cyclohexylpentan-3-ol is as follows:
Question 7.2(ix) Write structures of the compounds whose IUPAC names are as follows:
(ix) Cyclopent-3-en-1-ol
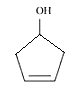
Answer :
structure of Cyclopent-3-en-1-ol
Question 7.2(x) Write structures of the compounds whose IUPAC names are as follows:
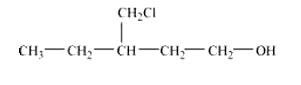
(x) 4-Chloro-3-ethylbutan-1-ol.
Answer :
structure of 4-Chloro-3-ethylbutan-1-ol
Question 7.3 (i) Draw the structures of all isomeric alcohols of molecular formula $C_5 H_{12}O$
and give their IUPAC names.
Answer :
The structures of all isomeric alcohols of C 5 H 12 O are given below:
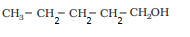 |
Pentan-1-ol
|
.png) |
Pentan-2-ol
|
.png) |
Pentan-3-ol
|
.png) |
3-Methylbutan-1-ol
|
.png) |
2,2-Dimethylpropan-1-ol
|
.png) |
3-Methylbutan-2-ol
|
.png) |
2-Methylbutan-2-ol
|
Question 7.3 (ii) Classify the isomers of alcohols in question 11.3 (i) as primary, secondary and tertiary alcohols.
Answer :
Primary Alcohol: Pentan-1-ol; 2-Methylbutan-1-ol; 3-Methylbutan-1-ol; 2,2-Dimethylpropan-1-ol
Secondary Alcohol: Pentan-2-ol; 3-Methylbutan-2-ol; Pentan-3-ol
Tertiary Alcohol: 2-Methylbutan-2-ol
Question 7.4 Explain why propanol has higher boiling point than that of the hydrocarbon, butane?
Answer :
Propanol forms intermolecular H-bonds because of the presence of -OH group while butane cannot. To break these bonds, extra energy will be required. This causes a higher boiling point for propanol as compared to butane.
Question 7.5 Alcohols are comparatively more soluble in water than hydrocarbons of comparable molecular masses. Explain this fact.
Answer :
Alcohols form hydrogen bonds with water due to the presence of –OH group whereas hydrocarbons cannot. Due to this inter molecular hydrogen bonding, alcohols are more soluble in water.
Question 7.6 What is meant by hydroboration-oxidation reaction? Illustrate it with an example.
Answer :
Hydroboration-oxidation reaction also called HBO reaction is the addition of borane followed by oxidation to produce alcohol.
Eg: Hydroboration-oxidation reaction of propene. In this reaction, propene reacts with diborane (BH3)2 to form trialkyl borane. This addition product is oxidized by hydrogen peroxide in the presence of aqueous sodium hydroxide to form propan-1-ol.
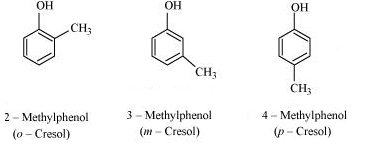
Question 7.7 Give the structures and IUPAC names of monohydric phenols of molecular formula, $C_7 H_8 O$ .
Answer :
The structures and IUPAC names of monohydric phenols of molecular formula, $C_7 H_8 O$ .
Phenol: $C_6 H_5 OH$
$C_7 H_8 O \rightarrow (C_6 H_5 OH)(C H_2)$
Answer :
Due to inter-molecular H bonding in para-nitrophenol, it gets tightly bonded with water. But ortho nitrophenol has intra-molecular H bonding and hence is steam volatile.
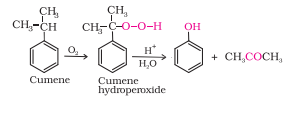
Question 7.9 Give the equations of reactions for the preparation of phenol from cumene.
Answer :
Cumene (isopropylbenzene) is oxidised in the presence of air to form cumene hydroperoxide. On treating with dilute acid it is converted to phenol and acetone.
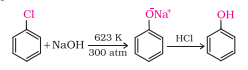
Question 7.10 Write chemical reaction for the preparation of phenol from chlorobenzene.
Answer :
Chlorobenzene, when fused with NaOH, produces sodium phenoxide which on acidification produces Phenol.
Question 7.11 Write the mechanism of hydration of ethene to yield ethanol.
Answer :
Ethanol is yielded from ethene by acid catalysed hydration.
The mechanism:
Step 1. Protonation of alkene to form carbocation by electrophilic attack of hydronium ion.
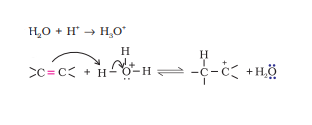
Step 2. Nucleophilic attack of water on carbocation .
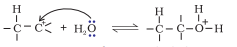
Step 3. Deprotonation to form an alcohol.
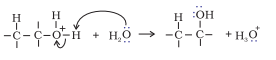
Question 7.12 You are given benzene, $conc. H_2SO_4$ and NaOH. Write the equations for the preparation of phenol using these reagents .
Answer :
When benzene reacts with $conc. \:H_2SO_4$ and heat it gives benzene sulphonic acid ,and after sulphonic this acid with NaOH then it gives phenol
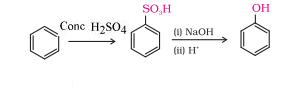
Question 7.13 Show how will you synthesise:
(i) 1-phenylethanol from a suitable alkene.
Answer :
Styrene on acid catalysed hydration gives 1-phenylethanol.
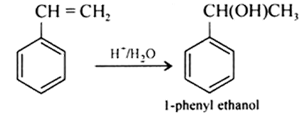
Question 7.13 (2) Show how will you synthesise:(ii) cyclohexylmethanol using an alkyl halide by an $SN^{2}$ reaction.
Answer :
On adding NaOH to chloromethylcyclohexane, cyclohexy methanol is formed.
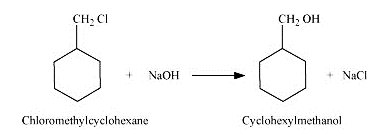
Question 7.13 Show how will you synthesise:
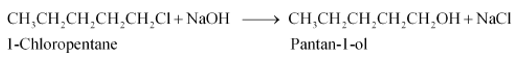
(iii) pentan-1-ol using a suitable alkyl halide?
Answer :
when 1-chloropentane reacts with NaOH it gives pantan-1-ol
Question 7.14 Give two reactions that show the acidic nature of phenol. Compare acidity of phenol with that of ethanol.
Answer :
1. Phenol reacts with sodium to give sodium phenoxide, liberating hydrogen gas.
.png)
2. Phenol reacts with sodium hydroxide to give sodium phenoxide and water.
.png)
Phenol is more acidic than ethanol. This is because phenol after losing a proton becomes phenoxide ion which is highly stable due to resonance whereas ethoxide ion does not.
Question 7.15 Explain why is ortho nitrophenol more acidic than ortho methoxyphenol ?
Answer :
Ortho-nitrophenol is more acidic than ortho- methoxyphenol . The presence of the nitro group, which is an electron withdrawing group, at the ortho position decreases the electron density in the O-H bond. Also, the o- nitrophenoxide ion formed after the loss of protons is stabile due to resonance. Hence, ortho nitrophenol is a stronger acid. Whereas the methoxy group is an electron-releasing group. Thus, it increases the electron density in the O-H bond.
Question 7.16 Explain how does the –OH group attached to a carbon of benzene ring activate it towards electrophilic substitution?
Answer :
The -OH group is an electron-donating group (EDG). Thus, it increases the electron density in the benzene ring in the resonance structure of phenol. As a result, the benzene ring is activated towards electrophilic substitution.
Question 7.17(i) Give equations of the following reactions:
(i) Oxidation of propan-1-ol with alkaline $KMnO_4$ solution.
Answer :
Oxidation of propane-1-ol with alkaline $KMnO_4$ solution gives propanoic acid.
$\\CH_{3}CH_{2}CH_{2}OH \rightarrow CH_{3}CH_{2}COOH\\$
Question 7.17(ii) Give equations of the following reactions:
(ii) Bromine in $CS_2$ with phenol.
Answer :
Bromine in $CS_2$ with phenol produces a mixture of o-bromo phenol and p-bromo phenol is formed.
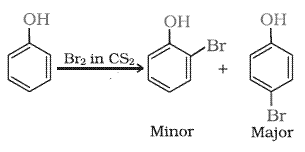
Question 7.17(iii) Give equations of the following reactions:
(iii) Dilute HNO3 with phenol.
Answer :
When dilute HNO3 reacts with phenol it gives o-bromo phenol and p-bromo phenol
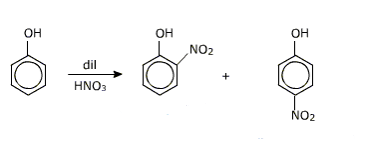
Question 7.17(iv) Give equations of the following reactions:
(iv) Treating phenol with chloroform in presence of aqueous NaOH.
Answer :
Treating phenol with chloroform in presence of aqueous NaOH.
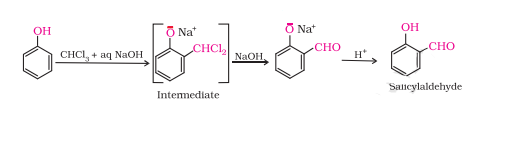
This reaction is known as the Reimer-Tiemann reaction.
Question 7.18(i) Explain the following with an example.
(i) Kolbe’s reaction.
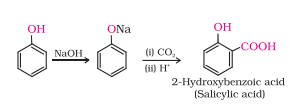
Answer :
Kolbe’s reaction: Phenol with carbon dioxide under pressure followed by treating the product with sulphuric acid produces Ortho-hydroxybenzoic acid (salicylic acid). Phenoxide ion generated is more reactive than phenol towards electrophilic aromatic substitution. Hence, it undergoes electrophilic substitution with carbon dioxide, a weak electrophile.
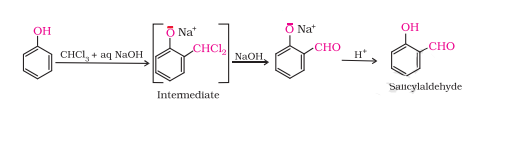
Question 7.18(ii) Explain the following with an example.
(ii) Reimer-Tiemann reaction.
Answer :
On treating phenol with chloroform in the presence of sodium hydroxide, a –CHO group is introduced at the ortho position of the benzene ring. This reaction is known as Reimer - Tiemann reaction
Question 7.18(iii) Explain the following with an example. (iii) Williamson ether synthesis.
Answer :
Williamson ether synthesis is a reaction forming ether from a primary alkyl halide via SN2 reaction.
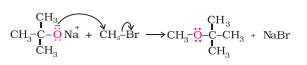
Question 7.18(iv) Explain the following with an example.(iv) Unsymmetrical ether.
Answer :
If the alkyl or aryl groups attached to the oxygen atom are different, then it is mixed or unsymmetrical ether.
Eg: $C_2H_5-O-CH_3\ and\ C_2H_5-O-C_6H_5$
Question 7.19 . Write the mechanism of acid dehydration of ethanol to yield ethene.
Answer :
Alcohols undergo dehydration (removal of a molecule of water) to form alkenes on treating with a protic acid. Ethanol undergoes dehydration by heating it with concentrated sulphuric acid at 443 K.
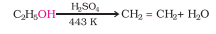
Question 7.20(i) How are the following conversions carried out?
(i) Propene $\rightarrow$ Propan-2-ol.
Answer :
Acid catalysed hydration of propene produces propan-2-ol.
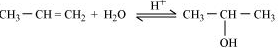
Question 7.20(ii) How are the following conversions carried out?
(ii) Benzyl chloride $\rightarrow$ Benzyl alcohol.
Answer :
Benzyl chloride treated with NaOH followed by acidification produces benzyl alcohol.
.jpg)
Question 7.20(iii) How are the following conversions carried out?
(iii) Ethyl magnesium chloride $\rightarrow$ Propan-1-ol.
Answer :
Ethyl magnesium chloride treated with formaldehyde followed by hydrolysis produces propan-1-ol.
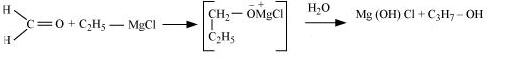
Question 7.20(iv) How are the following conversions carried out?
(iv) Methyl magnesium bromide $\rightarrow$ 2-Methylpropan-2-ol.
Answer :
Methyl magnesium bromide treated with propane, gives 2-methylpropane-2-ol on hydrolysis.
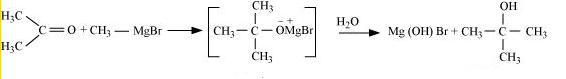
Question 7.21(i) Name the reagents used in the following reactions:
(i) Oxidation of a primary alcohol to carboxylic acid.
Answer :
Acidic/neutral/alkaline potassium permanganate $(KMnO_4)$ or acidified $K_2Cr_2O_7$
Question 7.21(ii) Name the reagents used in the following reactions:
(ii) Oxidation of a primary alcohol to aldehyde.
Answer :
The reagent used for oxidation of primary alcohol to aldehyde is Pyridinium chlorochromate (PCC) .
Question 7.21(iii) Name the reagents used in the following reactions:
(iii) Bromination of phenol to 2,4,6-tribromophenol.
Answer :
Reagents used in the bromination of phenol to 2,4,6-tribromophenol is Bromine water
Question 7.21(iv) Name the reagents used in the following reactions:
(iv) Benzyl alcohol to benzoic acid.
Answer :
Reagent used in the benzyl alcohol to benzoic acid isAcidified $KMnO_4$ (potassium permanganate)
Question 7.21(v) Name the reagents used in the following reactions:
(v) Dehydration of propan-2-ol to propene.
Answer :
Reagents used in the dehydration of propan-2-ol to propene is Concentrated Phosphoric acid.
Question 7.21(vi) Name the reagents used in the following reactions:
(vi) Butan-2-one to butan-2-ol.
Answer :
Reagent used in the butan-2-one to butan-2-ol is $LiAlH_4\ or\ NaBH_4$
Question 7.22 Give reason for the higher boiling point of ethanol in comparison to methoxymethane.
Answer :
Ethanol undergoes intermolecular hydrogen bonding due to the presence of -OH group. Therefore, extra energy is required to break those hydrogen bonds. Whereas methoxymethane does not make H-bonds and hence ethanol has a higher boiling point than methoxymethane.
Question 7.23(i) Give IUPAC names of the following ethers:
Answer :
IUPAC names of the given ether is 1-Ethoxy-2-methylpropane
Question 7.23(ii) Give IUPAC names of the following ethers:
Answer :
IUPAC names of the given ether is 2-Chloro-1-methoxyethane
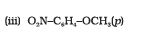
Question 7.23(iii) Give IUPAC names of the following ethers:
Answer :
IUPAC names of the given ether is 4-Nitroanisole
Question 7.23(iv) Give IUPAC names of the following ethers:
Answer :
IUPAC names of the given ether is 1-Methoxypropane
Question 7.23(v) Give IUPAC names of the following ethers:
Question 7.23(vi) Give IUPAC names of the following ethers:
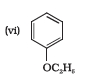
Answer :
IUPAC names of the given ether is Ethoxybenzene
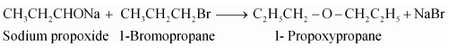
Question 7.24(i) Write the names of reagents and equations for the preparation of the following ethers by Williamson’s synthesis:
(i) 1-Propoxypropane
Answer :
Names of reagents and equations for the preparation of the 1-Propoxypropane ether by Williamson’s synthesis:-
Question 7.24(ii) Write the names of reagents and equations for the preparation of the following ethers by Williamson’s synthesis:
(ii) Ethoxybenzene
Answer :
Names of reagents and equations for the preparation of the Ethoxybenzene ether by Williamson’s synthesis:-
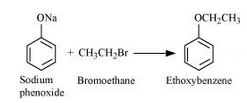 with NaBr as a side product.
with NaBr as a side product.
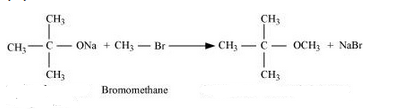
Question 7.24(iii) Write the names of reagents and equations for the preparation of the following ethers by Williamson’s synthesis:
(iii) 2-Methoxy-2-methylpropane
Answer :
Names of reagents and equations for the preparation of the 2-Methoxy-2-methylpropane ether by Williamson’s synthesis:-
Question 7.24(iv) Write the names of reagents and equations for the preparation of the following ethers by Williamson’s synthesis: (iv) 1-Methoxyethane
Answer :
Names of reagents and equations for the preparation of the1-Methoxyethane ether by Williamson’s synthesis:-
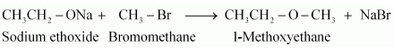
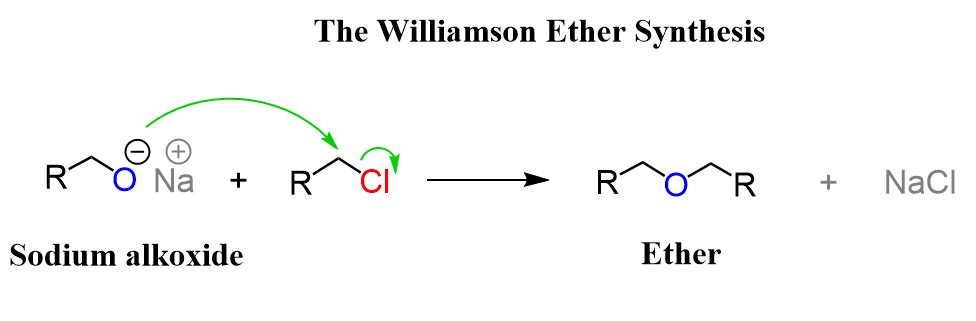
Question 7.25 Illustrate with examples the limitations of Williamson synthesis for the preparation of certain types of ethers.
Answer :
Williamson synthesis involves $S_N2$ attack by alkoxide ion on a primary alkyl halide. But if secondary or tertiary alkyl halides are taken then alkenes would be produced because elimination would take place. This is because alkoxides are nucleophiles as well as strong bases.
Question 7.26 How is 1-propoxypropane synthesised from propan-1-ol? Write mechanism of this reaction.
Answer :
Propan-1-ol on dehydration using protic acids such as sulphuric acid gives 1-propoxypropane.
Mechanism of this reaction:
Formation of protonated alcohol.
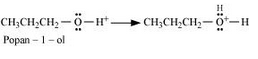
Formation of carbocation: It is the slowest step and hence, the rate determining step of the reaction.
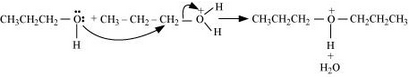
Formation of ethene by the elimination of a proton.
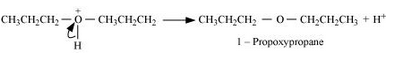
Question 7.27 Preparation of ethers by acid dehydration of secondary or tertiary alcohols is not a suitable method. Give reason.
Answer :
The formation of ethers by dehydration of a primary alcohol is an SN2 reaction. In case of secondary or tertiary alcohols, the alkyl group is hindered and hence elimination dominates substitution. Therefore alkenes are formed in place of ethers.
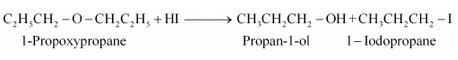
Question 7.28(i) Write the equation of the reaction of hydrogen iodide with:
(i) 1-propoxypropane
Answer :
1-propoxypropane reacts with HI to give propan-1-ol and 1-iodopropane as the products.
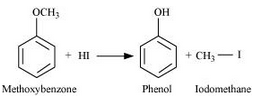
Question 7.28(ii) Write the equation of the reaction of hydrogen iodide with: (ii) methoxybenzene
Answer :
Methoxybenzene reacts with HI to give phenol and iodomethane.
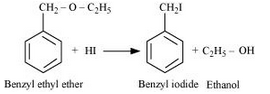
Question 7.28(iii) Write the equation of the reaction of hydrogen iodide with: (iii) benzyl ethyl ether.
Answer :
Benzyl ethyl ether reacts with HI to give benzyl iodide and ethanol.
Question 7.29(i) Explain the fact that in aryl alkyl ethers (i) the alkoxy group activates the benzene ring towards electrophilic substitution
Answer :
Due to the +R effect of the alkoxy group, it increases the electron density of the benzene ring pushing electrons into the ring making the benzene ring activated towards electrophilic substitution reactions.
Question 7.29(ii) Explain the fact that in aryl alkyl ethers (ii) it directs the incoming substituents to ortho and para positions in benzene ring.
Answer :
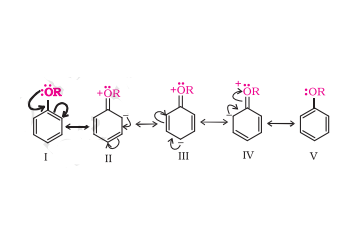
The above resonating structures shows that the electron density increases more at the ortho and para positions as compared to the meta positions. Hence, the alkoxy group directs the incoming substituents to ortho and para positions in the benzene ring.
7.30. Write the mechanism of the reaction of HI with methoxymethane.
Answer :
Following is the mechanism:
1. Protonation of methoxymethane
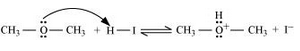
2. Nucleophilic attack of $I^-$
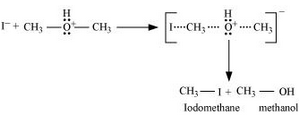
3. If HI is in excess, then methanol formed in step 2 reacts with another HI molecule and gets converted to methyl iodide at a high temperature.
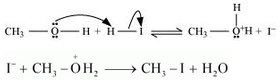
Question 7.31(i) Write equations of the following reactions:
(i) Friedel-Crafts reaction – alkylation of anisole.
Answer :
Fridel Craft reaction(Alkylation):
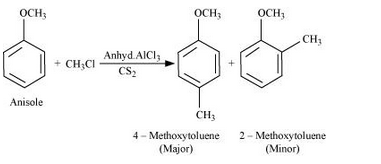
Question 7.31(ii) Write equations of the following reactions: (ii) Nitration of anisole.
Answer :
Nitration of anisole:
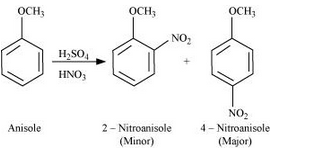
Question 7.31(iii) Write equations of the following reactions: (iii) Bromination of anisole in ethanoic acid medium.
Answer :
Bromination of anisole in ethanoic acid medium:
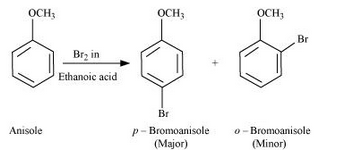
Question 7.31(iv) Write equations of the following reactions: (iv) Friedel-Craft’s acetylation of anisole.
Answer :
Friedel-Craft’s acetylation of anisole:
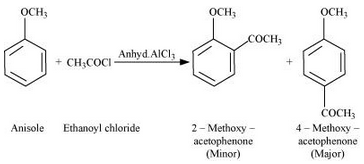
Question 7.32 Show how would you synthesise the following alcohols from appropriate alkenes?
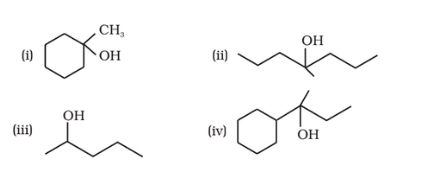
Answer :
(i) 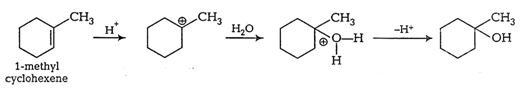
(ii) 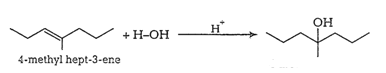
(iii) 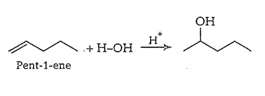
(iv) 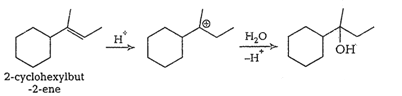
Question 7.33 When 3-methylbutan-2-ol is treated with HBr, the following reaction takes
place:
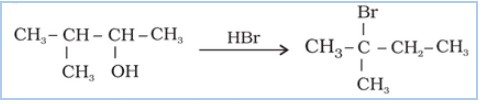
Give a mechanism for this reaction.
(Hint : The secondary carbocation formed in step II rearranges to a more stable tertiary carbocation by a hydride ion shift from 3rd carbon atom.
Answer :
Mechanism for the reaction 3-methylbutan-2-ol is treated with HBr
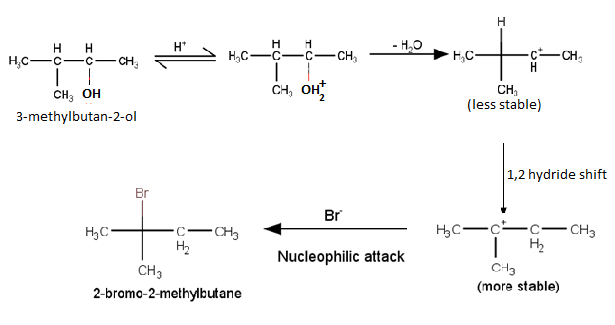
Class 12 Chemistry NCERT Chapter 7: Higher Order Thinking Skills (HOTS) Questions
Selected Higher Order Thinking Skills questions from alcohols, phenols and ethers ncert solutions to strengthen your concepts are given below. These solutions of NCERT will help you practise advanced problems and improve exam readiness.
Question 1. Which one of the following, with HBr, will give a phenol?
Answer:

Hence, the correct answer is option (2).
Question 2. Select the incorrect statement about Phenol.
(1) Phenol is a white crystalline solid.
(2) Phenol is soluble in hot water
(3) Phenol does not have antifungal and antibacterial properties.
(4) Phenol is soluble in NaOH.
Answer:
Properties of phenols:
- Phenols are either colourless liquids or white crystalline solids.
- Phenols are soluble in hot water.
- The boiling points of phenols are much higher than the corresponding aromatic hydrocarbons and haloarenes.
- Phenols have antifungal and antibacterial properties. Thus used as disinfectants and antiseptics.
- Phenols are poisonous in nature but act as antiseptics and disinfectants.
Hence, the correct answer is option (3).
Question 3. Given below are two statements:
Statement I:
The acidic strength of monosubstituted nitrophenol is higher than phenol because of the electron-withdrawing nitro group.
Statement II:
o-nitrophenol, m-nitrophenol and p-nitrophenol will have the same acidic strength as they have one nitro group attached to the phenolic ring
In light of the above statements, choose the most appropriate answer from the options given below:
Both Statement I and Statement II are incorrect.
Statement I is correct, but Statement II is incorrect.
Statement I is incorrect, but Statement II is correct.
Both Statement I and Statement II are correct.
Answer:
Electron-withdrawing group, O-Phenol increases its acidic strength
Statement I is correct
Now, the pKa values of nitro-substituted Phenols are given below:

Thus acidic strength of nitrophenols is
Para > Ortho > Meta
Hence, statement I is correct while statement II is incorrect
Hence, the correct answer is option (2).
Question 4:
When 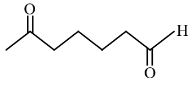
(1)
(2)
(3)
(4)
Answer:
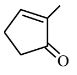
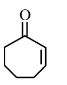
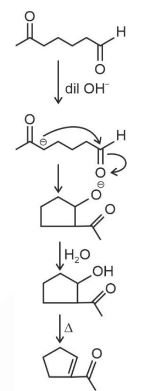
The first step of the scheme shows aldol condensation in the presence of a base, resulting in the formation of $\beta$-hydroxy ketone with a 5-membered ring. On heating, water is eliminated and the product $\alpha, \beta$-Unsaturated cyclopentanone is obtained.
Hence, the correct answer is option (1).
Question 5. Match List-I with List-II
| List-I Conversion | List-II Reagents, Conditions used | ||
| (A) | 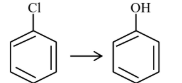 | (I) | Warm, $\mathrm{H}_2 \mathrm{O}$ |
| (B) | 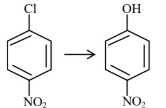 | (II) | (a) $\mathrm{NaOH}, 368 \mathrm{~K}$; (b) $\mathrm{H}_3 \mathrm{O}^{+}$ |
| (C) | 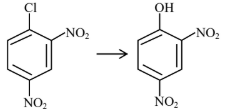 | (III) | (a) $\mathrm{NaOH}, 443 \mathrm{~K}$; (b) $\mathrm{H}_3 \mathrm{O}^{+}$ |
| (D) | 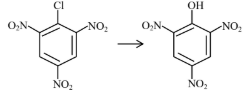 | (IV) | (a) $\mathrm{NaOH}, 623 \mathrm{~K}$, 300 atm ; (b) $\mathrm{H}_3 \mathrm{O}^{+}$ |
Choose the correct answer from the options given below :
(1) (A)-(II), (B)-(III), (C)-(I), (D)-(IV)
(2) (A)-(III), (B)-(IV), (C)-(II), (D)-(I)
(3) (A)-(IV), (B)-(III), (C)-(II), (D)-(I)
(4) (A)-(IV), (B)-(III), (C)-(I), (D)-(II)
Answer:
Presence of EWG at ortho and para-position of chlorobenzene will increase rate of aromatic nucleophilic substitution due to stable intermediate.
A-IV, B-III, C-II, D-I
Hence, the correct answer is option (3).
Question 6: Which among the following compounds give yellow solid when reacted with $\mathrm{NaOI} / \mathrm{NaOH}$ ?
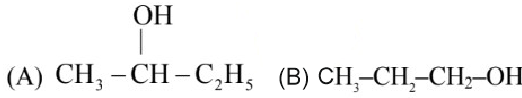
Choose the correct answer from the options given below :
(1) (B), (C) and (E) Only
(2) (A) and (C) Only
(3) (C) and (D) Only
(4) (A), (C) and (D) Only
Answer:
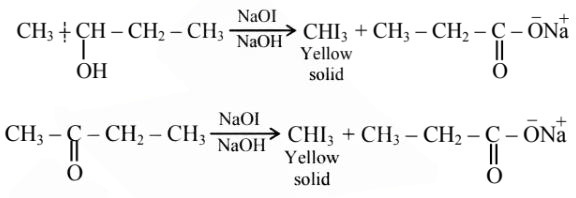
Hence, the correct answer is option (2).
Question 7: What will the major product of the following reaction?
$\mathrm{A} \xrightarrow[443 \mathrm{~K}]{\text { conc. } \mathrm{H}_2 \mathrm{SO}_4} \mathrm{~B} \xrightarrow[\text { cold, dilute, alkaline }]{\mathrm{KMnO}_4} \mathrm{C} \underset{\mathrm{H}^{+}, \text {heat }}{\longrightarrow} \mathrm{D}$
Compound A is an alcohol. Compound D on treatment with excess bromine water gives a white precipitate.Which of the following correctly represents compound A?
(1) Ethanol
(2) 2-propanol
(3) Phenol
(4) Benzyl alcohol
Answer:
White ppt with bromine water ⇒ phenol
(Product = 2,4,6-tribromophenol)
So D = phenol
Phenol is formed by acidic rearrangement of benzene diol / benzene derivative, not from aliphatic compounds. Cold alkaline $\mathrm{KMnO}_4$ oxidises side-chain of aromatic compounds, not alkenes completely.
C must be an aromatic oxidised intermediate
onc. $\mathrm{H}_2 \mathrm{SO}_4$ at 443 K causes:
- Alcohol → alkene (aliphatic)
- Benzyl alcohol → styrene
Only aromatic alcohol can maintain benzene ring
So:
$$
B=\text { styrene (vinyl benzene) }
$$
Alcohol which on dehydration gives styrene is: Benzyl alcohol
Hence, the correct answer is option (4).
Question 8: The number of molecules from below that cannot give an iodoform reaction is :
Ethanol, Isopropyl alcohol, bromoacetone, 2-Butanol, 2-Butanone, Butanal, 2-Pentanone, 3-Pentanone, Pentanal and 3-Pentanol
(1) 5
(2) 4
(3) 3
(4) 2
Answer:
A compound gives a positive iodoform test if it contains either:
- A methyl ketone group $\left(\mathrm{COCH}_3\right)$
- A secondary alcohol that can be oxidized to a methyl ketone
The following will not give an iodoform reaction/test.
- Butanal- Butanal is an aldehyde; butanal does not have a $\mathrm{COCH}_3$ (methyl ketone) group.
- 3-Pentanone - This does have a $-\mathrm{COCH}_3$ group a methyl ketone in its structure.
- Pentanal - Pentanal is also an aldehyde like butanal, but does not have the $\mathrm{CH}_3-\mathrm{CO}-$ group.
- 3-Pentanol- This is a secondary alcohol, but it does not have the structure $-\mathrm{CH}(\mathrm{OH}) \mathrm{CH}_3$, which is required for oxidation to a methyl ketone.
Hence, the correct answer is option (2).
Approach to Solve Questions of Class 12 Chemistry Chapter 7
These class 12 chemistry chapter 7 alcohols, phenols and ethers solutions is an essential study material that is required for all students preparing for Class 12 board exams as well as competitive exams. Here are some tips that students can follow to effectively solve questions:
1. Questions related to nomenclature are often asked in exams, hence knowing the IUPAC naming rules for alcohols, phenols, and ethers becomes important. For ethers, name the smaller alkyl group as a prefix, followed by the main chain
2. Preparation reactions of Alcohols, Phenols, and Ethers from haloalkanes, alkenes, carbonyl compounds, etc. Learn about reagents and conditions, like the hydration of alkenes and Williamson synthesis for ethers
3. Compare boiling points, solubility, and hydrogen bonding.
4. Questions related to reaction mechanisms play a very important role and are often asked in exams. Practice reaction mechanisms like dehydration of alcohols, oxidation, and acidic behaviour of phenols.
5. Students can refer to the solved examples in the textbook and then solve the in-text questions. Students can also access the class 12 chemistry chapter 7 alcohols, phenols and ethers question answer pdf for quick revision of the concepts.
Topics and Subtopics Covered in the NCERT Textbook
All the topics and subtopics covered in the NCERT solutions for Class 12 Chemistry Chapter 7 Alcohols, Phenols and Ethers are listed below:
7.1 Classification
7.1.1 Alcohols - Mono, Di, Tri, or Polyhydric Alcohols
7.1.2 Phenols- Mono, Di, and Trihydric Phenols
7.1.3 Ethers
7.2 Nomenclature
7.3 Structures of Functional Groups
7.4 Alcohols and Phenols
7.4.1 Preparation of Alcohols
7.4.2 Preparation of Phenols
7.4.3 Physical Properties
7.4.4 Chemical Reactions
7.5 Some Commercially Important Alcohols
7.6 Ethers
7.6.1 Preparation of Ethers
7.6.2 Physical Properties
7.6.3 Chemical Reactions
What Extra Should Students Study Beyond the NCERT for JEE?
Along with alcohols, phenols and ethers class 12 question answer students preparing for JEE should study advanced reference books and practise higher-level numerical and conceptual problems. Students need to focus on important concepts and reactions according to the table given below:
What Students Learn from NCERT Solutions for Class 12 Chemistry Chapter 7 Alcohols, Phenols and Ethers
These alcohols, phenols and ethers class 12 question answer help students understand the structures, properties, and reactions of alcohols, phenols, and ethers. Here are some important points highlighting what students learn from this chapter.
- These solutions help students to understand the naming of alcohols, phenols, and ethers according to IUPAC rules.
- Physical and chemical properties like boiling points, solubility, acidity, and reactivity are explained well in these solutions through a series of solved examples.
- Here preparation methods and reaction mechanisms like substitution, oxidation, and dehydration reactions are well explained.
- Using these alcohols, phenols and ethers ncert solutions students can learn about hydrogen bonding and intermolecular forces.
Importance of Class 12 Chemistry Chapter 7 Alcohols, Phenols and Ethers Solutions
The class 12 chemistry chapter 7 alcohols, phenols and ethers question answer is an important part of organic chemistry that deals with oxygen-containing compounds and their physical and chemical behaviour.
- These solutions introduce functional groups that are fundamental to many organic reactions.
- Learning about these compounds helps students to understand the uses of them in making medicines, disinfectants, perfumes, and fuels.
- The class 12 chemistry alcohols, phenols and ethers question answer are very important for CBSE board exams and competitive exams like JEE and NEET.
- Students can learn mechanisms like dehydration, oxidation, and substitution
NCERT Solutions Class 12 Chemistry Chapter-Wise
Apart from the class 12 chemistry alcohols, phenols and ethers question answer students can also explore the links provided below for solutions to other chapters.
NCERT Solutions for Class 12 Subject-wise
NCERT Subject-wise solutions are given below:
NCERT Books and NCERT Syllabus
The NCERT books and syllabus links for class 12 are given below:
Frequently Asked Questions (FAQs)
Chapter 7 of NCERT Class 12 Chemistry covers alcohols, phenols, and ethers, focusing on their structures, nomenclature, physical and chemical properties, methods of preparation, and reaction mechanisms. It explains how these compounds behave chemically and physically, their industrial and medicinal applications, and provides a foundation for understanding related organic chemistry concepts.
Phenols are compounds where the hydroxyl group is directly bonded to an aromatic benzene ring, whereas alcohols have the OH group attached to aliphatic carbon chains. This structural difference leads to varying physical and chemical properties.
The hydroxyl group is crucial as it imparts unique properties to alcohols and phenols. It makes these compounds polar, allowing them to form hydrogen bonds with water, which contributes to their solubility in water.
Class 12 chemistry chapter 7 alcohols, phenols and ethers solutions are detailed, step-by-step answers to all textbook questions from this chapter. They help students understand the concepts, reactions, and properties of these compounds clearly, making it easier to revise.
Common methods for preparing alcohols include fermentation, which converts sugars into ethanol; reduction of carbonyl compounds using reducing agents and hydration of alkenes in the presence of an acid.
The chapter discusses several important reactions of alcohols, including oxidation to form aldehydes, ketones, and carboxylic acids, dehydration to form alkenes, and esterification to form esters.
Isomerism in alcohols and phenols is significant because it leads to different compounds with varying properties. For instance, structural isomers have different structural arrangements while stereoisomers exhibit different spatial orientations. This can affect boiling points, solubility, and reactivity.
Alcohols typically have higher boiling points compared to alkanes due to hydrogen bonding. Phenols also have elevated boiling points for similar reasons but exhibit unique acidic properties due to their ability to donate hydrogen ions. Ethers generally have lower boiling points compared to alcohols due to weaker dipole-dipole interactions.
The class 12 chemistry chapter 7 alcohols, phenols and ethers question answer focuses primarily on three classes of organic compounds: alcohols, phenols, and ethers. Alcohols are characterized by the presence of one or more hydroxyl (-OH) groups, phenols have a hydroxyl group attached to an aromatic ring, and ethers contain an oxygen atom connected to two alkyl or aryl groups.
Ethers are classified based on their structure. The NCERT solutions classify ethers as simple ethers, which have the general formula R-O-R', where R and R' can be identical or different alkyl or aryl groups. They can also be symmetrical or asymmetrical. Understanding this classification helps in recognizing their properties and reactions.
Questions related to CBSE Class 12th
On Question asked by student community
Hello
You will be able to download the CBSE Previous Year Board Question Papers from our official website, careers360, by using the link given below.
https://school.careers360.com/boards/cbse/cbse-previous-year-question-papers
I hope this information helps you.
Thank you.
Hello
You will be able to download the CBSE Pre-Board Class 12 Question Paper 2025-26 from our official website by using the link which is given below.
https://school.careers360.com/boards/cbse/cbse-pre-board-class-12-question-paper-2025-26
I hope this information helps you.
Thank you.
Hello,
Yes, it's completely fine to skip this year's 12th board exams and give them next year as a reporter or private candidate, allowing you to prepare better; the process involves contacting your current school or board to register as a private candidate or for improvement exams during the specified
HELLO,
Yes i am giving you the link below through which you will be able to download the Class 12th Maths Book PDF
Here is the link :- https://school.careers360.com/ncert/ncert-book-for-class-12-maths
Hope this will help you!
Hello,
Here is your Final Date Sheet Class 12 CBSE Board 2026 . I am providing you the link. Kindly open and check it out.
https://school.careers360.com/boards/cbse/cbse-class-12-date-sheet-2026
I hope it will help you. For any further query please let me know.
Thank you.
Popular CBSE Class 12th Questions
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters