Chemistry Coordination Compounds notes in PDF format help students by providing a well-organised and easily accessible summary of the chapter. They save time during revision, strengthen conceptual understanding, and allow students to practise important questions anytime, even offline.
NCERT Class 12 Chemistry Chapter 9 Notes Coordination Compounds - Download PDF
Do you know 50 percent of enzymes in our body are coordination compounds and many life saving medicines are made up of them. These compounds are everywhere from the vibrant colour of transition metals complexes to the red colour of blood. These compounds are formed when a central metal atom combines with small atoms or ions called ligands. NCERT notes help you understand topics like coordination spheres, the role of ligands in stabilising metal ions, Valence Bond Theory, and Crystal Field Theory.
This Story also Contains
- NCERT Notes for Class 12 Chapter 5 Coordination Compounds: Download PDF
- NCERT Notes for Class 12 Chapter 5 Coordination Compounds
- Coordination Compounds Previous Year Questions And Answers
- How to Master Class 12 Chemistry Chapter 5 Coordination Compounds
- Advantages of Class 12 Chemistry Chapter 5 Coordination Compounds Notes
- CBSE Class 12 Chemistry Chapter-Wise Notes
- NCERT Solutions for Class 12 Chemistry
- Subject-Wise NCERT Solutions
- Subject-Wise NCERT Exemplar Solutions
NCERT Class 12 Chemistry Notes include important concepts, definitions, diagrams, and selective previous year solved questions, which helps students in scoring well in the CBSE board as well as competitive exams by simplifying complex concepts. Whether you are revising for an exam or learning the topic for the first time, NCERT Notes for Class 12 are an essential resource.
NCERT Notes for Class 12 Chapter 5 Coordination Compounds: Download PDF
These comprehensive class 12 chemistry chapter 5 coordination compounds notes cover all important concepts in a simple and exam-friendly format. Students can easily download the PDF by clicking the button given below.
Also refer,
NCERT Notes for Class 12 Chapter 5 Coordination Compounds
These coordination compounds class 12 ncert notes given below covers all concepts of NCERT textbook and explains them in a concise and easy-to-understand manner. All the essential topics like ligands, coordination number, isomerism, IUPAC naming rules and bonding theories like VBT, CFT are explained in a step by step and detailed manner. These notes are useful for last minute revision as well as exam preparation.
Werner's Theory of Coordination Compounds
The postulates from Werner’s theory are listed below:
-
The central metal or the metal atoms within a coordination compounds will always shows two types of valency. Among these two types, one is known as primary valency and another one is secondary valency.
-
The primary valency denotes the oxidation state of the atom, and the secondary valency will show the coordination number.
-
The secondary valences is not variable i.e. constant for every metal atom. Or we can say that the coordination number of any particular atom is fixed.
-
The metal atom will fulfil both its primary as well as secondary valencies. Generally, a negative ion i.e. anion, will satisfies the primary valency. And on the other hand, among these two i.e. negative ion or neutral molecules both can satisfy secondary valencies.
Definitions of Some Important Terms pertaining to Coordination Compounds
Coordination Entity
A coordination entity comprises a central metal atom or ion bonded with a fixed number of ions or molecules.
For example: $\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_6\right] \mathrm{Cl}_3$is a coordinate compound or we can say coordination entity in which six ammonia molecules are composed of 3 chlorine molecules.
Other examples of coordination entities are as follows:
$\begin{aligned} & {\left[\mathrm{Ni}(\mathrm{CN})_4\right]^{2-}} \\ & \left.\left.\left[\mathrm{Cr}(\mathrm{NH})_3\right)_4\right] \mathrm{Cl}_2\right]^{+}, \\ & {\left[\mathrm{Fe}(\mathrm{CN})_6\right]^{4-}}\end{aligned}$
Central atom/ion
Central metal atom/ ion to which one or more neutral molecules or ions are linked by coordinate bonds in a definite geometrical arrangement around it. It is also referred as Lewis's acid.
Ligands
The ions or molecules that are bound to the central atom or ion within the coordination entity are called ligands. They can be simple ions like Cl–, or small molecules like H2O or NH3, they can also be larger molecules like $\mathrm{H}_2 \mathrm{NCH}_2 \mathrm{CH}_2 \mathrm{NH}_2$ or $\mathrm{N}\left(\mathrm{CH}_2 \mathrm{CH}_2 \mathrm{NH}_2\right)_3$ or even macromolecules, like proteins.
Coordination number:
It is the total number of coordinate bonds through which the central metal atom/ion is attached to ligands.
Coordination sphere:
The central metal atom/ion and the ligands which are directly attached to it, are enclosed in square brackets and is collectively known as the coordination sphere. It is non-ionisable.
Coordination polyhedron:
It is the spatial arrangement of the ligands around the central metal/atoms or ions. The most common coordination Polyhedra are octahedral, square planar, and tetrahedral.
Oxidation number of the central atom:
It is the number that represents the charge on the central metal atom, if all the ligands are removed along with the electron pairs that are shared with the central atom.
Homoleptic and heteroleptic complexes
-
Homoleptic complex are those complexes in which the metal atom/ion is bound to only one kind of ligand.
-
Heteroleptic complex are those complexes in which a metal atom/ion is bound to more than one kind of ligand.
Nomenclature of coordination compounds:
Rules for writing the formula of coordination compounds:
-
Formula of the cation whether simple or complex must be written first followed by anion.
- The coordination sphere is written in square brackets.
- Within the coordination sphere the sequence of symbols is, first the metal atom followed by anionic ligand then neutral ligand finally cationic ligand. Ligands of same type are arranged alphabetically. Polyatomic ligands are enclosed in parentheses.
- The number of cations or anions to be written in the formula is calculated on the basis that total positive charge must be equal to the total negative charge, as the complex as a whole is electrically neutral.
Rules for naming coordination compounds:
- The cation is named first then the anion.
- In naming coordination sphere, ligands are named first in alphabetical order followed by metal atom and then oxidation state of metal by a roman numeral in parentheses.
- Name of coordination compounds is started with a small letter and the complex part is written as one word.
Naming of ligands:
- Name of anionic ligands end in -o. e.g., $\mathrm{Cl}^{-}$: Chlorido
- Neutral ligands (with a few exceptions) retain their names e.g., $\mathrm{NH}_3$ : Ammine
- Name of cationic ligands end in - ium. e.g., $\mathrm{NO}_2^{+}$: Nitronium
- Certain ligands are represented by abbreviations in parentheses instead of their complex structural formulae. e.g., ethylenediamine(en).
- Ambidentate ligands are named by using different names of ligands or by placing the symbol of donor atom. e.g., $-\mathrm{SCN}^{-}$(Thiocyanato-S or Thiocyanato), $-\mathrm{NCS}^{-}$(Thiocyanato-N or Isothiocyanato),$-\mathrm{ONO}^{-}$(Nitrito-O or Nitrito), $-\mathrm{NO}_2^{-}$(Nitrito-N or Nitro)
Isomerism in Coordination Compounds
Isomers are two or more compounds that have the same chemical formula but a different arrangement of atoms.
The two principal types of isomerism are:
- Stereoisomerism
- Structural isomerism
1). Stereoisomerism
Coordination compounds having different positions and arragements of ligands in space. They are further divided into two categories
a). Geometrical Isomerism
This isomerism is due to ligands occupying different positions around the central metal atom or ion. The ligands occupy positions either adjacent or opposite to one another. This type of isomerism is also known as cis-trans isomerism. When two same ligands are at right angle($90^{\circ}$), the form is cis- form and when they are present diagonally at $180^{\circ}$ to each other, the form is termed as trans- from. Geometrical isomerism is very common in coordination number 4 and 6 complexes.

Another type of geometrical isomerism occurs in octahedral coordination entities of the type $\left[M a_3 b_3\right]$ like $\left[\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_3\left(\mathrm{NO}_2\right)_3\right]\right.$. If three donor atoms of the same ligands occupy adjacent positions at the corners of an octahedral face, we have the facial (fac) isomer. When the positions are around the meridian of the octahedron, we get the meridional (mer) isomer.

b). Optical Isomerism
Optical isomers are mirror images that cannot be superimposed on one another. These are called as enantiomers. The molecules or ions that cannot be superimposed are called chiral. The two forms are called dextro (d) and laevo (l) depending upon the direction they rotate the plane of polarised light in a polarimeter (d rotates to the right, l to the left). Optical isomerism is common in octahedral complexes involving didentate ligands.

2). Structural isomerism
Coordination compounds having different ligands within their coordination spheres.
a). Linkage Isomerism
Linkage isomerism arises in a coordination compound containing ambidentate ligand. A simple example is provided by complexes containing the thiocyanate ligand, NCS–, which may bind through the nitrogen to give M–NCS or through sulphur to give M–SCN. Jørgensen discovered such behaviour in the complex $\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_5\left(\mathrm{NO}_2\right)\right] \mathrm{Cl}_2$, which is obtained as the red form, in which the nitrite ligand is bound through oxygen (–ONO), and as the yellow form, in which the nitrite ligand is bound through nitrogen (–NO2).
b). Coordination Isomerism
This type of isomerism arises from the interchange of ligands between cationic and anionic entities of different metal ions present in a complex. An example is provided by $\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_6\right]\left[\mathrm{Cr}(\mathrm{CN})_6\right]$, in which the NH3 ligands are bound to Co3+ and the CN– ligands to Cr3+. In its coordination isomer $\left[\mathrm{Cr}\left(\mathrm{NH}_3\right)_6\right]\left[\mathrm{Co}(\mathrm{CN})_6\right]$, the NH3 ligands are bound to Cr3+ and the CN– ligands to Co3+.
c). Ionisation isomerism
Complexes that give different ions in solution.
$
\text { e.g., }\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_5 \mathrm{Br}\right] \mathrm{SO}_4
$
$\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_5 \mathrm{SO}_4\right] \mathrm{Br}$
d). Solvate isomerism
Complexes that differ in number of water molecules present as ligands (inside the coordination sphere) and as free molecules (outside the coordination sphere).
$
\begin{gathered}
\text { e.g., }\left[\mathrm{Cr}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right] \mathrm{Cl}_3 \\
{\left[\mathrm{Cr}\left(\mathrm{H}_2 \mathrm{O}\right)_5 \mathrm{Cl}\right] \mathrm{Cl}_2 \cdot \mathrm{H}_2 \mathrm{O}}
\end{gathered}
$
Bonding in Coordination Compounds
Werner could not answer basic questions like:
(i) Why only certain elements possess the remarkable property of forming coordination compounds?
(ii) Why the bonds in coordination compounds have directional properties?
(iii) Why coordination compounds have characteristic magnetic and optical properties?
To explain the nature of bonding in coordination compounds various theories are proposed such as Valence Bond Theory (VBT), Crystal Field Theory (CFT), Ligand Field Theory (LFT) and Molecular Orbital Theory (MOT). Students can understand these concepts better by using coordination compounds Class 12 Chemistry Chapter 5 CBSE notes.
Valence Bond Theory
According to this theory, the metal atom or ion under the influence of ligands can use its (n-1)d or nd orbitals along with its ns and np for hybridisation to yield a set of equivalent orbitals of definite geometry such as octahedral, tetrahedral, square planar and so on. These hybridised orbitals are allowed to overlap with ligand orbitals that can donate electron pairs for bonding. The different types of hybridisation and their respective shapes are given below.
| Coordination Number | Type of Hybridisation | Shape |
| 4 | sp3 | Tetrahedral |
| 4 | dsp2 | Square Planar |
| 5 | sp3d | Trigonal Bipyramidal |
| 6 | sp3d2 | Octahedral |
| 6 | d2sp3 | Octahedral |
Magnetic Properties of Coordination Compound
The transition metals have a unique ability to form magnets. Metal complexes show paramagnetism due to unpaired electrons as the last electrons will reside in the d orbitals. By considering only monometallic complexes, they have unpaired electrons or an odd number of electrons in which each electron has a magnetic moment associated with spin angular momentum and causes destabilization.
Limitations of Valence Bond Theory
Some limitations of VBT are given below:
- It does not give quantitative interpretation of magnetic data.
- It does not explain the colour exhibited by coordination compounds.
- It does not give a quantitative interpretation of the thermodynamic or kinetic stabilities of coordination compounds.
- It does not make exact predictions regarding the tetrahedral and square planar structures of 4-coordinate complexes.
- It involves a number of assumptions
Crystal Field Theory
Crystal Field Theory (CFT) is not a basic theory but an electrostatic model. As the theory is based on the electrostatic model of hard spheres and the interaction is done in a purely electrostatic way. The central atom is having a positive charge and ligands have a negative charge and thus this negative charge approaches towards the positive charge and due to ligands point charge degeneracy has been created. This means that all the five “d” orbitals in an isolated gaseous metal atom or ion will have the same energy or we can be told that they are degenerate. However, when this negative field is formed due to ligands in a complex, this will become asymmetrical and then the degeneracy of d orbitals is lifted. It will result in the splitting of the d orbitals. The pattern of splitting will depend on the nature of the crystal field.
In an octahedral coordination entity with six ligands surrounding the metal atom/ion, there will be repulsion between the electrons in metal d orbitals and the electrons (or negative charges) of the ligands. Such a repulsion is more when the metal d orbital is directed towards the ligand than when it is away from the ligand. Thus, the $d_{x^2-y^2}$ and $d_{z^2}$ orbitals which are oriented towards the axes along the direction of approach of the ligand will experience more repulsion and will be raised in energy; and the $d_{x y}, d_{y z}$ and $d_{z x}$ orbitals which are directed between the axes will be lowered in energy relative to the average energy in the spherical crystal field. Thus, the degeneracy of the d orbitals has been removed due to ligand electron-metal electron repulsions in the octahedral complex to yield three orbitals of lower energy, $t_{2 g}$ set and two orbitals of higher energy, $e_g$ set. This splitting of the degenerate levels due to the presence of ligands in a definite geometry is termed as crystal field splitting and the energy separation is denoted by $\Delta_0$. Thus, the energy of the two $e_g$ orbitals will increase by $\frac{3}{5} \Delta_0$ and that of the three $t_{2 g}$ will decrease by $\frac{2}{5} \Delta_0$.

The crystal field splitting, $\Delta_0$, depends upon the field produced by the ligand and charge on the metal ion. Some ligands are able to produce strong fields in which case, the splitting will be large whereas others produce weak fields and consequently result in small splitting of d orbitals. In general, ligands can be arranged in a series in the order of increasing field strength as given below:
$\begin{aligned} I^{-} & <\mathrm{Br}^{-}<S C N^{-}<\mathrm{Cl}^{-}<S^{2-}<F^{-}<\mathrm{OH}^{-}<\mathrm{C}_2 \mathrm{O}_4^{2-}<\mathrm{H}_2 \mathrm{O}< \\ \mathrm{NCS}^{-} & <e \mathrm{edta} \mathrm{a}^{4-}<\mathrm{NH}_3<e n<\mathrm{CN}^{-}<\mathrm{CO}\end{aligned}$
Such a series is termed as spectrochemical series. It is an experimentally determined series based on the absorption of light by complexes with different ligands. Let us assign electrons in the d orbitals of metal ion in octahedral coordination entities. Obviously, the single d electron occupies one of the lower energy $t_{2 g}$ orbitals. In $d^2$ and $d^3$ coordination entities, the d electrons occupy the $t_{2 g}$ orbitals singly in accordance with the Hund’s rule. For $d^4$ ions, two possible patterns of electron distribution arise: (i) the fourth electron could either enter the $t_{2 g}$ level and pair with an existing electron, or (ii) it could avoid paying the price of the pairing energy by occupying the $e_g$ level. Which of these possibilities occurs, depends on the relative magnitude of the crystal field splitting, $\Delta_0$ and the pairing energy, P (P represents the energy required for electron pairing in a single orbital). The two options are:
- If $\Delta_0<P$, the fourth electron enters one of the $e_g$ orbitals giving the configuration $t_{2 g}^3 e_g^1$.. Ligands for which ∆o < P are known as weak field ligands and form high spin complexes.
- If $\Delta_0>P$, it becomes more energetically favourable for the fourth electron to occupy a $t_{2 g}$ orbital with configuration $t_{2 g}^4 e_g^0$. Ligands which produce this effect are known as strong field ligands and form low spin complexes.
Calculations show that $d^4$ to $d^7$ coordination entities are more stable for strong field as compared to weak field cases.
In tetrahedral coordination entity formation, the d orbital splitting is inverted and is smaller as compared to the octahedral field splitting. For the same metal, the same ligands and metal-ligand distances, it can be shown that $\Delta_t=\frac{4}{9} \Delta_0$. Consequently, the orbital splitting energies are not sufficiently large for forcing pairing and, therefore, low spin configurations are rarely observed. The ‘g’ subscript is used for the octahedral and square planar complexes which have centre of symmetry. Since tetrahedral complexes lack symmetry, ‘g’ subscript is not used with energy levels.

Applications of CFT
These are the various applications of crystal field theory.
- Configuration of metal ion: In weak field ligand, the crystal field splitting energy difference is very less. Thus, pairing of electrons is done by Hund's rule. But for strong field ligand, the splitting energy difference is high due to which the pairing of electrons is not done by Hund's rule.
- Magnetic nature of complex: On the basis of magnetism, all the complexes can be divided into two categories, paramagnetic and diamagnetic. Paramagnetic complexes are weakly attracted by magnetic field and have unpaired electron, while diamagnetic complexes are weakly repelled by magnetic field and these molecules have no unpaired electron.
- Colour in coordination compounds: The colour of the complex is complementary to that which is absorbed. The complementary colour is the colour generated from the wavelength left over; if green light is absorbed by the complex, it appears red. The colour in the coordination compounds can be readily explained in terms of the crystal field theory. Consider, for example, the complex $\left[\mathrm{Ti}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{3+}$, which is violet in colour. This is an octahedral complex where the single electron ($T i^{3+}$ is a $3 d^1$ system) in the metal d orbital is in the $t_{2 g}$ level in the ground state of the complex. The next higher state available for the electron is the empty $e_g$ level. If light corresponding to the energy of blue-green region is absorbed by the complex, it would excite the electron from $t_{2 g}$ level to the $e_g$ level i.e the configuration changes from $t_{2 g}^1 e_g^0$ to $t_{2 g}^0 e_g^1$. Consequently, the complex appears violet in colour. This phenomenon is called d-d transition and the crystal field theory attributes the colour of the coordination compounds this d-d transition of the electron.
Limitations of Crystal Field Theory
- Due to assumptions of CFSE that the ligands are point charges, it follows that anionic ligands should exert the greatest splitting effect. The anionic ligands actually are found at the low end of the spectrochemical series.
- It does not take into account the covalent character of bonding between the ligand and the central atom.
Colour in Coordination Compounds
-
The coordination compound is made up of a ligand and a metal ion, and ligands are responsible for the coloration of the complex compounds. This means that different types of ligands show different colors.
-
The energy is required to remove the electron from a lower energy state to a higher energy state.
-
A higher energy state absorbs the color of shorter wavelengths.
-
We know that the metals of complex compounds are basically from d- orbitals or transition elements which have half-filled or unfilled d orbitals which pull oy the electron from the lower state and forward it to a higher energy level which causes the d-d transition of the metal atom thus radiates the color depending on the type of ligands.
-
For example, considering the two ligands one is a strong field ligand and the other is a weak field ligand.
-
The complex [Cr(NH3)6]3+ has strong-field ligands, so it will absorb relatively high-energy photons. This corresponds to the blue-violet light zone, which will give it a yellow color.
-
The other complex is of weak-field ligands, the [Cr(H2O)6]3+ ion, which absorbs lower-energy photons. This corresponds to the yellow-green portion of the visible spectrum and leads to giving deep violet color.
-
Discussing other examples based upon the high and low spin is as follows:
-
The iron(II) complex [Fe(H2O)6]SO4 gives blue-green due to the high-spin complex which absorbs the photons in the red wavelengths.
-
On the other hand, the complex iron(II) K4[Fe(CN)6] gives pale yellow which absorbs photons in violet wavelengths.
-
In general, strong-field ligands can cause a large split in the energies of d orbitals, so ligands are generally yellow, orange, or red because they absorb higher-energy violet or blue light.
-
On the other hand, weak-field ligands are often blue-green, blue, or indigo because they absorb lower-energy yellow, orange, or red light.
-
400-nm Violet light if absorbed → Green-yellow colour will be observed
-
430-nm Blue light if absorbed → Orange colour will be observed
-
450-nm Blue light if absorbed → Yellow colour will be observed
-
490-nm Blue-green light if absorbed → Red colour will be observed
-
570-nm Yellow-green light if absorbed → Violet colour will be observed
-
580-nm Yellow light if absorbed → Dark blue colour will be observed
-
600-nm Orange light if absorbed → Blue colour will be observed
-
650-nm Red light if absorbed → Green colour will be observed
Bonding in Metal carbonyls:
Compounds that contain at least one carbon-metal bond are called organometallic compounds. Zeise, in 1830, prepared the first organometallic compound by the action of ethylene on a solution of potassium chloroplatinate(II). In the last four decades, enormous work has been done in this field and many fascinating compounds have been synthesized and investigated. Grignard reagent, RMgX is a familiar example of organometallic compounds where R is an alkyl group. Diethyl zinc [Zn(C2H5)2], lead tetraethyl [Pb(C2H5)4], ferrocene [Fe(C5H5)2], dibenzene chromium[Cr(C6H6)2], metal carbonyls are other examples of organometallic compounds. The compounds of metalloids such as germanium and antimony and mon-metallic elements such as boron and silicon are also included under this classification.
Organometallic compounds may be classified in three classes:
Sigma(σ) bonded complexes: These complexes contain a metal and carbon atom attached with a sigma bond e.g. Tetramethyl Tin, Trimethyl aluminium etc.
Bonding in Trimethyl aluminium is shown below

Pi(π) bonded complexes: These complexes contain a metal and carbon atom attached with a Pi bond. e.g. Ferrocene, Dibenzene Chromium etc. Bonding in Ferrocene and Dibenzene Chromium is shown below:

Complexes containing both $\sigma$ and $\pi$ bonding characteristics: These complexes contain both $\sigma$ as well as $\pi$ bonding characteristics. e.g. Metal Carbonyls. The $M-C \sigma$ bond is formed by the donation of the lone pair of electrons of carbonyl group into the vacant d orbital of metal while the $M-C \pi$ bond is formed by back donation of lone pair of electrons from the metal into vacant antibonding $\pi^*$ molecular orbital of CO. This synergic bonding leads to the formation of stronger bonds and stable metal carbonyl complexes. The bonding in metal carbonyls is shown below:

Importance and Applications of coordination Compounds
Coordination compounds are of great importance. These compounds are widely present in the mineral, plant and animal worlds and are known to play many important functions in the area of analytical chemistry, metallurgy, biological systems, industry and medicine.
- Coordination compounds find use in many qualitative and quantitative chemical analysis.
- The selective estimation of ions can be done due to difference in the stability constants complexes.
- Purification of metals can be achieved through formation and subsequent decomposition of their coordination compounds.
- Coordination compounds are of great importance in biological systems. The pigment responsible for photosynthesis, chlorophyll, is a coordination compound of magnesium. Haemoglobin, the red pigment of blood which acts as oxygen carrier is a coordination compound of iron. Vitamin B12 , cyanocobalamine, the anti pernicious anaemia factor, is a coordination compound of cobalt.
- These compounds are used as catalysts for many industrial processes.
Coordination Compounds Previous Year Questions And Answers
Practising previous year questions on coordination compounds helps students understand the exam pattern, commonly asked concepts, and the level of difficulty. Students can refer to ncert class 12 chemistry chapter 5 coordination compounds notes to solve these questions effectively.
Question 1: The diamagnetic species is:
[At. $\mathrm{No} . \mathrm{Co}=27, \mathrm{Fe}=26, \mathrm{Ni}=28$ ]
(1) $\left[\mathrm{Ni}(\mathrm{CN})_4\right]^{2-}$
(2) $\left[\mathrm{NiCl}_4\right]^{2-}$
(3) $\left[\mathrm{Fe}(\mathrm{CN})_6\right]^{3-}$
(4) $\left[\mathrm{CoF}_6\right]^{3-}$
Answer:
1) $\left[N i(C N)_4\right]^{2-}$
The atomic number of Ni is 28
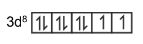
Ground state electronic configuration of Ni is $[A r] 3 d^8 4 s^2$
In complex $\left[\mathrm{Ni}(\mathrm{CN})_4\right]^{2-}$, Ni is in +2 oxidation state.
So, the electronic configuration becomes $3 d^8 $
In $\left[N i(C N)_4\right]^{2-}$, $C N^{-}$is a strong field ligand which cause pairing of electrons in d-orbital. So no unpaired electron left.
Therefore it is diamagnetic in nature.
2) $\left[\mathrm{NiCl}_4\right]^{2-}$
Ni is in +2 oxidation state, so the electronic configuration becomes $3 d^8 $. But Cl- a weak field ligand, does not cause a pairing of electrons in the orbital. So there are unpaired electrons, and it is not diamagnetic.
3) $
\left[\mathrm{Fe}(\mathrm{CN})_6\right]^{3-}
$
Fe atomic number 26
$\mathrm{CN}^{-}$ligand charge $-1,6 \times-1=-6$
Overall charge -3
So, Fe oxidation state $x+(-6)=-3 \Rightarrow x=+3$
$
\operatorname{Fe}(\text { III })=\mathrm{d}^5
$
$\mathrm{CN}^{-}$is strong field ligand $\rightarrow$ low spin
Low spin $d^5$ has one unpaired electron $t_{2 g}{ }^5$ eg $\left.^0\right)$
So, it not diamagnetic cause it has one unpaired electron.
4) $\left[\mathrm{CoF}_6\right]^{3-}$
Co atomic number 27 and the charge is -3
So, $\operatorname{Co}($ III $)=d^6$
$\mathrm{F}^{-}$is weak field ligand does not cause pairing of electrons $\rightarrow$ high spin
$d^6$ has unpaired electrons, so it is also not diamagnetic.
Hence, the correct answer is option (1).
Question 2: Write the electronic configuration of $\mathrm{d}^5$ ion when $\Delta_0>P$.
Answer:
$\Delta_0>P \rightarrow$ Low-spin configuration
In an octahedral field, the d-orbitals split into:
- $\mathrm{t}_{\mathbf{2}} \mathrm{g}$ (lower energy): 3 orbitals
- $\mathbf{e}_{\mathrm{g}}$ (higher energy): 2 orbitals
Low-spin $d^5$ configuration:
All five electrons occupy the lower-energy $\mathrm{t}_{\mathbf{2}} \mathrm{g}$ orbitals, pairing up before entering the higher-energy $\mathrm{e}_{\mathrm{g}}$ orbitals.
$e_g$ 0
$t_{2 g}$ - $\uparrow \downarrow, \uparrow \downarrow, \uparrow(2 \text { pairs }+1 \text { unpaired })$

Electronic configuration: $t_{2 g}^5 e_g^0$
Hence, the answer is $t_{2 g}^5 e_g^0$
Question 3: Write IUPAC names of the following coordination entity :
$\left[\mathrm{Ni}(\mathrm{CN})_4\right]^{2-}$
Answer:
$\left[\mathrm{Ni}(\mathrm{CN})_4\right]^{2-}$ Anionic complex
Central metal: Ni (nickel)
Ligands: $\mathrm{CN}^{-}$(cyanide), 4 of them $\rightarrow$ tetracyanido
Oxidation state of Ni
Let oxidation state of $\mathrm{Ni}=\boldsymbol{x}$
$
x+4(-1)=-2 \Rightarrow x=+2
$
$\mathrm{So}, \mathrm{Ni}$ is in the +2 oxidation state
Since the complex ion is anionic (charge $=-2$ ), the name of the metal ends in -ate.
Nickel $\rightarrow$ nickelate
IUPAC Name: Tetracyanidonickelate(II) ion
Hence, the answer is Tetracyanidonickelate(II) ion
Question 4: Match the LIST-I with LIST-II
| LIST-I (Complex/Species) | LIST-II (Shape \& magnetic moment) | ||
| A. | $\left[\mathrm{Ni}(\mathrm{CO})_4\right]$ | I. | Tetrahedral, 2.8 BM |
| B. | $\left[\mathrm{Ni}(\mathrm{CN})_4\right]^{2-}$ | II. | Square planar, 0 BM |
| C. | $\left[\mathrm{NiCl}_4\right]^2$ | III. | Tetrahedral, 0 BM |
| D. | $\left[\mathrm{MnBr}_4\right]^{2-}$ | IV. | Tetrahedral, 5.9 BM |
Choose the correct answer from the options given below :
(1) A-III, B-IV, C-II, D-I
(2) A-I, B-II, C-III, D-IV
(3) A-III, B-II, C-I, D-IV
(4) A-IV, B-I, C-III, D-II
Answer:
A) $\mathrm{Ni}(\mathrm{CO})_4 \rightarrow \mathrm{Ni}(0) \Rightarrow \mathrm{sp}^3$, tetrahedral, 0 BM
$\left(3 d^{10}\right)$ (pairing)
B) $\left[\mathrm{Ni}(\mathrm{CN})_4\right]^{2-} \rightarrow \mathrm{Ni}^{2+} \Rightarrow \mathrm{dsp}^2$, square planar, 0 BM
$\left(3 \mathrm{~d}^8\right)$ (pairing)
C) $\left[\mathrm{NiCl}_4\right]^{2-} \rightarrow \mathrm{Ni}^{2^{+}}$(no pairing) $\Rightarrow \mathrm{sp}^3$, tetrahedral,
2 unpaired electrons therefore, $2.8 \text { BM }$
D) $\left[\mathrm{MnBr}_4\right]^{2^{-}} \Rightarrow \mathrm{Mn}^{2+} \Rightarrow 3 \mathrm{~d}^5$ (no pairing)
Hence, the correct answer is option (3).
Question 5: Match List-I with List-II
| List-I Complex | List-II Primary valency and Secondary valency | ||
| (A) | $\left[\mathrm{Co}(\mathrm{en})_2 \mathrm{Cl}_2\right] \mathrm{Cl}$ | (I) | 3 6 |
| (B) | $\left[\mathrm{Pt}\left(\mathrm{NH}_3\right)_2 \mathrm{Cl}\left(\mathrm{NO}_2\right)\right]$ | (II) | 3 4 |
| (C) | $\mathrm{Hg}\left[\mathrm{Co}(\mathrm{SCN})_4\right]$ | (III) | 2 6 |
| (D) | $[\mathrm{Mg}(\mathrm{EDTA})]^{2-}$ | (IV) | 2 4 |
Choose the correct answer from the options given below :
(1) (A)-(III), (B)-(I), (C)-(II), (D)-(IV)
(2) (A)-(I), (B)-(IV), (C)-(II), (D)-(III)
(3) (A)-(I), (B)-(III), (C)-(II), (D)-(IV)
(4) (A)-(II), (B)-(III), (C)-(IV), (D)-(I)
Answer:
| P.V | S.V | ||
| (A) | $\left[\mathrm{Co}(\mathrm{en})_2 \mathrm{Cl}_2\right] \mathrm{Cl}$ | 3 | 6 |
| (B) | $\left[\mathrm{Pt}\left(\mathrm{NH}_3\right)_2 \mathrm{Cl}\left(\mathrm{NO}_2\right)\right]$ | 2 | 4 |
| (C) | $\mathrm{Hg}\left[\mathrm{Co}(\mathrm{SCN})_4\right]$ | 3 | 4 |
| (D) | $[\mathrm{Mg}(\mathrm{EDTA})]^{2-}$ | 2 | 6 |
(A)-(I), (B)-(IV), (C)-(II), (D)-(III)
Hence, the correct answer is option (2).
How to Master Class 12 Chemistry Chapter 5 Coordination Compounds
These ncert class 12 chemistry chapter 5 coordination compound notes help to understand the basic concepts from your NCERT book. Below are some points on how students master chapter 5.
- Firstly understand the Basic concepts like ligands, coordination number, oxidation states, and nomenclature to build a strong foundation.
- Then learn about Werner’s theory, Valence Bond Theory (VBT), and Crystal Field Theory (CFT) to understand the bonding and structure of coordination compounds. Students can also refer to coordination compounds class 12 ncert notes for understanding these concepts better through a series of solved examples.
- Nomenclature and Isomerism plays an important role in this chapter and questions are often asked in exams. Students must practise regularly naming coordination compounds and identifying structural and stereoisomers to avoid confusion during exams.
- Students must focus on learning important formulas, colours of common complexes and stability order of ligands.
- After that students can solve previous year questions from this chapter.
Advantages of Class 12 Chemistry Chapter 5 Coordination Compounds Notes
NCERT Class 12 Chemistry Chapter 5 Notes Coordination Compounds provide a clear explanation of concepts that helps in mastering topics and scoring well in board and competitive exams. The advantages of using these notes are given below:
- These coordination compound notes cover all important topics like ligands, coordination number, Werner’s theory, isomerism, valence Bond Theory and Crystal Field Theory.
- These notes are prepared by subject experts in a well structured and organised manner.
- NCERT notes include important derivations, and examples commonly asked in board and competitive exams.
- Students can understand the formation, structure, and stability of coordination compounds using ncert class 12 chemistry chapter 5 coordination compounds notes.
CBSE Class 12 Chemistry Chapter-Wise Notes
Along with coordination compounds Class 12 Chemistry Chapter 5 CBSE notes, students can also refer to other Class 12 Chemistry notes for additional practice and deeper understanding.
NCERT Solutions for Class 12 Chemistry
Along with cert class 12 chemistry chapter 5 coordination compounds notes students can also refer to solutions of NCERT for Class 12 Chemistry chapters.
Subject-Wise NCERT Solutions
Students can refer to the links given below for NCERT Solutions:
Subject-Wise NCERT Exemplar Solutions
Students can refer to the links given below for NCERT Exemplar Solutions:
| NCERT Exemplar Class 12 Solutions |
| NCERT Exemplar Class 12 Maths |
| NCERT Exemplar Class 12 Physics |
| NCERT Exemplar Class 12 Chemistry |
| NCERT Exemplar Class 12 Biology |
Frequently Asked Questions (FAQs)
The coordination number refers to the number of ligand atoms that are bonded to the central metal atom. It is determined by the number of donor atoms in the ligands that are directly attached to the metal. Common coordination numbers are 2, 4, and 6, which correspond to various geometrical arrangements like linear, tetrahedral, and octahedral, respectively.
The naming of coordination compounds follows specific IUPAC rules. First, the ligands are named in alphabetical order, followed by the name of the central metal. If the complex is an anion, the name of the metal is modified by adding the suffix "-ate." The oxidation state of the metal is indicated in Roman numerals in parentheses.
Isomerism in coordination compounds is crucial as it leads to different compounds with varying properties, despite having the same formula. Isomers can differ in spatial arrangements, coordination of ligands.
Students can find coordination compounds Class 12 Chemistry Chapter 5 CBSE notes on educational websites, online learning platforms, and the official NCERT website. They are also available on various exam preparation portals that offer downloadable PDF notes for quick and easy access.
The coordination compounds Class 12 Chemistry Chapter 5 CBSE notes are concise study materials that summarise all key concepts, formulas, and reactions from the chapter. These notes help students revise quickly, understand complex topics easily, and prepare effectively for board exams.
Studying coordination compounds is crucial because they play significant roles in biological systems, catalysis, and materials science. Understanding their properties and behaviors helps students comprehend complex chemical interactions and applications relevant to various fields.
Coordination compounds differ from other compounds in their structural composition, properties, and stability. The presence of a central metal atom and its interaction with ligands leads to unique electron configurations, which affect their chemical behavior differently than typical ionic or covalent compounds
A ligand is a molecule or ion that donates a pair of electrons to a central metal atom or ion to form a coordination complex. Ligands can vary in size and charge; they can be simple ions like chloride or complex molecules like ethylenediamine.
Coordination compounds are found in various applications, including in medicine, fertilizers, dyes, and catalysts in chemical reactions.
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters