NCERT Solutions for Class 12 Chemistry Chapter 8 Aldehydes, Ketones and Carboxylic Acids
CBSE Class 12th Exam Date:17 Feb' 26 - 17 Feb' 26
Do you know why the smell of a ripe fruit differs so sharply from that of nail polish remover or vinegar, even though all of them contain carbon, hydrogen, and oxygen, how can compounds with such similar formulas behave so differently, some with pleasant fragrances, others with irritating odours? These aldehydes, ketones and carboxylic acids ncert solutions answer all these questions. This chapter explains how these compounds are formed and how they react. These carbonyl compounds are really important in both our day-to-day life and industries. For instance, aldehydes and ketones are common in perfumes, medicines, and food preservatives. Carboxylic acids, such as acetic acid and citric acid, are common organic acids we come across in daily life.

NCERT Solutions for Class 12 Chemistry are designed in a structured way to improve conceptual clarity and problem solving ability. The cover essential reactions of Aldehydes, Ketones and Carboxylic acids like nucleophilic addition, oxidation, and reduction, which are crucial for grasping organic chemistry. This article includes some HOTS questions and effective approaches to solve questions that are beyond memorization and promote conceptual understanding.
NCERT Solutions for Class 12 Chemistry Chapter 8: Download PDF
Students can download the class 12 chemistry chapter 8 aldehydes, ketones and carboxylic acids solution pdf for free. These NCERT solutions for class 12 are designed to help you understand the fundamental concepts and solve textbook questions with ease.
Also Read,
NCERT Solutions for Class 12 Chemistry Chapter 8 (In-text Question Exercise)
NCERT Solutions provide detailed explanations to help students learn the basic concepts of class 12 chemistry chapter 8 aldehydes, ketones and carboxylic acids question answer. These answers are designed to strengthen conceptual clarity and enhance problem-solving skills.
Page no. 231
Question 8.1(i) Write the structures of the following compounds.
Answer :
The structure of the compound α -methoxy propionaldehyde is shown here-

Question 8.1(ii) Write the structures of the following compounds.
Answer :
The structure of the compound 3-Hydroxy butanal is shown here-

Question 8.1(iii) Write the structures of the following compounds.
2-Hydroxy cyclopentane carbaldehyde
Answer :
The structure of the compound 2-Hydroxycyclopentane carbaldehyde is shown here-

Question 8.1(iv) Write the structures of the following compounds.
Answer :
The structure of the compound 4-oxopentanal is shown here-

Question 8.1(v) Write the structures of the following compounds.
Answer :
The structure of the compound Di-sec. butyl ketone is shown here-

Question 8.1(vi) Write the structures of the following compounds.
Answer :
The structure of the compound 4-Fluoro acetophenone is shown here-

Page no.234
Question 8.2(i) Write the structures of products of the following reactions:
(i) 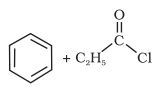 →CS2Anhyd.AlCl3
→CS2Anhyd.AlCl3
Answer :
When benzene is treated with acid chloride in the presence of anhydrous aluminum chloride (−COCH3) group attached to the benzene ring. this reaction is known as friedel craft acylation reaction.

Question 8.2(ii) Write the structure of products of the following reactions;
(ii) $\left(\mathrm{C}_6 \mathrm{H}_5 \mathrm{CH}_2\right)_2 \mathrm{Cd}+2 \mathrm{CH}_3 \mathrm{COCl} \rightarrow$
Answer :
Reaction of acyl chloride with dialkylcadmium$\left(\left(\mathrm{C}_6 \mathrm{H}_5 \mathrm{CH}_2\right)_2 \mathrm{Cd}\right)$, prepared from reaction of cadmium chloride and grignard reagents ,gives ketone

Question 8.2(iii) Write the structures of products of the following reactions:
(iii) $\mathrm{H}_3 \mathrm{C}-\mathrm{C} \equiv \mathrm{C}-\mathrm{H} \rightarrow \mathrm{Hg}^{+}{ }_2, \mathrm{H}_2 \mathrm{SO}_4$
Answer :
When propyne reacts with $\mathrm{Hg}_2^{+}$ in presence of dil. sulphuric acid, water molecules as a nucleophile attack on suitable postion[ $\mathrm{CH}_3-\mathrm{C}^{+}=\mathrm{CH}^{-}$ , primary anion are stable] and then tautomerisation occurs to get the final product

Question 8.2(iv) Write the structures of products of the following reactions:
(iv) 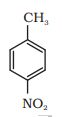 $\rightarrow 2 \cdot \mathrm{H}_3 \mathrm{O}+1 \cdot \mathrm{CrO}_2 \mathrm{Cl}_2$
$\rightarrow 2 \cdot \mathrm{H}_3 \mathrm{O}+1 \cdot \mathrm{CrO}_2 \mathrm{Cl}_2$
Answer :
Chromyl chloride oxidise the methyl group into a chromium complex, which on hydrolysis give aldehyde group.

Page no. 236
Question 8.3 Arrange the following compounds in increasing order of their boiling points.
$\mathrm{CH}_3 \mathrm{CHO}, \mathrm{CH}_3 \mathrm{CH}_2 \mathrm{OH}, \mathrm{CH}_3 \mathrm{OCH}_3, \mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}_3$
Answer :
Increasing order in their boiling points-
$\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}_3<\mathrm{CH}_3 \mathrm{OCH}_3<\mathrm{CH}_3 \mathrm{CHO}<\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{OH}$
Alcohol has the highest boiling point due to more extensive intermolecular H-bonding. Aldehyde is more polar than ether so, ethanal has high BP than ethyl ether. And alkane has the lowest BP.
Page no. 243
Question 8.4(i) Arrange the following compounds in increasing order of their reactivity in nucleophilic addition reactions.
Ethanal, Propanal, Propanone, Butanone.
Answer :

By the above structure, we can see that, due to +I effect of alkyl group te electron density at Carbonyl carbon increase from ethanal to bytanone. And so the tendency of attacking nucleophile is decreased.
Thus the increasig order (reactivity towards nucleophile)-
Butanone < propanone < propanal < ethanal
Question 8.4(ii) Arrange the following compounds in increasing order of their reactivity in nucleophilic addition reactions.
Benzaldehyde, p-Tolualdehyde, p-nitrobenzaldehyde, Acetophenone.
Answer :
If +I effect is more its reactivity towards nucleophilic addition is less. and -I is more, more reactive toward addition.

So, according to this concept, increasing order of their reactivity in nucleophilic addition reactions-
Acetophenone < p-tolualdehyde < benzaldehyde <p-nitrobenzaldehyde
Question 8.5(i) Predict the products of the following reactions:
(i) $\mathrm{HO}-\mathrm{NH}_2 \rightarrow \mathrm{H}^{+}$
Answer :
The product of the reaction is-
Water molecule is removed as a by-product in this reaction.

Question 8.5(ii) Predict the products of the following reactions:
(ii) 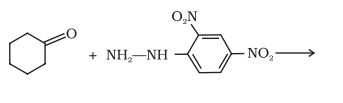 →
→
Answer :
The product of the reaction is a hydrazone, which is formed when ketone and 2, 4-dinitrophenyl hydrazine derivative reacts with each other.

Question 8.5(iii) Predict the products of the following reactions:
R−CH=CH−CHO + 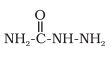 →H+
→H+
Answer :
The product of the above reaction is -
Water molecule is eleminated as a by-product in this reaction

Question 8.5(iv) Predict the products of the following reactions:
(iv) $\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{NH}_2 \rightarrow \mathrm{H}^{+}$
Answer :
The product of the above reaction is -
water is also obtained in this reaction as a by-product

Question 8.6(i) Give the IUPAC names of the following compounds:
$\mathrm{PhCH}_2 \mathrm{CH}_2 \mathrm{COOH}$
Answer :
The IUPAC name of the compound $\mathrm{PhCH}_2 \mathrm{CH}_2 \mathrm{COOH}$ is-
3-phenyl propanoic acid
Question 8.6(ii) Give the IUPAC names of the following compounds:
$\left(\mathrm{CH}_3\right)_2 \mathrm{C}=\mathrm{CHCOOH}$
Answer :
The IUPAC name of the compound $\left(\mathrm{CH}_3\right)_2 \mathrm{C}=\mathrm{CHCOOH}$ is -
3-methyl but-2-en-1-oic acid
Question 8.6(iii) Give the IUPAC names of the following compounds:
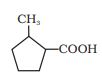
Answer :
The IUPAC name of the compound is-
2-methyl cyclopentane carboxylic acid
Question 8.6(Iv) Give the IUPAC names of the following compounds:
(iv) 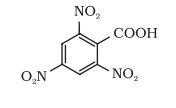
Answer :
The IUPAC name of the compound is-
2, 4, 6-trinitrobenzoic acid
Page no. 248
Question 8.7(i) Show how each of the following compounds can be converted to benzoic acid?
Answer :
Strong oxidising agents like potassium permanganate in the presence of KOH, followed by acidic hydrolysis give benzoic acid.

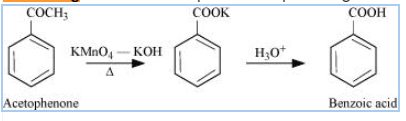
Question 8.7(ii) Show how each of the following compounds can be converted to benzoic acid.
Answer :
On strong oxidation with potassium permanganate in presenc of KOH followed by acidic hydrolysis, give benzoic acid
Question 8.7(iii) Show how each of the following compounds can be converted to benzoic acid?
Answer :
Make bromobenzene first Grignard reagent, react it with dry ice (carbon dioxide) followed by acidic hydrolysis gives benzoic acid.

Question 8.7(iv) Show how each of the following compounds can be converted to benzoic acid?
Answer :
On strong oxidation with potassium permanganate ( $\mathrm{KMnO}_4$ ) in the presence of strong alkali (KOH) followed by acidic hydrolysis gives benzoic acid.

Page no. 254
Question 8.8(i) Which acid of each pair shown here would you expect to be stronger?
$\mathrm{CH}_3 \mathrm{CO}_2 \mathrm{H}$ or $\mathrm{CH}_2 \mathrm{FCO}_2 \mathrm{H}$
Answer :
$\mathrm{CH}_2 \mathrm{FCO}_2 \mathrm{H}$ is stronger than $\mathrm{CH}_3 \mathrm{COOH}$ due to -I effect of Fluorine decreases the electron density at OH bond, which makes it easier to lose proton ($\mathrm{H}^{+}$). The conjugate base of $\mathrm{CH}_2 \mathrm{FCO}_2^{-}$ is more stable than $\mathrm{CH}_3 \mathrm{COO}^{-}$ .
Question 8.8(ii) Which acid of each pair shown here would you expect to be stronger?
$\mathrm{CH}_2 \mathrm{FCO}_2 \mathrm{H}$ or $\mathrm{CH}_2 \mathrm{ClCO}_2 \mathrm{H}$
Answer :
$\mathrm{CH}_2 \mathrm{FCO}_2 \mathrm{H}$ is a stronger acid.
Fluorine has more -I effect than chlorine. So, $\mathrm{CH}_2 \mathrm{FCO}_2 \mathrm{H}$ can release proton easily than $\mathrm{CH}_2 \mathrm{ClCO}_2 \mathrm{H}$.
Question 8.8(iii) Which acid of each pair shown here would you expect to be stronger?
(iii) $\mathrm{CH}_2 \mathrm{FCH}_2 \mathrm{CH}_2 \mathrm{CO}_2 \mathrm{H}$ or $\mathrm{CH}_3 \mathrm{CHFCH}_2 \mathrm{CO}_2 \mathrm{H}$
Answer :
Since we know that inductive effect depends on distance. Greater is the distance lesser is the effect. So, -I effect in $\mathrm{CH}_3 \mathrm{CHFCH}_2 \mathrm{CO}_2 \mathrm{H}$ is more.
$\mathrm{CH}_3 \mathrm{CHFCH}_2 \mathrm{CO}_2 \mathrm{H}$ is more acidic than $\mathrm{CH}_2 \mathrm{FCH}_2 \mathrm{CH}_2 \mathrm{CO}_2 \mathrm{H}$.
Question 8.8(iv) Which acid of each pair shown here would you expect to be stronger?
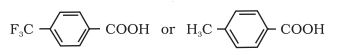
Answer :
Due to -I effect of Fluorine in A, it is easy to release proton ( H+ ) but in compound B due to +I effect of methyl group it becomes difficlt te release protons. Therefore, compound A is more acidic than compound B.

NCERT Solutions for Class 12 Chemistry Chapter 8 (Exercise Questions with Answers)
Detailed aldehydes, ketones and carboxylic acids class 12 question answer, are given below. These NCERT Solutions are designed to strengthen conceptual clarity and enhance problem-solving skills for exam preparation.
Question 8.1 (i). What is meant by the following terms ? Give an example of the reaction in each case.
Answer:
Cyanohydrin -
When ketone and aldehyde react with the hydrogen cyanide ( HCN ) to yield cyanohydrin. The Reaction is very slow with the pure HCN, so it can be catalyzed by using a base.

Question 8.1 (ii). What is meant by the following terms? Give an example of the reaction in each case.
Answer:
Acetal -
When aldehyde reacts with one molecule of monohydric alcohol in presence of dry HCl, it gives intermediate compound known as hemiacetals, which on further reaction with one more molecule of alcohol gives a product (gem-dialkoxy compound), known as acetal.
For example:

Question 8.1(iii). What is meant by the following terms? Give an example of the reaction in each case.
Answer:
Semicarbazone -
This is the derivative of the aldehyde and ketone and it is derived from the condensation reaction between aldehyde and ketone. For example:

Question 8.1(iv). What is meant by the following terms? Give an example of the reaction in each case.
Answer:
Aldol -
β -hydroxy aldehyde is known as aldol and it can be prepared from condensation of aldehyde and ketone, having atleast one alpha-hydrogen atom in presence of dil. alkali as a catalyst.
For example:

Question 8.1(v) What is meant by the following terms? Give an example of the reaction in each case.
Answer:
Hemiacetal -
Aldehyde reacts with one equivalent of a monohydric alcohol, to yield an alkoxy alcohol intermediate compound, in presence of dry hydrochloric acid. The intermediate is called hemiacetal.
For example:

Question 8.1 (vi) What is meant by the following terms? Give an example of the reaction in each case.
Answer:
Oxime -
Aldehyde and ketone on reacting with the hydroxylamine in a weakly basic medium give oximes.
The general form of oxime-

For example:

Question 8.1 (vii) What is meant by the following terms? Give an example of the reaction in each case.
Answer:
Ketal-
It is a cyclic compound, which is formed when a ketone reacts with the ethylene glycol in the presence of dry hydrochloric acid.
For example:

Question 8.1(viii) What is meant by the following terms? Give an example of the reaction in each case.
Answer:
Imine -
These are the chemical compounds having carbon-nitrogen double bonds. It is formed when aldehyde and ketone react with ammonia and its derivatives.
For example:

Question 8.1(ix) What is meant by the following terms? Give an example of the reaction in each case.
Answer:
2,4-DNP-derivative-
These are produced when aldehyde and ketone are reacted with the 2, 4-dinitrophenylhydrazine in a weak basic medium. 2, 4DNP test is used for distinguishing between aldehyde and ketone.
For example:

Question 8.1 (x) What is meant by the following terms? Give an example of the reaction in each case.
Answer:
Schiff’s base-
When aldehyde and ketone are treated with the primary aliphatic or aromatic amines in the presence of acid produces Schiff's base.
For example:

Question 8.2(i) Name the following compounds according to IUPAC system of nomenclature:
$\mathrm{CH}_3 \mathrm{CH}\left(\mathrm{CH}_3\right) \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{CHO}$
Answer:
CH3CH(CH3)CH2CH2CHO
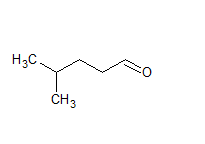 IUPAC name of this compound is 4-methyl pentanal
IUPAC name of this compound is 4-methyl pentanal
Question 8.2(ii) Name the following compounds according to IUPAC system of nomenclature:
$\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{COCH}\left(\mathrm{C}_2 \mathrm{H}_5\right) \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{Cl}$
Answer:
$\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{COCH}\left(\mathrm{C}_2 \mathrm{H}_5\right) \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{Cl}$
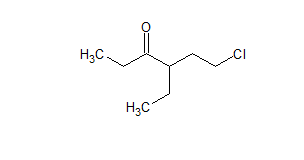 The IUPAC name of the compound is 6-chloro-4ethyl hexane-3-one
The IUPAC name of the compound is 6-chloro-4ethyl hexane-3-one
Question 8.2(iii) Name the following compounds according to IUPAC system of nomenclature:
$\mathrm{CH}_3 \mathrm{CH}=\mathrm{CHCHO}$
Answer:
$\mathrm{CH}_3 \mathrm{CH}=\mathrm{CHCHO}$
The structure of the compound is
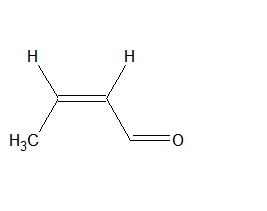
The IUPAC name of the compound is But-2-en-1-al
Question 8.2(iv) Name the following compounds according to IUPAC system of nomenclature:
$\mathrm{CH}_3 \mathrm{COCH}_2 \mathrm{COCH}_3$
Answer:
$\mathrm{CH}_3 \mathrm{COCH}_2 \mathrm{COCH}_3$
The structure of the given compound is-
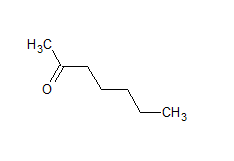
The IUPAC name of the compound is pentan-2, 4-dione.
Question 8.2 (v) Name the following compounds according to IUPAC system of nomenclature:
$\mathrm{CH}_3 \mathrm{CH}\left(\mathrm{CH}_3\right) \mathrm{CH}_2 \mathrm{C}\left(\mathrm{CH}_3\right)_2 \mathrm{COCH}_3$
Answer:
$\mathrm{CH}_3 \mathrm{CH}\left(\mathrm{CH}_3\right) \mathrm{CH}_2 \mathrm{C}\left(\mathrm{CH}_3\right)_2 \mathrm{COCH}_3$
The structure of the compound is -
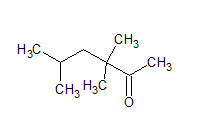
The IUPAC name of the given compound is 3, 3, 5-trimethyl hexan-2-one.
Question 8.2 (vi) Name the following compounds according to IUPAC system of nomenclature:
$\left(\mathrm{CH}_3\right)_3 \mathrm{CCH}_2 \mathrm{COOH}$
Answer:
$\left(\mathrm{CH}_3\right)_3 \mathrm{CCH}_2 \mathrm{COOH}$
The structure of the compound is -
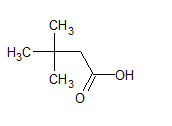
The IUPAC name of the compound is 3, 3-dimethyl butanoic acid .
Question 8.2 (vii) Name the following compounds according to IUPAC system of nomenclature:
$\mathrm{OHCC}_6 \mathrm{H}_4 \mathrm{CHO}-\mathrm{p}$
Answer:
$\mathrm{OHCC}_6 \mathrm{H}_4 \mathrm{CHO}-\mathrm{p}$
The structure of the compound is-
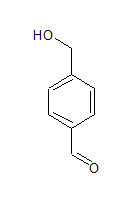
The IUPAC name of the compound is Benzene-1, 4-dicarbaldehyde.
Question 8.3(i) Draw the structures of the following compounds.
Answer:
The structure of the 3-methylbutanal is shown here-

Question 8.3 (ii) Draw the structures of the following compounds.
Answer:
The structure of p-nitro propiophenone is shown here-

Question 8.3 (iii) Draw the structures of the following compounds.
Answer:
The strcuture of the p -methyl benzaldehyde is shown here-

Question 8.3(iv) Draw the structures of the following compounds.
Answer:
The structure of the compound 4-methylpent-3-en-2-one is shown here-

Question 8.3(v) Draw the structures of the following compounds.
Answer:
The structure of the compound 4-chloro-pentan-2-one is shown here-

Question 8.3 (vi) Draw the structures of the following compounds.
3-Bromo-4-phenylpentanoic acid
Answer:
The structure of the compound 3-Bromo-4-phenyl pentanoic acid is shown here-

Question 8.3 (vii) Draw the structures of the following compounds.
Answer:
The structure of the compound p,p’-dihydroxy benzophenone acid is shown here-

Question 8.3 (viii) Draw the structures of the following compounds.
Answer:
The structure of the compound Hex-2-en-4-ynoic acid acid is shown here-

Question 8.4 (i) Write the IUPAC names of the following ketones and aldehydes. Wherever possible, give also common names.
$\mathrm{CH}_3 \mathrm{CO}\left(\mathrm{CH}_2\right)_4 \mathrm{CH}_3$
Answer:
$\mathrm{CH}_3 \mathrm{CO}\left(\mathrm{CH}_2\right)_4 \mathrm{CH}_3$
The structure of the compound is
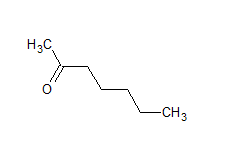 The IUPAC name of the compound is hept-2-one Common name is methyl-n-propyl ketone
The IUPAC name of the compound is hept-2-one Common name is methyl-n-propyl ketone
Question 8.4 (ii) Write the IUPAC names of the following ketones and aldehydes. Wherever possible, give also common names.
$\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CHBrCH}_2 \mathrm{CH}\left(\mathrm{CH}_3\right) \mathrm{CHO}$
Answer:
$\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CHBrCH}_2 \mathrm{CH}\left(\mathrm{CH}_3\right) \mathrm{CHO}$
The structure of the compound is
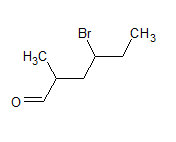 The IUPAC name of the compound is 4-bromo-2-methylhexanal
The IUPAC name of the compound is 4-bromo-2-methylhexanal
The common name is - γ -bromo- α -methyl-caproaldehyde
Question 8.4 (iii) Write the IUPAC names of the following ketones and aldehydes. Wherever possible, give also common names.
$\mathrm{CH}_3\left(\mathrm{CH}_2\right)_5 \mathrm{CHO}$
Answer:
$\mathrm{CH}_3\left(\mathrm{CH}_2\right)_5 \mathrm{CHO}$
structure os the compound is
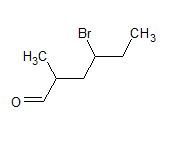 The IUPAC name of the compound is heptanal
The IUPAC name of the compound is heptanal
Question 8.4 (iv) Write the IUPAC names of the following ketones and aldehydes. Wherever possible, give also common names.
Ph−CH=CH−CHO
Answer:
Ph−CH=CH−CHO
structur eof the compound is
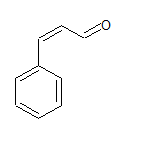 The IUPAC name of the sompound is 3-phenylprop-2-ene-al
The IUPAC name of the sompound is 3-phenylprop-2-ene-al
Common name is β -phenylacrolein
Question 8.4 (v). Write the IUPAC names of the following ketones and aldehydes. Wherever possible, give also common names.
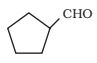
Answer:
 The IUPAC name of the compound is cyclopentane carbaldehyde.
The IUPAC name of the compound is cyclopentane carbaldehyde.
Question 8.4 (vi) Write the IUPAC names of the following ketones and aldehydes. Wherever possible, give also common names.
PhCOPh
Answer:
PhCOPh
structure of the compound is
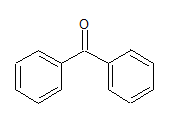 The IUPAC name of the compound is Diphenyl mentanone.
The IUPAC name of the compound is Diphenyl mentanone.
The common name is - benzophenone
Question 8.5 (i) Draw structures of the following derivatives.
The 2,4-dinitro phenyl hydrazone of benzaldehyde
Answer:
The structure of 2,4-dinitro phenyl hydrazone of benzaldehyde

Question 8.5 (ii) Draw structures of the following derivatives.
Answer:
The structure of the Cyclopropanone oxime

Question 8.5 (iii) Draw structures of the following derivatives.
Answer:
The structure of Acetaldehyde dimethylacetal

Question 8.5 (iv) Draw structures of the following derivatives.
The semicarbazone of cyclobutanone
Answer:
The structure of the semicarbazone of cyclobutanone

Question 8.5 (v) Draw structures of the following derivatives.
The ethylene ketal of hexan-3-one
Answer:
The structure of the ethylene ketal of hexan-3-one

Question 8.5 (vi) Draw structures of the following derivatives.
The methyl hemiacetal of formaldehyde
Answer:
The structure of the compound methyl hemiacetal of formaldehyde

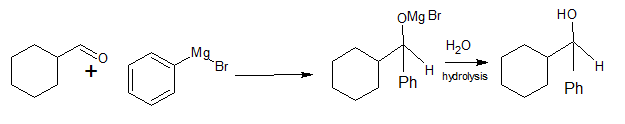
Question 8.6 (i) Predict the products formed when cyclohexane carbaldehyde reacts with following reagents.
PhMgBr and then $\mathrm{H}_3 \mathrm{O}^{+}$
Answer:
When cyclohexane carbaldehyde reacts with PhMgBr in presence of dry ether and then $\mathrm{H}_3 \mathrm{O}^{+}$ (hydrolysis)
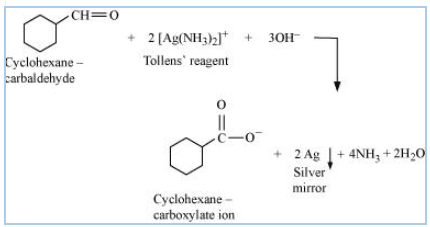
Question 8.6 (ii) Predict the products formed when cyclohexane carbaldehyde reacts with the following reagents.
Answer:
Structure of cyclohexanecarbaldehyde
Cyclohexanecarbaldehyde reacts with Tollen's reagent and reduces it to silver ( Ag ) and oxidizes itself to cyclohexane carboxylate ion
So the reaction is-
Question 8.6 (iii) Predict the products formed when cyclohexane carbaldehyde reacts with following reagents.
Answer:
Structure of cyclohexanecarbaldehyde

The reaction of cyclohexane carbaldehyde with semicarbazide and weak acid is-
The $-\mathrm{NH}_2$ group, which is not involved in resonance with the carbonyl group, acts as a nucleophile and attacks on -CHO group to form the product.

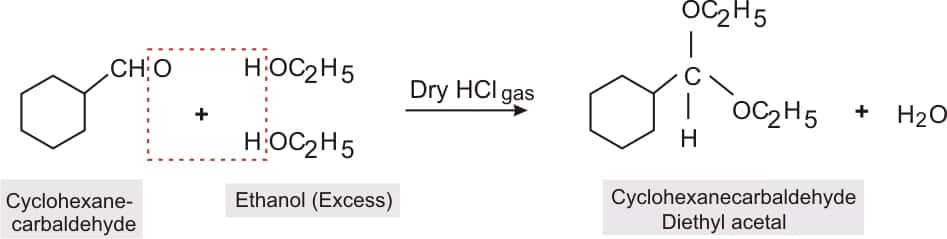
Question 8.6 (iv) Predict the products formed when cyclohexane carbaldehyde reacts with following reagents.
Answer:
Structure of cyclohexanecarbaldehyde
.png)
reaction of cyclohexanecarbaldehyde with an excess of ethanol and acid to form cyclohexanecarbaldehyde diethyl acetal.
Question 8.6 (v) Predict the products formed when cyclohexane carbaldehyde reacts with following reagents.
Zinc amalgam and dilute hydrochloric acid
Answer:
Structure of cyclohexane carbaldehyde

When cyclohexane carbaldehyde reacts with zinc amalgam( Zn−Hg ) and dil. HCl , the carbonyl group of reactant is reduced to CH2 , it is also known as clemmensen reduction.
.png)
Question 8.7 (i) Which of the following compounds would undergo aldol condensation, the Cannizzaro reaction, and which neither? Write the structures of the expected products of aldol condensation and Cannizzaro reaction.
Methanal
Answer:
The compounds of ketones or aldehydes having at least one α - hydrogen atom give aldol condensation reaction. Here, methanal has an alpha-hydrogen atom. So, it gives a Cannizzaro reaction.
The products of the reactions are, in which one product is reduced and another is oxidised to a carboxylic acid salt-
.png)
Answer:
In 2-methylpentanal, there is one alpha-hydrogen atom, so it gives an aldol condensation reaction. Hence aldol product is-

Answer:
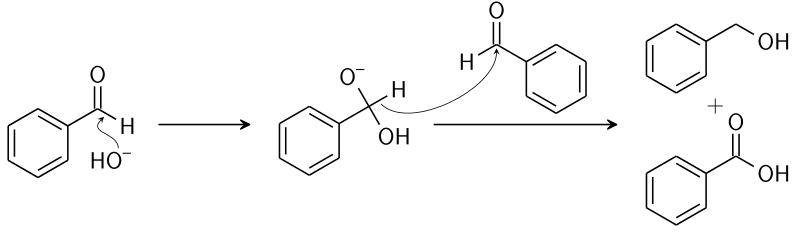
Benzaldehyde Structure
In this structure, we can clearly see that there is no alpha-hydrogen atom. So, it gives the Cannizzaro reaction.
Answer:
It does not give any of reaction because it is a keto compound and has no alpha-hydrogen atom. Hence, it does not give the Cannizzaro as well as the aldol reaction.
Structure -
.png)
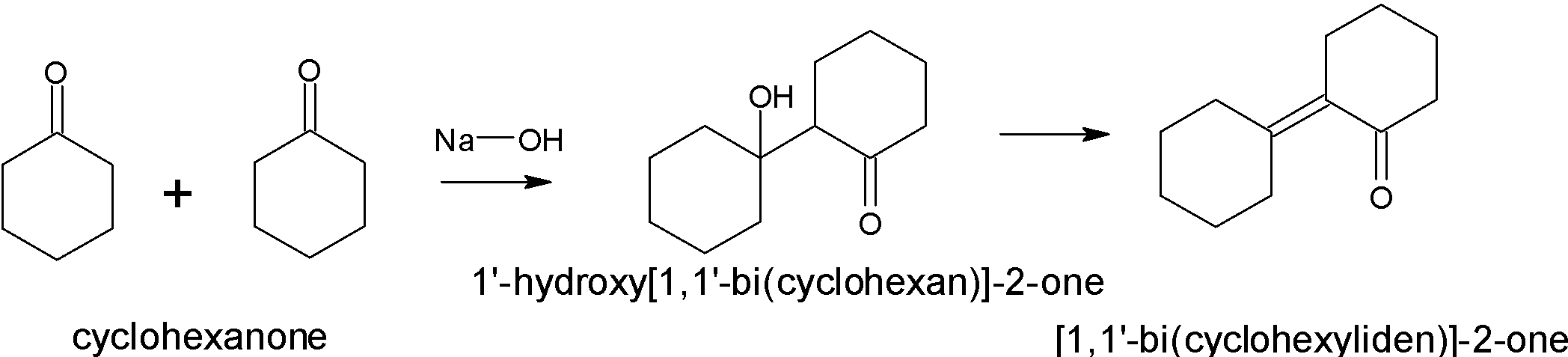
Answer:
Yes, this compound will give an aldol condensation reaction due to the presence of an alpha-hydrogen atom. Two moles of cyclohexanone react with each other in which one molecule act as a nucleophile and the other act as an electrophile.
Answer:
1-Phenylpropanone has two alpha-hydrogen atoms, so it gives an aldol condensation reaction. It reacts with another molecule of 1-Phenylpropanone in the presence of dil. NaOH .
.png)
Answer:
Phenylacetaldehyde it has two alpha-hydrogens, which makes it possible to perform the aldol condensation reaction. So, the reaction is shown here;
.png)
Answer:
It does not give any of the reactions, either the aldol reaction or Cannizzaro reaction, because it is an alcohol.
Answer:
In this compound, there is no alpha hydrogen, which means it gives the Cannizzaro reaction in the presence of conc. sodium hydroxide ( NaOH ). One molecule of it is oxidised and the other molecule is reduced.
.png)
Question 8.8 (i) How will you convert ethanal into the following compounds?
Answer:
On treating ethanal with dil. alkali it gives 3-hydroxybutanal, which on further reduction with NaBH4 gives the product Butane-1,3-diol
.png)
Question 8.8 (ii) How will you convert ethanal into the following compounds?
Answer:
On treating with he presence of dil. alkali ( NaOH ), it gives 3-hydroxybutanal, which on further dehydration gives but-2-ene-al.
.png)
Question 8.8 (iii) How will you convert ethanal into the following compounds?
Answer:
When the given substrate is reacting with the tollens reagent, it produces but-2-enoic acid.
.png)
Answer:
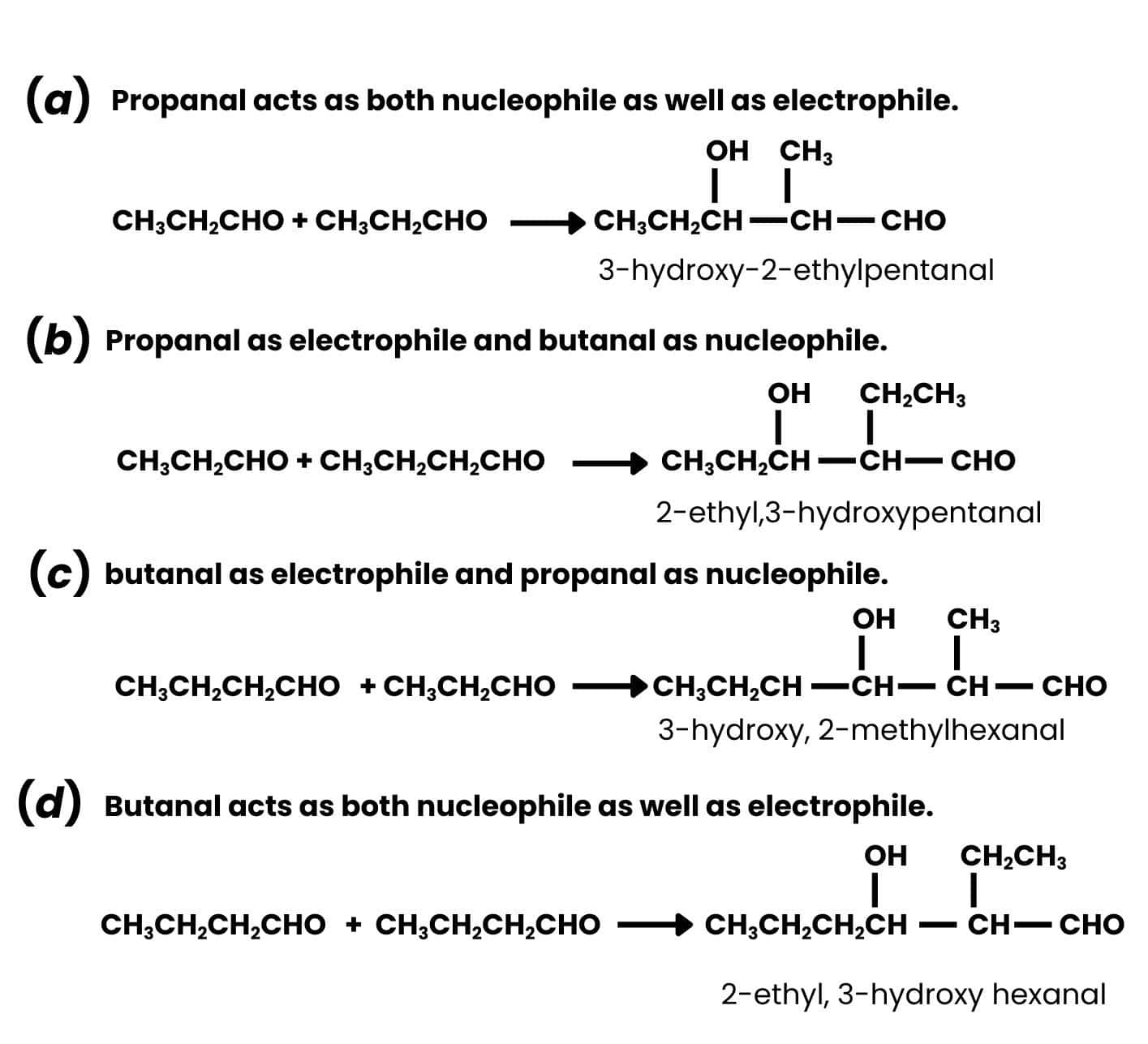
Propanal - $\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CHO}$
butanal - $\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{CHO}$
In cross-aldol condensation, there are a total of four cases of reaction-
- When two molecules of propanal react with each other
- When two molecules of butanal react with each other
- When propanal acts as a nucleophile and attacks butanal(as an electrophile)
- Butanone acts as a nucleophile and attacks propanal(as an electrophile)
Answer:
According to the given information, the molecular formula is $\mathrm{C}_9 \mathrm{H}_{10} \mathrm{O}$ and it reduces Tollens reagent. So, there must be an aldehyde group. And also it gives Cannizzaro reaction, it means the given compound has no alpha-hydrogen atom. On oxidation, it gives 1,2-benzenedicarboxylic acid. Thus, the aldehyde group ( −CHO ) is directly attached to the benzene ring, and it should also be para-substituted.
Hence, the structure of the compound (2-ethylbenzaldehyde) is-
.png)
By following the reaction we can understand this problem-
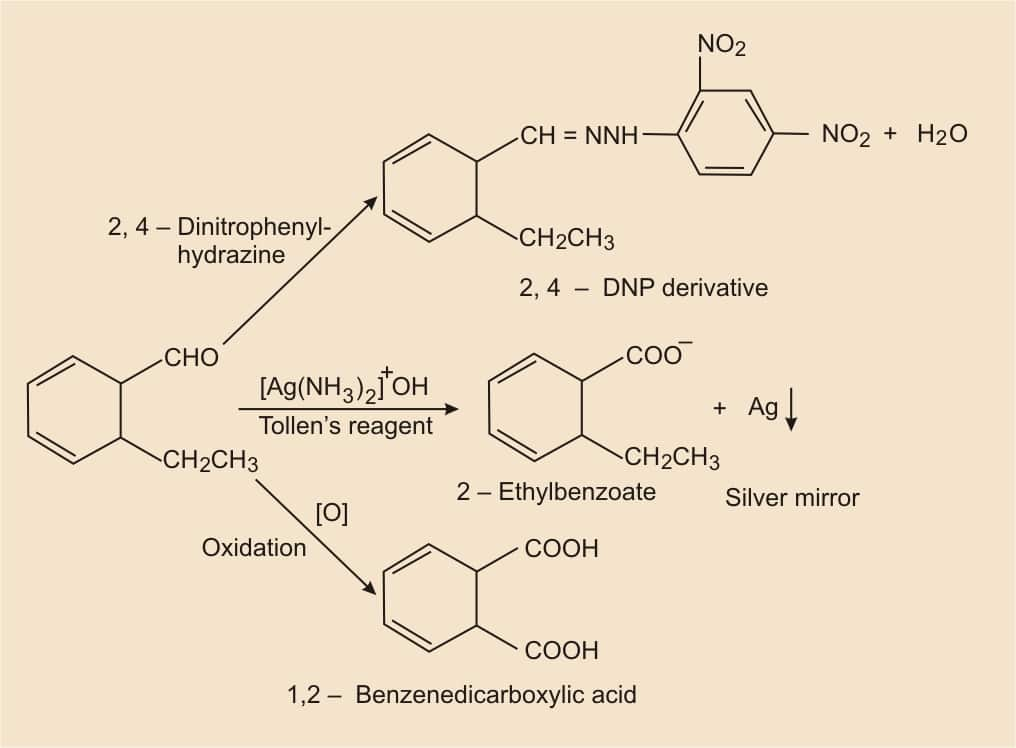
Question 8.12 (i) Arrange the following compounds in increasing order of their property as indicated:
Acetaldehyde, Acetone, Di-tert-butyl ketone, Methyl tert-butyl ketone (reactivity towards HCN)
Answer:
Increasing order (reactivity towards HCN)-
Di-tert-butyl ketone < Methyl tert-butyl ketone < Acetone < Acetaldehyde
The attacking power of CN nucleophile can be affected in two ways-
- hindrance near the carbonyl group
- negative charge on −CO group (through +I effect of methyl)
By observing the structure of all compounds, we can arrange them in order to reactivity towards HCN.
Question 8.12 (ii) Arrange the following compounds in increasing order of their property as indicated:
Answer:
increasing order of their acidic strength-
$\left(\mathrm{CH}_3\right)_2 \mathrm{CHCOOH}<\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{COOH}<\mathrm{CH}_3 \mathrm{CH}(\mathrm{Br}) \mathrm{CH}_2 \mathrm{COOH}<\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}(\mathrm{Br}) \mathrm{COOH}$
Due to the presence of Bromine, which shows -I effect, and we know that -I grows weaker as distance increases. And in case of n-propyl and isopropyl, the +I effect is more in isopropyl, so it is weaker in acidic strength.
−I∝acidic nature
+I∝1/acidic
Question 8.12 (iii) Arrange the following compounds in increasing order of their property as indicated:
Benzoic acid, 4-Nitrobenzoic acid, 3,4-Dinitrobenzoic acid, 4-Methoxybenzoic acid (acid strength)
Answer:
Increasing order of acidic strength -
4-methoxybenzoic acid < Benzoic acid < 4-Nitrobenzoic acid < 3,4-Dinitrobenzoic acid
As we know, electron-withdrawing groups increase the strength of the acid, and electron-donating groups decrease the strength of the acid. Therefore, 4-methoxybenzoic acid is the weakest acid among them, and 3,4-Dinitrobenzoic acid is the strongest due to the presence of 2 electron-withdrawing groups and then by 4-nitrobenzoic acid.
Question 8.13 (i) Give simple chemical tests to distinguish between the following pairs of compounds.
Answer:
Both compounds can be distinguished by the following tests-
- tollens test
- iodoform test
- fehlings test
Propanal reduces the Tollens reagent, but propanone does not.
Acetaldehyde responds to Fehling's test, but ketone does not. So, Propanal gives a positive Fehling's test but not propanone.
Question 8.13 (ii) Give simple chemical tests to distinguish between the following pairs of compounds.
Answer:
Acetophenone gives a positive iodoform test; it reacts with NaOI to give yellow ppt. But benzophenone does not give this test.
$\mathrm{C}_6 \mathrm{H}_5 \mathrm{COCH}_3+\mathrm{NaOI} \rightarrow \mathrm{C}_6 \mathrm{H}_5 \mathrm{COONa}+\mathrm{CHI}_3+\mathrm{NaOH}$Ṁ
benzophenone + NaOI → no yellow ppt.
Question 8.13 (iii) Give simple chemical tests to distinguish between the following pairs of compounds.
Answer:
Phenol gives ferric chloride test ( $\mathrm{FeCl}_3$ ), on reaction with ferric chloride, phenol gives violet coloured solution, but benzoic acid does not.
.png)
Question 8.13 (iv) Give simple chemical tests to distinguish between the following pairs of compounds.
Benzoic acid and Ethyl benzoate
Answer:
Both can be distinguished by the sodium bicarbonate test. In this test, benzoic acid reacts with $\mathrm{NaHCO}_3$ to give brisk effervescence due to the evolution of carbon dioxide ( $\mathrm{CO}_2$), but Ethyl benzoate does not.
.png)
Question 8.13 (v) Give simple chemical tests to distinguish between the following pairs of compounds.
Answer:
They can be distinguished by the iodoform test,
This test is given by compounds having a methyl ketone group. So, pentan-2-one gives this test, and pentan-3-one does not.
.png)
Question 8.13 (vi) Give simple chemical tests to distinguish between the following pairs of compounds.
Answer:
Both can be distinguished by
- Iodoform test
In acetophenone, the methyl ketone group is present. So it gives a positive iodoform test by forming a yellow ppt.
.png)
- Tollen's test
Aldehydes give a positive Tollens test. Benzaldehyde reduces the Tollens reagent and gives red brown precipitate. But acetophenone does not give this test.
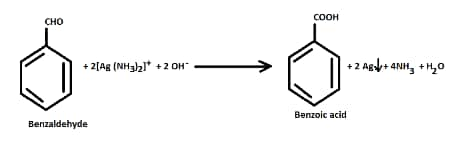
Question 8.13 (vii) Give simple chemical tests to distinguish between the following pairs of compounds.
Answer:
Ethanal and Propanal can be distinguished by the Iodoform test.
Ethanal has one methyl group attached to the Carbonyl group, so it gives a positive iodoform test, but propanal does not give this test.
.png)
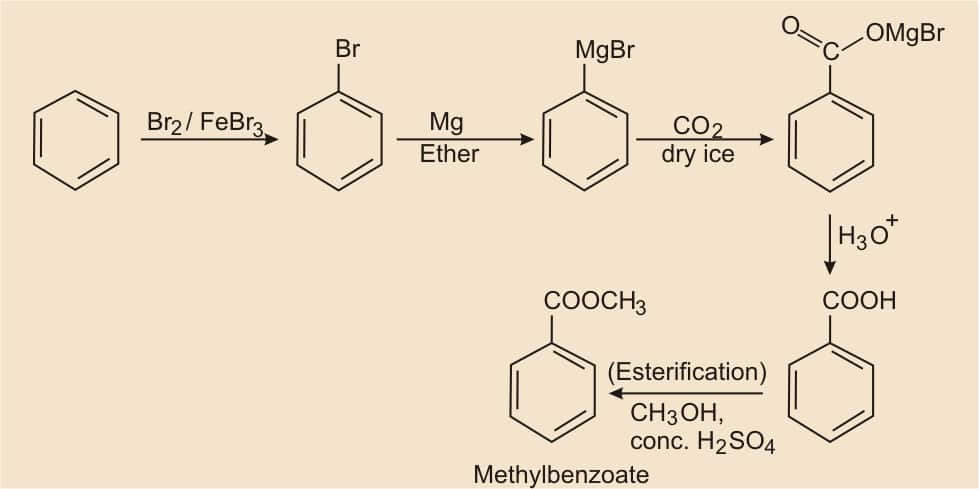
Question 8.14(i) How will you prepare the following compounds from benzene? You may use any inorganic reagent and any organic reagent having not more than one carbon atom
Answer:
Benzene, on bromination, formed bromobenzene, and after that, we have to react with Mg in the presence of ether, it becomes Grignard reagents ( R−MgX ). Grignard reagents react with carbon dioxide (dry ice) to form salts of carboxylic acids, which on acidic hydrolysis give benzoic acid. On esterification, it gives methyl benzoate.
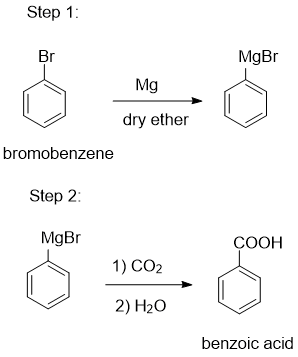
Question 8.14 (ii) How will you prepare the following compounds from benzene? You may use any inorganic reagent and any organic reagent having not more than one carbon atom.
Answer:
Benzene on bromination, formed bromobenzene, and after that, we have to react with Mg in the presence of ether, it becomes a Grignard reagent ( R−MgX ). Grignard reagents react with carbon dioxide (dry ice) to form salts of carboxylic acids, which on acidic hydrolysis give benzoic acid. Finally, after doing nitration, we get our desired product (m-Nitrobenzoic acid).
.png)
Question 8.14 (iii) How will you prepare the following compounds from benzene? You may use any inorganic reagent and any organic reagent having not more than one carbon atom
Answer:
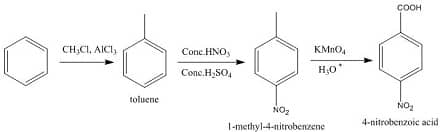
By friedel craft alkylation of benzene give methyl benzene, which on nitration give meta and para substituted methyl benzene, para substituted is major product. Oxidation of para substituted methyl benzene( KMnO4/KOH ) gives salts of carboxylic acid, which on further acidic hydrolysis gives p-nitrobenzoic acid.
Question 8.14 (iv) How will you prepare the following compounds from benzene You may use any inorganic reagent and any organic reagent having not more than one carbon atom
Answer:
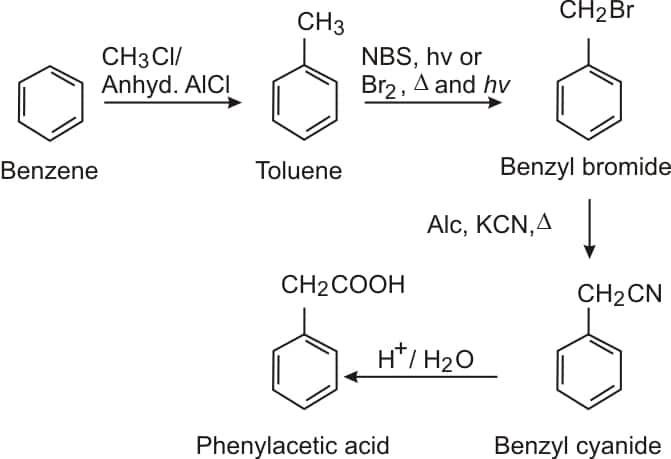
Alkylation of benzene in presence of anhydrous $\mathrm{AlCl}_3$ gives toluene, which on further reaction with Br2/Δ/hv gives benzyl bromide, and after that reacts with alc. KCN, CN replace the Br and form benzyl cyanide, which on acidic hydrolysis gives phenyl acetic acid.
Question 8.14(v) How will you prepare the following compounds from benzene? You may use any inorganic reagent and any organic reagent having not more than one carbon atom
Answer:
Alkylation of benzene gives toluene, which on further reaction with $\mathrm{HNO}_3 / \mathrm{H}_2 \mathrm{SO}_4$ gives p- nitrotoluene and react it with the the carbon disulphide / $\mathrm{CrO}_2 \mathrm{Cl}_2$ followed by acidic hydrolysis to form p -nitrobenzaldehyde.
.png)
Question 8.15(i) How will you bring about the following conversions in not more than two steps?
Answer:
First, reduce propanone with the help of lithium aluminum hydride ($\mathrm{LiAlH}_4$) to get propan-2-ol, and then by dehydration, remove the water molecule to get propene.
.png)
Question 8.15(ii) How will you bring about the following conversions in not more than two steps?
Answer:
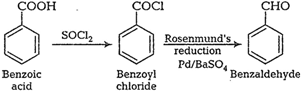
First react it with $\mathrm{SOCl}_2$ which replace OH group and then hydogenation reaction.
Question 8.15 (iii) How will you bring about the following conversions in not more than two steps?
Answer:
Ethanol on heating at 573K in the presence of copper it converts into ethanol, and then by aldol condensation, we get 3-hydroxybutanal.
.png)
Question 8.15 (iv) How will you bring about the following conversions in not more than two steps?
Benzene to m-nitroacetophenone
Answer:
Benzene, on reacting with acetic anhydride $\left(\mathrm{CH}_3 \mathrm{CO}\right)_2 \mathrm{CO}$, gives acetophenone. Further reacting acetophenone with nitric acid in the presence of sulphuric acid gives a meta-substituted product, m-nitroacetophenone.
.png)
Question 8.15 (v) How will you bring about the following conversions in not more than two steps?
Answer:
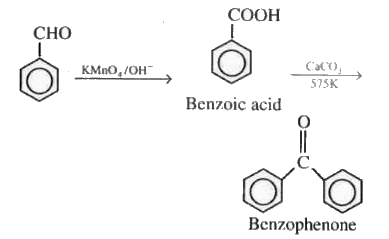
On oxidation of benzaldehyde, it converted into benzoic acid, which on further reacting with $\mathrm{CaCO}_3$ it formed calcium benzoate. After distillation, we get benzophenone.
Question 8.15 (vi) How will you bring about the following conversions in not more than two steps?
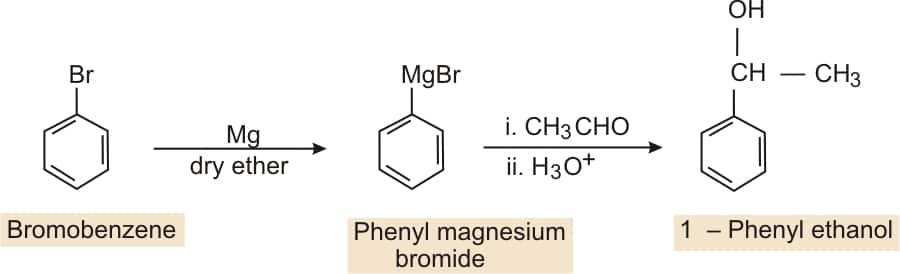
Bromobenzene to 1-phenylethanol
Answer:
Bromobenzene on reacting with Mg in the presence of dry ether it forms Grignard reagents ( R−Mg−X ), which, on further reacting with ethanol, followed by acidic hydrolysis, gives 1-phenyl ethanol
Question 8.15 (vii) How will you bring about the following conversions in not more than two steps?
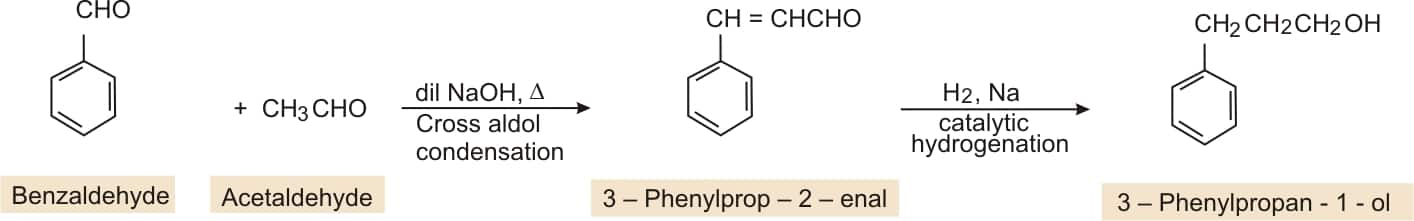
Benzaldehyde to 3-Phenylpropan-1-ol
Answer:
BY cross aldol condensation of benzaldehyde and acetaldehyde gives 3-phenylprop-2-ene-al, which on catalytic hydrogenation gives 3-Phenylpropan-1-ol
Question 8.15 (viii) How will you bring about the following conversions in not more than two steps?
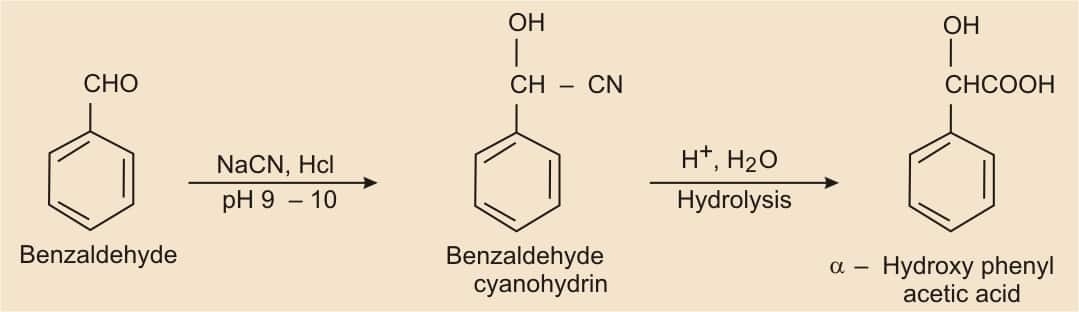
Benzaldehyde to α-hydroxy phenyl acetic acid
Answer:
Nucleophilic reaction of benzaldehyde with NaCN in the presence of HCl gives benzaldehyde cyanohydrine, which on acidic hydrolysis forms α-Hydroxyphenylacetic acid
Question 8.15(ix) How will you bring about the following conversions in not more than two steps?
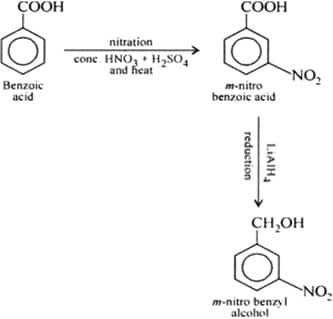
Benzoic acid to m-nitrobenzyl alcohol
Answer:
On nitration of benzoic acid gives meta-substituted nitrobenzoic acid, which, on further reacting with a reducing agent and followed by acidic hydrolysis, gives nitrobenzyl alcohol.
Question 8.16 (i) Describe the following.
Answer:
Acetylation-
The addition of an acetyl functional group ($\mathrm{CH}_3 \mathrm{CO}^{-}$) in an organic compound is called acetylation. Acetic anhydride( $\left(\mathrm{CH}_3 \mathrm{CO}\right)_2 \mathrm{CO}$ ) and acetyl chloride ($\mathrm{CH}_3 \mathrm{COCl}$ ) are mostly used as acetylating agents. This reaction happens in the presence of a base such as pyridine, etc.
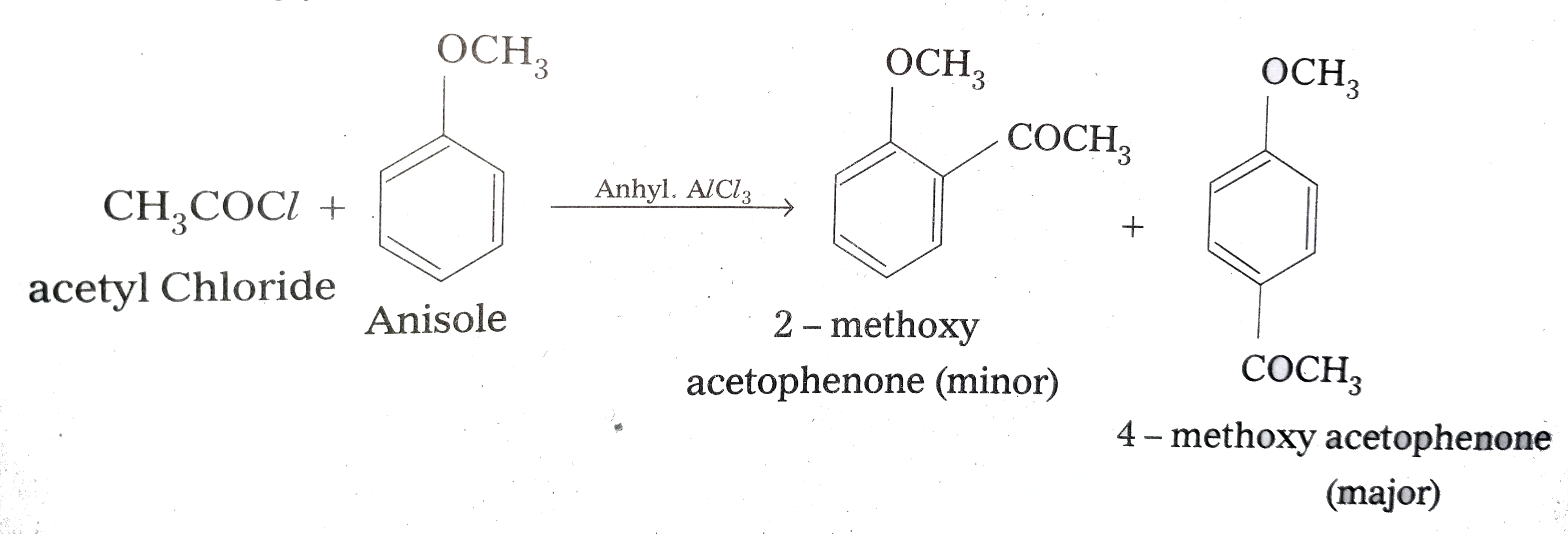
Question 8.16 (ii) Describe the following.
Answer:
Cannizzaro reaction-
Aldehydes that do not have any alpha-hydrogen undergo self-oxidation as well as reduction reaction on treatment with concentrated alkalis. In this reaction, one molecule gets reduced and the other is oxidised to a carboxylic acid salt.
.png)
Question 8.16 (iii) Describe the following.
Answer:
Cross aldol condensation -
In aldol condensation, if the reactants are two different aldehydes or ketones, then it is called cross-aldol condensation. If both of the reactants contain alpha-hydrogen atoms, then it gives a mixture of four products.
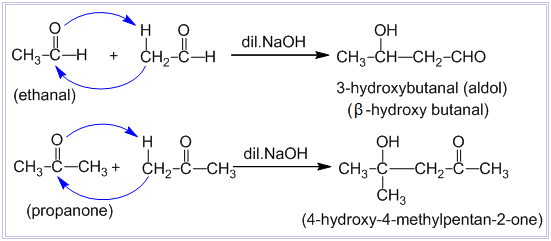
Question 8.16 (iv) Describe the following.
Answer:
Decarboxylation -
The reaction in which the carboxylic acid loses carbon dioxide to hydrocarbon, in the presence of sodalime( NaOH and CaO in the ratio of 3:1). This reaction is known as decarboxylation.
.png)
Question 8.17(i) Complete each synthesis by giving missing starting material, reagent or products.

Answer:
Potassium permanganate oxidizes the substituted ethyl group to −COOH group, and due tothe presence of KOH, forms carboxylic acid salts (potassium benzoate)

Question 8.17(ii) Complete each synthesis by giving the missing starting material, reagent, or products.

Answer:
The -OH groups of both carboxylic acid is replaced by the chlorine
So, the overall reaction is

Question 8.17 (iii) Complete each synthesis by giving the missing starting material, reagent, or products
$\mathrm{C}_6 \mathrm{H}_5 \mathrm{CHO} \rightarrow \mathrm{H}_2 \mathrm{NCONHNH}_2$
Answer:
In this reaction, the −NH2 group, which is not directly attached to the carbonyl group, acts as a nucleophile and attacks on -CO group of benzaldehyde, and a water molecule is also produced as a by-product.
So, the final product is-

Question 8.17(iv) Complete each synthesis by giving the missing starting material, reagent or products.
Answer:
Benzene reacts with benzoyl chloride in the presence of anhydrous aluminium chloride ($\mathrm{AlCl}_3$) to give benzophenone and also HCl as a by-product.

Question 8.17(v) Complete each synthesis by giving the missing starting material, reagent or products.

Answer:
Since the given reactant is an aldehyde compound, it gives a positive Tollens' test by reducing the Tollens reagent.

Question 8.17 (vi) Complete each synthesis by giving missing starting material, reagent or products.

Answer:
In this reaction, the nucleophile CN attacks on -CHO group because it has more reactivity towards CN− .
So the reaction gives

Question 8.17 (vii) Complete each synthesis by giving the missing starting material, reagent, or products.
$\mathrm{C}_6 \mathrm{H}_3 \mathrm{CHO}+\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CHO} \rightarrow$ dil. NaOH
Answer:
The given reaction is a cross aldol reaction between benzaldehyde and propanal in the presence of dil. sodium hydroxide

Question 8.17 (viii) Complete each synthesis by giving the missing starting material, reagent, or products.
Answer:
In this reaction NaBH4 is a reducing agent, which reduces the CO group of the compound.

Question 8.17(ix) Complete each synthesis by giving the missing starting material, reagent, or products.

Answer:
$\mathrm{CrO}_2$ oxidises the OH(alcohol) group into keto-group (-CO) and finally the product produced is cyclohexanone

Question 8.17(x) Complete each synthesis by giving the missing starting material, reagent, or products.

Answer:
Diborane reacts with an alkene to give trialkyl boranes (addition product), and this is oxidised to alcohol by using hydrogen peroxide.
After the pyridinium chlorochromate (PCC) is reacted with alcohol to convert it into an Aldehyde group.

Question 8.17 (xi) Complete each synthesis by giving the missing starting material, reagent or products.
$\xrightarrow[\text { (ii) } \mathrm{Zn}-\mathrm{H}_2 \mathrm{O}]{\text { (i) } \mathrm{O}_3}$
Answer:
The above reaction is ozonolysis reaction in which ozone molecule breaks the double bond of the alkene and formed a ketone or aldehyde as a product.

Question 8.18(i) Give plausible explanation for each of the following:
Cyclohexanone forms cyanohydrin in good yield but 2,2,6-trimethylcyclohexanone does not.
Answer:
Structure of 2,2,6-trimethylcyclo hexanone

Cyclohexanone forms cyanohydrin when the nucleophile ( HCN/CN- ) attack on cyclohexanone.

In this case, there is less hindrance around the CO group so the nucleophile can attack easily. But in case of 2,2,6-trimethylcyclo hexanone at the alpha ( α ) position, there is a hindrance because of the methyl groups. Thus the nucleophile cannot attack effectively.
Question 8.18(ii) Give plausible explanation for each of the following:
Answer:
In Semicarbazide,

One of the $-\mathrm{NH}_2$ groups is involved in resonance with the carbonyl group, which is directly attached. Therefore, it cannot act as a nucleophile (its electrons are less available or have less electron density at that $-\mathrm{NH}_2$ group). But the other $-\mathrm{NH}_2$ group is not participating in resonance, so it can act as a nucleophile and attack the carbonyl carbon of the ketone and aldehyde.
Question 8.18(iii) Give a plausible explanation for each of the following:
Answer:
$\mathrm{RCOOH}+\mathrm{R}^{\prime} \mathrm{OH} \rightleftharpoons \mathrm{RCOOR}{ }^{\prime}+\mathrm{H}_2 \mathrm{O}$
If either water or the ester is not removed immediately, then the ester can react with water and again and give back the reactants.
Thus, to shift the reaction in the forward direction, we need to produce more ester or remove either of the two.
Answer:
In the question, it is given that,
% of carbon = 69.77
% of Hydrogen = 11.63
% of Oxygen = (100-69.77-11.63) = 18.6
Now, the no. of moles of the components are-
nc=69.7712=5.81
nH=11.631=11.63
nO=18.616=1.16
Thus ration of C:H:O = 5.81:11.63:1.16
To get empirical formula we need to divide them by 1.16 .So,
C:H:O= 5:10:1
So, the empirical formula is $\mathrm{C}_5 \mathrm{H}_{10} \mathrm{O}$
The molecular mass of the compound = (5×12)+(10×1)+(1×16)=86
Since the compound does not give tollens test it means it is not an aldehyde. It is given that, the compound gives positive iodoform test it means the compound must be methyl ketone.
On oxidation, it gives ethanoic acid and propanoic acids. Therefore, the compound should be
Pentan-2-one
the structure of the compound is - $\mathrm{CH}_3-\mathrm{CO}-\left(\mathrm{CH}_2\right)_2-\mathrm{CH}_3$
Answer:
In the carboxylate ion, the electron is delocalised between the oxygen, which is electronegative in nature. But in the case of phenoxide ion, the electrons are delocalised between the less electronegative atom, and also phenoxide ion has non-equivalent resonance structures. Therefore,the carboxylate ion is more resonance stable than the phenoxide ion, and we know that the more stable the conjugate base of an acid, high stronger is the acid

Class 12 Chemistry NCERT Chapter 8: Higher Order Thinking Skills (HOTS) Questions
HOTS questions of aldehydes, ketones and carboxylic acids ncert solutions are given below to help students think deeply and apply their knowledge of Aldehydes, Ketones, and Carboxylic Acids in solving tricky problems.
Question 1. $\mathrm{CH}_3-\mathrm{CH}_2-\mathrm{OH} \xrightarrow[\mathrm{KMnO}_4]{\text { Joner reagent }\left(\mathrm{CrO}_3+\mathrm{H}^{\oplus}\right)}$
Consider the above reaction sequence and identify the major product P.
(1) Methane
(2) Methanal
(3) Methoxymethane
(4) Methanoic acid
Answer.

Hence, the correct answer is option (1).
Question 2. Fehling’s solution ‘A’ is:
(1) aqueous copper sulphate
(2) alkaline copper sulphate
(3) alkaline solution of sodium potassium tartrate (Rochelle’s salt)
(4) aqueous sodium citrate
Answer:
Fehling's solution A is a deep blue aqueous solution of copper(II) sulfate. It's also known as cupric sulfate, blue vitriol, or bluestone.
Fehling solution ‘A’ = Aqueous copper sulphate
Fehling solution ‘B’ = Alkaline sodium potassium tartrate (Rochelle salt)
Hence, the correct answer is option (1).
Question 3. Anhydrides react with alcohols and ammonia to form
(i) Carboxylic Acid and Amine
(ii) Esters and Oximes
(iii) Esters and Amides
(iv) Carboxylic acid and amide
Answer:
Anhydrides undergo nucleophilic substitution at the acyl carbon to form Esters and Amides, respectively.
The reactions are given below
Hence, the correct answer is option (3).
Approach to Solve Questions of Class 12 Chemistry Chapter 8
To solve class 12 chemistry chapter 8 aldehydes, ketones and carboxylic acids question answer it is important to follow a systematic approach. Given below the approaches to solve these questions effectively.
1. Learn about Nomenclature and Structure:
Nomenclature and structure plays an important role while solving questions of Chapter 8. Be confident with naming CHO and CO groups. Differentiate between aldehydes and ketones.
2. Understand Preparation Methods:
Study oxidation of alcohols, ozonolysis of alkenes, and hydration of alkynes. Students can follow aldehydes, ketones and carboxylic acids ncert notes for conceptual clarity.
3. Nucleophilic Addition Reactions:
Questions related to Nucleophilic Addition Reactions are often asked in exams. The key mechanism includes HCN, $\mathrm{NaHSO}_3$, Grignard reagent addition to C=O.
4. Learn how to Identify Aldehydes:
Various tests to identify aldehydes are Tollens test, Fehlings solution, and Schiffs test. These are only for aldehydes, not ketones.
5. Solve questions
Practice is the key to success, solve aldehydes, ketones and carboxylic acids class 12 question answer as they are often asked in exams. Students can also follow additional sources and previous years questions.
Topics and Subtopics Covered in the NCERT Textbooks
All the topics and subtopics covered in the NCERT Solutions for Class 12 Chemistry Chapter 8 Aldehydes, Ketones and Carboxylic Acids are listed below:
8.1 Nomenclature and Structure of Carbonyl Compounds
8.1.1 Nomenclature
8.1.2 Structure of Carbonyl Compounds
8.2 Preparation of Aldehydes And Ketones
8.2.1 Preparation of Aldehydes And Ketones
8.2.2 Preparation of Aldehydes
8.2.3 Preparation of Ketones
8.3 Physical Properties of Aldehydes and Ketones
8.4 Chemical Reactions of Aldehydes and Ketones
8.5 Uses of Aldehydes and Ketones
8.6 Nomenclature and Structure of Carboxyl Group
8.6.1 Nomenclature
8.6.2 Structure of Carboxyl Group
8.7 Methods of Preparation of Carboxylic Acids
8.8 Physical Properties of Carboxylic Acids
8.9 Chemical Reactions of Carboxylic Acids
8.9.1 Reaction Involving Cleavage of O-H Bond
8.9.2 Reaction Involving Cleavage of C-OH Bond
8.9.3 Reaction Involving -COOH Group
8.9.4 Substitution Reaction in the Hydrocarbon Part
8.10 Uses of Carboxylic Acids
What Extra Should Students Study Beyond the NCERT for JEE?
Beyond the NCERT, students should focus on the important concepts and reactions according to the table given below. To understand these concepts better students can follow aldehydes, ketones and carboxylic acids ncert notes.
What Students Learn from NCERT Solutions for Class 12 Chemistry Chapter 8 Aldehydes, Ketones and Carboxylic Acids
These aldehydes, ketones and carboxylic acids class 12 question answer help students understand the structure, properties, and reactions of aldehydes, ketones, and carboxylic acids. Given below some points on what students will learn using these solutions of NCERT:
- Using these solutions students will understand the nomenclature and classification of aldehydes, ketones, and carboxylic acids.
- They will learn how to identify and write structural formulas for different compounds
- Important reactions such as oxidation, reduction, nucleophilic addition, and substitution reactions are well explained in these aldehydes, ketones and carboxylic acids ncert solutions through a series of solved examples.
- Mechanisms of key reactions are explained well in these solutions.
NCERT Solutions for Class 12 Chemistry Chapter-Wise
Apart from the class 12 chemistry chapter 8 aldehydes, ketones and carboxylic acids solutions students can also explore the links provided below for solutions to other chapters
NCERT Solutions for Class 12 Subject-wise
Class 12 NCERT subject-wise solutions are given below:
NCERT Exemplar Solutions Subject-Wise
Class 12 NCERT subject-wise exemplar solutions are given below:
NCERT Books and NCERT Syllabus
The NCERT books and syllabus links for class 12 are given below:
Frequently Asked Questions (FAQs)
They are step by step answers to all textbook questions from this chapter, helping students understand the structure, properties, reactions, and mechanisms of aldehydes, ketones, and carboxylic acids for better exam preparation.
You can find them on educational websites like Careers360 which offer free step by step solutions in PDF format for easy study and revision.
Carboxylic acids can be prepared by oxidising primary alcohols or aldehydes, or by hydrolysing nitriles, esters, and amides under acidic or basic conditions.
Esterification is the reaction of a carboxylic acid with an alcohol in the presence of an acid catalyst.
Due to the presence of both a carbonyl (C=O) and a hydroxyl (-OH) group, carboxylic acids exhibit strong hydrogen bonding. This results in:
- High boiling points
- Dimer formation
- Solubility in water
Questions related to CBSE Class 12th
On Question asked by student community
Hello,
The date of 12 exam is depends on which board you belongs to . You should check the exact date of your exam by visiting the official website of your respective board.
Hope this information is useful to you.
Hello,
Class 12 biology questions papers 2023-2025 are available on cbseacademic.nic.in , and other educational website. You can download PDFs of questions papers with solution for practice. For state boards, visit the official board site or trusted education portal.
Hope this information is useful to you.
Hello Pruthvi,
Taking a drop year to reappear for the Karnataka Common Entrance Test (KCET) is a well-defined process. As a repeater, you are fully eligible to take the exam again to improve your score and secure a better rank for admissions.
The main procedure involves submitting a new application for the KCET through the official Karnataka Examinations Authority (KEA) website when registrations open for the next academic session. You must pay the required application fee and complete all formalities just like any other candidate. A significant advantage for you is that you do not need to retake your 12th board exams. Your previously secured board marks in the qualifying subjects will be used again. Your new KCET rank will be calculated by combining these existing board marks with your new score from the KCET exam. Therefore, your entire focus during this year should be on preparing thoroughly for the KCET to achieve a higher score.
For more details about the KCET Exam preparation,
CLICK HERE.
I hope this answer helps you. If you have more queries, feel free to share your questions with us, and we will be happy to assist you.
Thank you, and I wish you all the best in your bright future.
Yes, you can switch from Science in Karnataka State Board to Commerce in CBSE for 12th. You will need a Transfer Certificate from your current school and meet the CBSE school’s admission requirements. Since you haven’t studied Commerce subjects like Accountancy, Economics, and Business Studies, you may need to catch up before or during 12th. Not all CBSE schools accept direct admission to 12th from another board, so some may ask you to join Class 11 first. Make sure to check the school’s rules and plan your subject preparation.
Hello
For the 12th CBSE Hindi Medium board exam, important questions usually come from core chapters like “Madhushala”, “Jhansi ki Rani”, and “Bharat ki Khoj”.
Questions often include essay writing, letter writing, and comprehension passages. Grammar topics like Tenses, Voice Change, and Direct-Indirect Speech are frequently asked.
Students should practice poetry questions on themes and meanings. Important questions also cover summary writing and translation from Hindi to English or vice versa.
Previous years’ question papers help identify commonly asked questions.
Focus on writing practice to improve handwriting and presentation. Time management during exams is key to answering all questions effectively.
Popular CBSE Class 12th Questions
Applications for Admissions are open.
This ebook serves as a valuable study guide for NEET 2025 exam.
NEET Previous 10 Year Questions
Get nowThis e-book offers NEET PYQ and serves as an indispensable NEET study material.
JEE Main Important Physics formulas
Get nowAs per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters



