Polymers Class 12th Notes- Free NCERT Class 12 Chemistry Chapter 15 Notes- Download PDF
The chapter, polymers is the continuation of the NCERT chapter, biomolecules. The NCERT Class 12 Chemistry chapter 15 notes covers a brief outline of the chapter on polymers. The main topics covered in Class 12 Chemistry chapter 15 notes are the classification of polymers, types of polymerization reactions, molecular masses of polymers, biodegradable polymers and polymers of commercial importance.
This Story also Contains
- NCERT Class 12 Chemistry Chapter 15 Notes
- Polymers :
- Polymers Class 12 Notes - Topic 1:
- Classification of Polymers:
- NCERT Class 12 Chemistry Chapter 15 Notes- Topic 2:
- Types of Polymerization Reactions:
- The Molecular Mass of Polymers
- NCERT Class 12 Chemistry Chapter 15 Notes - Topic 4
- Biodegradable Polymers
- Polymers of Commercial Importance:
- NCERT Class 12 Notes Chapter-Wise
- NCERT Books and Syllabus
NCERT Class 12 Chemistry chapter 15 notes also include a brief introduction to some important polymers like polyethene, nylon, rubber, etc. Class 12 Chemistry chapter 15 notes also cover the basic chemical equations in the chapter. The properties and preparations of polymers of commercial importance like Nylon 6,6, Buna-S, Polythene, Nylon 6, Neoprene, Polyvinylchloride, Polyesters, etc are also covered in the CBSE Class 12 Chemistry chapter 15 notes. The polymers Class 12 notes comprise of some solved examples on these important topics. All mentioned material is downloadable in pdf format and are available in Class 12 Chemistry chapter 15 notes pdf download.
Also, students can refer,
NCERT Class 12 Chemistry Chapter 15 Notes
Polymers :
Also referred to as macromolecules, polymers are very large molecules of high molecular mass formed by the combination of numerous structural units on a large scale.
The monomer(s) undergoes a polymerization reaction to form polymers.
Polymers Class 12 Notes - Topic 1:
Classification of Polymers:
Polymers can be classified on the basis of:
Source
Structure
Mode of polymerization
Molecular forces
Growth polymerization
Classification based on source:
On the basis of source, polymers can be classified as below :
Type of polymer | Description | Example |
Natural | Found in plants & animals | Starch, proteins, cellulose, and rubber. |
Semi-synthetic | Modified natural polymers | cellulose nitrate & cellulose acetate (rayon) |
Synthetic | Man-made or artificial polymers | plastic (polythene), synthetic rubbers (Buna - S), synthetic fibres (nylon 6,6) |
Classification Based on Structure of Polymers
On the basis of structure, polymers can be classified as below :
Type of polymer | Description | Example |
Linear |
| polyvinyl chloride, high-density polythene |
Branched-chain |
| low-density polythene |
Cross-linked / network |
| Melamine, bakelite |
Classification Based on Mode of Polymerisation:
On the basis of Mode of Polymerisation, polymers can be classified as below :
Addition polymers
The repeated addition of monomer molecules having double or triple bonds leads to the formation of the addition polymers.
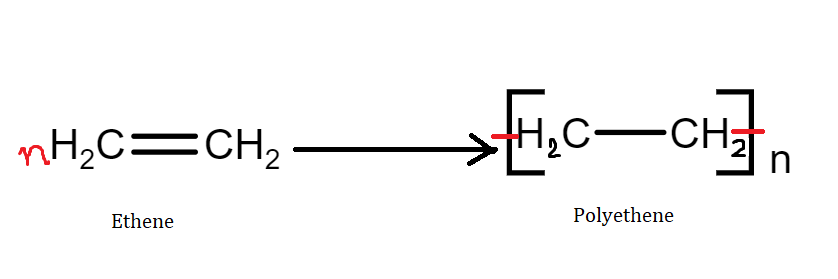
Example - The formation of polythene from ethene and the formation of polypropene from propene.
Homopolymers are said to be the addition polymers formed by the polymerization of a single monomeric species.
Example - polythene
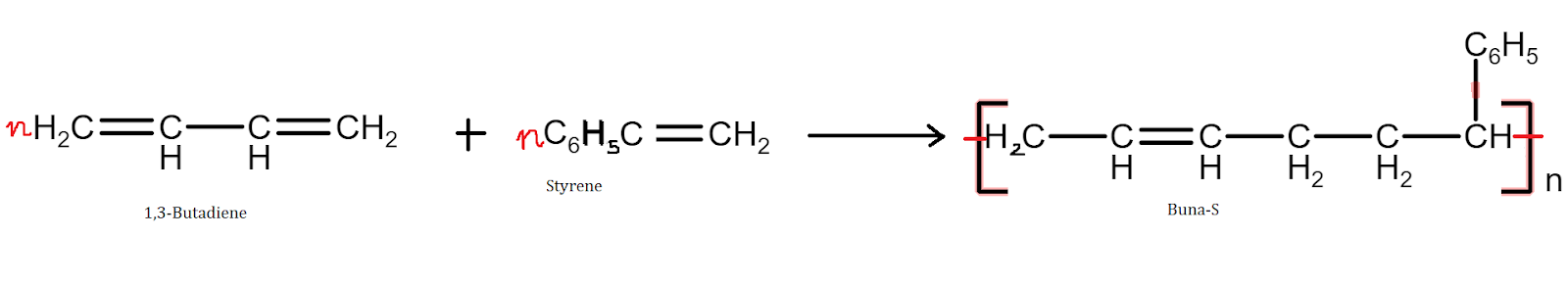
Copolymers are said to be the addition polymers formed by the polymerization of two different monomeric species.
Example - Buna-N, Buna-S, etc.
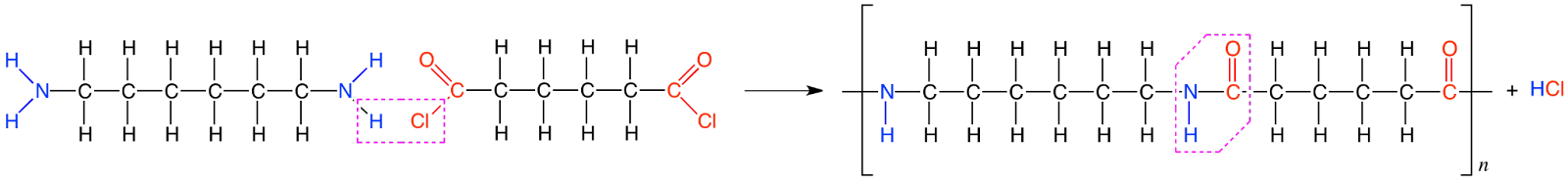
Condensation polymers
The repeated condensation of bi-functional or tri-functional monomer molecules leads to the formation of condensation polymers.
Elimination of small molecules like water takes place in condensation polymers.
Example - nylon 6, 6 is formed by the condensation of hexamethylene diamine with adipic acid.
Other examples include nylon 6, 6, terylene (dacron), nylon 6, etc.
Classification Based on Molecular Forces
On the basis of Molecular Forces, polymers can be classified as below :
Type of polymer | Description | Examples |
Elastomers |
| Neoprene, Buna-N, Buna-S |
Fibers |
| polyesters (terylene), polyamides (nylon 6, 6) |
Thermoplastic |
| polyvinyls, polythene, polystyrene |
Thermosetting |
| urea-formaldelyde resins, bakelite |
Classification Based on Growth Polymerisation:
The addition polymers are referred to as chain-growth polymers and the condensation polymers are referred to as step-growth polymers. This is based on the type of polymerization mechanism addition and condensation polymers undergo.
NCERT Class 12 Chemistry Chapter 15 Notes- Topic 2:
Types of Polymerization Reactions:
Addition Polymerisation or Chain Growth Polymerisation
The molecules of the same monomer or different monomers add together to form additional polymers.
Some characteristics of addition polymers are given below:
The monomers used are unsaturated compounds.
Increase in chain length or chain growth
Formation of free radicals or ionic species.
Free radical addition is the most common.
Free Radical Mechanism:
Free radical generating initiators (catalysts) are used in the polymerization of alkene/diene & derivatives.
Benzoyl peroxide, acetyl peroxide, tert-butyl peroxide, etc are used as free radical generating initiators (catalysts).
Chain initiation - Benzoyl peroxide disintegrates into phenyl free radical. This phenyl free radical adds to the ethene double bond resulting in a new and larger free radical.
Chain propagation - The process of addition of free radicals is repeated to form bigger free radical molecules.
Chain termination - The radical formes as the product reacts with another radical to form the polymerized product.
Preparation of some important addition polymer :
Polyethene
High-density polyethene
In hydrocarbon solvent, ethene undergoes addition polymerization in the presence of a catalyst such as triethylaluminium and titanium tetrachloride (Ziegler-Natta catalyst). The temperature is 333 K to 343 K and pressure of 6-7 atmosphere is maintained. High-density polythene (HDP) is produced. HDP consists of :
linear molecules
has a high density due to close packing
chemically inert
more tough and hard
used for manufacturing buckets, dustbins, bottles, pipes, etc.
Low-density polyethene
ethene undergoes free radical addition & H atom abstraction in the presence of a catalyst of dioxygen or a peroxide initiator. The temperature is 350 K to 570 K and pressure of 1000 to 2000 atmosphere is maintained. Low-density polythene (HDP) is produced. LDP is:
chemically inert
tough but flexible
poor conductor of electricity
used manufacture of squeeze bottles, toys, and flexible pipes and in the insulation of electricity-carrying wires.
Polytetrafluoroethene (Teflon)
Tetrafluoroethene at high pressure is heated with a free radical or persulphate catalyst to form Teflon. Teflon is :
chemically inert
resistant to attack by corrosive reagents
used in making oil seals and gaskets
used for non – stick surface coated utensils
Polyacrylonitrile
Acrylonitrile undergoes addition polymerization in presence of a peroxide catalyst to form polyacrylonitrile. It is used as a substitute for wool in making commercial fibers as Acrilan or orlon.
Condensation Polymerisation or Step Growth Polymerisation
The repeated condensation of bi-functional or tri-functional monomer molecules leads to the formation of condensation polymers. Simple molecules as water, alcohol, etc., are expelled and high molecular mass condensation polymers. Each step produces a distinct functionalized species. These species are independent of each other thus this process is also called step-growth polymerization.
Example - ethylene glycol and terephthalic acid to form terylene or dacron.
On the basis of their linking units, some condensation polymerization reactions are described below:
Polyamides
Polyamides are polymers possessing amide linkages and are termed nylon. Preparation of some important nylons are tabulated below:
Type | Preparation Reaction | Uses |
Nylon 6,6 | Obtained at high temperature and under high pressure by condensation polymerization of hexamethylenediamine with adipic acid | making bristles for brushes, sheets, and in the textile industry |
Nylon 6 | Obtained at a high temperature by heating caprolactam with water. | manufacture of tire cords, fabrics, and ropes. |
Polesters
Heating a mixture of ethylene glycol & terephthalic acid (at 420 to 460 Kelvin) produce a polycondensation products of dicarboxylic acids and diol called Polyesters. zinc acetateantimony trioxide is used as the catalyst.
Example - Dacron or terylene. Dacron is used as glass reinforcing materials in safety helmets, etc. and is crease-resistant, and is used in blending with cotton and wool fibers.
Phenol - formaldehyde polymer (Bakelite and related polymers)
Phenol - formaldehyde polymers obtained by the condensation reaction of phenol with formaldehyde in the presence of either an acid or a base catalyst.
These are the oldest synthetic polymers. The initial product formed in the condensation reaction could be a linear product called Novolac. Novalac is used in paints.
A cross-linking infusible solid polymer, Bakelite is formed when this Novolac is heated with formaldehyde. This solid polymer is used for making handles of utensils, electrical switches, combs, & phonograph records.
Melamine – formaldehyde polymer
Melamine and formaldehyde undergo condensation polymerization to form Melamine formaldehyde polymer which is used in the manufacture of unbreakable crockery.
Copolymerization
Copolymers are said to be the addition polymers formed by the polymerization of two different monomeric species.
The copolymer can be made by both chain-growth polymerizations and step-growth polymerization.
For example, the butadiene-styrene copolymer is quite tough and is a good substitute for natural rubber, and is used for the manufacture of auto tires, floor tiles, footwear components, cable insulation, etc.
Rubber
Natural rubbers
Some characteristics of rubber are given below:
natural polymer
possesses elastic properties
also termed elastomer
manufactured from rubber latex
also called cis - 1, 4 - polyisoprene.
has a coiled structure.
weak van der Waals forces held chain together
Vulcanization of rubber
Natural rubber turns soft at high temperatures and brittle at low temperatures.
Natural rubber shows a high water absorption capacity.
Natural rubber is soluble in non-polar solvents and is non-resistant to attack by oxidizing agents.
vulcanization is carried out to improve upon these physical properties.
This process is done by heating a mixture of raw rubber with sulfur. An appropriate additive is added at a temperature ranging from 373 Kelvin to 415 Kelvin. Rubber is stiffened when at the reactive sites of double bonds sulfur forms cross-links.
Synthetic rubbers
Any vulcanisable rubber-like polymer, which is competent of getting stretched to twice its length is Synthetic rubber. Preparation of some important synthetic rubbers is tabulated below:
Type | Description | Uses |
Neoprene |
| manufacturing conveyor belts, gaskets, and hoses |
Buna – N |
| making oil seals, tank lining |
polymers Class 12th Notes- Topic 3:
The Molecular Mass of Polymers
The molecular mass is always expressed as an average polymer sample because it contains chains of varying lengths. Physical and chemical methods are used to determine the molecular mass of polymers.
NCERT Class 12 Chemistry Chapter 15 Notes - Topic 4
Biodegradable Polymers
Aliphatic polyesters are biodegradable polymers:
Type | Description | Uses |
Poly β-hydroxybutyrate – co-β-hydroxy valerate (PHBV) |
| used in controlled release of drugs, specialty packaging, orthopedic devices |
Nylon 2–Nylon 6 | alternating polyamide copolymer of glycine and aminocaproic acid. |
Class 12 chemistry chapter 15 notes - Topic 5
Polymers of Commercial Importance:
Name of Polymer | Monomer | Uses |
Polypropene | Propene | Manufacture of pipes, ropes, fibers, toys, etc |
Polystyrene | Styrene | As an insulator, manufacture of toys, wrapping material, radio, television cabinets |
Polyvinyl chloride | Vinyl chloride | Manufacture of water pipes, raincoats, handbags, vinyl flooring |
Urea-formaldehyde resin |
| In the making of unbreakable cups, laminated sheets |
Glyptal |
| Manufacture of lacquers & paints |
Bakelite |
| For making electrical switches, combs, handle of utensils and computer discs. |
Significance of ncert class 12 chemistry chapter 15 notes
Polymers Class 12 notes will be helpful to revise the chapter and to get an idea about the main topics covered in the chapter. Also, this ncert class 12 chemistry chapter 15 notes are useful to cover the main topics of the class 12 CBSE chemistry syllabus and also for competitive exams like VITEEE, BITSAT, JEE Core, NEET, etc. Class 12 chemistry chapter 15 notes pdf download can be used to prepare in offline mode.
NCERT Class 12 Notes Chapter-Wise
Subject Wise NCERT Exemplar Solutions
- NCERT Exemplar Class 12 Solutions
- NCERT Exemplar Class 12 Maths
- NCERT Exemplar Class 12 Physics
- NCERT Exemplar Class 12 Chemistry
- NCERT Exemplar Class 12 Biology
Subject Wise NCERT Solutions
NCERT Books and Syllabus
Frequently Asked Questions (FAQs)
Ans-The Polymers can be classified on the basis of Source, Structure, Mode of polymerization, Molecular forces, Growth polymerization as covered in the ncert notes for Class 12 Chemistry chapter 15. This NCERT Class 12 Chemistry chapter 15 notes is a brief of the main subtopics covered in the chapter and can be used for revising the polymers.
Ans- The main differences as covered in the NCERT book are given in the tabular form.
| Type of polymer | Description | Example |
| Natural | Found in plants & animals | Starch, proteins, cellulose, and rubber. |
| Semi-synthetic | Modified natural polymers | cellulose nitrate & cellulose acetate (rayon) |
| Synthetic | Man-made or artificial polymers | plastic (polythene), synthetic rubbers (Buna - S), synthetic fibres (nylon 6,6) |
Ans- Students can expect none to 2 mark questions from the chapter polymers.
Ans- As stated in Class 12 polymers notes, on the basis of structure, polymers can be classified as below :
| Type of polymer | Description | Example |
| Linear | Long, straight-chain polymers | polyvinyl chloride, high-density polythene |
| Branched-chain | Linear chain polymers with a slight branching | low-density polythene |
| Cross-linked / network |
| Melamine, bakelite |
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
