CBSE Class 12th Chemistry Question Paper 2025, Exam Pattern, Marking Scheme
The CBSE Class 12 Board Exams 2025 are conducted by the Central Board of Secondary Education (CBSE) for the students of the Science, Commerce, and Arts streams. The examinations are very significant for students in their academic and career lives, as their marks are considered for admission to colleges as well as in competitive exams like JEE, NEET, and CUET. Students have to read NCERT books thoroughly, practice previous year's papers, and take mock tests to perform better in exams. Conceptual clarity and time management are the mantras to get a high mark.
Electronic gadgets or devices such as Mobile phones, smartwatches, calculators, and more are not allowed inside the CBSE 2026 exam hall.

CBSE Chemistry Question Paper 2025 Class 12 – Overview
CBSE Class 12 Chemistry 2025 exam is a high-scoring paper for Science Stream students, and this paper serves as a basis for higher studies pertaining to pharmacy, medicine, engineering, and research. The knowledge of students in terms of Physical, Organic, and Inorganic Chemistry is tested through this paper, and the syllabus includes Electrochemistry concepts, Chemical Kinetics, Coordination Compounds, Organic Reactions, and Polymers.
This examination is very important for those students who are preparing for competitive exams like JEE, NEET, CUET, and other entrance exams because Chemistry forms the bulk of these exams. A clear understanding of the subject enhances problem-solving and scientific thinking.
| Exam Details | Information |
|---|---|
Exam Date | February 27, 2025 |
Exam Mode | Pen and Paper (Offline) |
Marks Distribution | 70 Marks (Theory) + 30 Marks (Practical) |
To excel, students need to pay extra attention to NCERT textbooks, last year's question papers, sample papers, and numerical problems. Conceptual clarity and time management will be the key to success in the exam.
This article will help the CBSE Class 12 students prepare well for the Chemistry Exam 2025 by providing a detailed exam pattern, marking scheme, key topics, and preparation advice. It will help students develop an understanding of the question paper pattern, conceptual understanding, numerical, and key topics. Apart from this, it provides advice on time management and past years' exam trends to help in confidence-building and performance improvement
CBSE Chemistry Exam Pattern 2025
The CBSE Class 12 Chemistry Exam 2025 is designed to assess the clarity of concepts, application ability, and problem-solving ability of students. The exam pattern is as provided below:
| Exam Detail | Information |
|---|---|
Number of Sections | Three (Section A, Section B, Section C) |
Types of Questions | MCQs, Short-Answer, Long-Answer, Case-Based |
Marking Scheme | 70 Marks (Theory) + 30 Marks (Practical) |
Exam Duration | 3 Hours (180 Minutes) |
Key Takeaways of the Paper
- MCQs & Objective Questions examine fundamental concepts and definitions.
- Short-answer questions assess problem-solving and explanation ability.
- Long-answer & Case-Based Questions stress application, reasoning, and experimental analysis.
- Internal Choice is provided in some questions to provide response flexibility.
Class 12 CBSE Chemistry Question Paper 2025 PDF
Students can download the CBSE 12th chemistry question paper PDF here. The question paper PDF link is provided below. Click on the link given bwlow to download it.
| Question Paper | Download PDF |
|---|---|
CBSE Class 12 Chemistry question paper 2025 |
CBSE Class 12 Chemistry 56/2/1, Set 1 Paper and Answer Key
Check CBSE 12 chemistry 56/2/1, set 1 answer key, along with the question given below.
1. The charge required for the reduction of 1 mol of MnO4 to MnO2 is
(A) 1 F
(Q) 5 F
(B) 3 F
(D) 6 F
Answer: (B) 3 F
2. Which among the following is a false statement?
(A) Rate of zero order reaction is independent of initial concentration of reactant.
(B) Half-life of a zero order reaction is inversely proportional to the rate constant.
(C) Molecularity of a reaction may be zero.
(D) For a first order reaction, t1/2=0.693/k.
Answer: (C) Molecularity of a reaction may be zero. (False statement)
3. The number of molecules that react with each other in an elementary reaction is a measure of the :
(A) activation energy of the reaction (B) stoichiometry of the reaction
(Q) molecularity of the reaction
(D) order of the reaction
Answer: (C) Molecularity of the reaction
4. Thgelement having [Ar]3d 104 s1 electronic configuration is
(2) Cu
(B) Zn
(C) Cr
(D) Mn
Answer: (A) Cu
5. The complex ions [Co(NH3)5(NO2)]2+ and [Co(NH3)5(ONO)]2+ are called
(A) Ionization isomers
(B) Linkage isomers
(C) Co-ordination isomers
(D) Geometrical isomers
Answer: (B) Linkage isomers
6. The diamagnetic species is :
(A) [Ni(CN)4]2−
(B) [NiCl4]2−
(C) [Fe(CN)6]3−
(D) [CoF6]3−
Answer: (A) [Ni(CN)4]2−
7. Which is the correct IUPAC name for
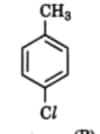
(A) Methylchlorobenzene
(B) Toluene
(C) 1-Chloro-4-Methylbenzene
(D) 1-Methyl-4-Chlorobenzene
Answer: (C) 1-Chloro-4-Methylbenzene
8. What will be formed after oxidation reaction of secondary alcohol with chromic anhydride (CrO3) ?
(A) Aldehyde
(B) Ketone
(C) Carboxylic acid
(D) Ester
Answer: (B) Ketone
9. The conversion of phenol to salicylic acid can be accomplished by
(A) Reimer-Tiemann reaction
(B) Friedel-Crafts reaction
(C) Kolbe reaction
(D) Coupling reaction
Answer: (C) Kolbe reaction
10. Which of the following is/are examples of denaturation of protein?
(A) Coagulation of egg white
(B) Curdling of milk
(C) Clotting of blood
(D) Both (A) and (B)
Answer: (D) Both (A) and (B)
11. Nucleotides are joined together by
(A) Glycosidic linkage
(B) Peptide linkage
(C) Hydrogen bonding
(D) Phosphodiester linkage
Answer: (D) Phosphodiester linkage
CBSE Class 12 Chemistry 56/2/2, Set 2 Paper and Answer Key
For questions number 1 to 4, two statements are given - one labelled as Assertion (A) and the other labelled as Reason (R). Select the correct answer to these questions from the codes (A), (B), (C) and (D) as given below :
(A) Both Assertion (A) and Reason (R) are true and Reason (R) is the correct explanation of the Assertion (A).
(B) Both Assertion (A) and Reason (R) are true, but Reason (R) is not the correct explanation of the Assertion (A).
(C) Assertion (A) is true, but Reason (R) is false.
(D) Assertion (A) is false, but Reason (R) is true.
1. Assertion (A) : Vitamin D cannot be stored in our body.
Reason (R) : Vitamin D is fat soluble vitamin and is not excreted from the body in urine.
2. Assertion (A) : Aromatic primary amines cannot be prepared by Gabriel Phthalimide synthesis.
Reason (R) : Aryl halides do not undergo nucleophilic substitution reaction with the anion formed by phthalimide.
(A)
3. Assertion (A) : Cu cannot liberate H2 on reaction with dilute mineral acids.
Reason (R) : Cu has positive electrode potential.
4. Assertion (A) : In a first order reaction, if the concentration of the reactant is doubled, its half-life is also doubled.
Reason (R) : The half-life of a reaction does not depend upon the initial concentration of the reactant in a first order reaction.
5. Scurvy is caused due to deficiency of
(A) Vitamin B1
(B) Vitamin B2
(C) Ascorbic acid
(D) Glutamic acid
6. Nucleotides are joined together by
(A) Glycosidic linkage
(B) Peptide linkage
(C) Hydrogen bonding
(D) Phosphodiester linkage
7. Which of the following is/are examples of denaturation of protein?
(A) Coagulation of egg white
(B) Curdling of milk
(C) Clotting of blood
(D) Both (A) and (B)
8. The conversion of phenol to salicylic acid can be accomplished by
(A) Reimer-Tiemann reaction
(B) Friedel-Crafts reaction
(C) Kolbe reaction
(D) Coupling reaction
9. What will be formed after oxidation reaction of secondary alcohol with chromic anhydride (CrO3) ?
(A) Aldehyde
(B) Ketone
(C) Carboxylic acid
(D) Ester
CBSE Class 12 Chemistry 56/2/3, Set 3 Paper and Answer Key
1. Which among the following is a false statement ?
(A) Rate of zero order reaction is independent of initial concentration of reactant.
(B) Half-life of a zero order reaction is inversely proportional to the rate constant.
(C) Molecularity of a reaction may be zero.
(D) For a first order reaction, t1/2=0.693/k.
2. The charge required for the reduction of 1 molof MnO4−to MnO2 is
(A) 1 F
(B) 3 F
(C) 5 F
(D) 6 F
3. The element having [Ar]3 d104 s1 electronic configuration is
(A) Cu
(B) Zn
(C) Cr
(D) Mn
4. The number of molecules that react with each other in an elementary reaction is a measure of the :
(A) activation energy of the reaction
(B) stoichiometry of the reaction
(C) molecularity of the reaction
(D) order of the reaction
5. The diamagnetic species is :
(A) [Ni(CN)4]2−
(B) [NiCl4]2−
(C) [Fe(CN)6]3−
(D) [CoFF6]3−
[At. No. Co=27,Fe=26,Ni=28 ]
6. The complex ions [Co(NH3)5(NO2)]2+ and [Co(NH3)5(ONO)]2+ are called
(A) Ionization isomers
(B) Linkage isomers
(C) Co-ordination isomers
(D) Geometrical isomers
Important Topics For CBSE Chemistry 2025
CBSE Class 12 Chemistry Exam 2025 has three key sections: Physical Chemistry, Inorganic Chemistry, and Organic Chemistry. The following are the most significant topics that the students have to practice for better scoring.
1. Physical Chemistry
- Solid State – Crystal structures, Packing efficiency, Defects in solids.
- Solutions – Raoult’s law, Colligative properties, Van’t Hoff factor.
- Electrochemistry – Nernst equation, Conductance, Electrochemical cells.
- Chemical Kinetics – Rate law, Arrhenius equation, Order & Molecularity.
- Surface Chemistry – Adsorption, Catalysis, Colloids, Emulsions.
2. Inorganic Chemistry
- Isolation of Elements – Metallurgy, Extraction of metals, Refining.
- p-Block Elements – Group 15-18 elements, Oxoacids, Allotropes.
- d- and f-Block Elements – Transition Elements, Properties, Lanthanoids & Actinoids.
- Coordination Compounds – Nomenclature, Isomerism, Bonding theories.
3. Organic Chemistry
- Haloalkanes and Haloarenes – SN1 & SN2 reactions, Preparation methods.
- Alcohols, Phenols, and Ethers – Properties, Preparation, Reactions.
- Aldehydes, Ketones, and Carboxylic Acids – Nucleophilic addition, Oxidation, Reduction.
- Amines – Basicity, Preparation, Chemical reactions.
- Biomolecules – Carbohydrates, Proteins, DNA, RNA.
- Polymers – Classification, Polymerization types, Biodegradable polymers.
- Chemistry in Everyday Life – Drugs, Cleansing agents, Food additives.
Key Areas of Concentration for 2025
- Physical Chemistry Numericals (Electrochemistry, Solution, Chemical Kinetics)
- Organic Chemistry Mechanisms (SN2, SN1, Electrophilic & Nucleophilic reactions)
- Periodic Trends & Coordination Chemistry (p-block, d-block, and f-block elements)
- NCERT-based conceptual understanding is required for theory & reactions.
- Application-based and case-based questions can be expected.
Tips to Prepare for the CBSE Chemistry 2025 Exam
Following are some efficient preparation techniques through which students can perform well in the CBSE Class 12 Chemistry Exam 2025:
1. Familiarise yourself with Exam Patterns & Syllabus
- Go through the CBSE syllabus and highlight important topics of Physical, Organic, and Inorganic Chemistry.
- Understand the marking scheme and pattern of the question paper.
2. NCERT textbooks to Concentrate on
- NCERT is the key – Read each chapter, theory, reactions, and numerical problems carefully.
- All the board questions are directly framed from NCERT concepts.
3. Develop Key Reactions & Mechanisms
- Remember major Organic Chemistry reactions, mechanisms, and conversions.
- Emphasize the name reaction, its catalyst, and conditions.
- Revise Periodic Trends, Coordination Compounds, and p/d-block elements.
4. Master Number Problems
Practice Numerical questions from:
- Electrochemistry – Conductivity, Nernst equation.
- Chemical Kinetics – Half-life equations, Rate law.
- Solutions – Molarity, Colligative properties.
5. Practice Sample Papers & Previous Year Papers
- Practice sample papers & previous year questions of CBSE to understand trends & difficulty levels.
- Focus on time management and develop answer-writing.
6. Practice Assertion-Reason Questions from a Case-Based Style
- Get ready for 2025 case-based, assertion-reasoning, and application-based questions.
- Strengthen conceptual understanding to respond rationally.
7. Revise Often & Note
- Make brief notes of formulas, reactions, and main points.
- Re-learn basic definitions, legislative statutes, and mathematical equations daily.
8. Time Management During Exam
- Spend time on every section and do not waste too much time on one question.
- Try the high-scoring & easier questions first to gain maximum marks.
9. Focus on Practical Chemistry
30 Practical marks – ensure Salt Analysis, Titration, and Organic Functional Group tests are accurately carried out.
Last Tip: Practice and consistency are key! Stick to a study schedule and stay calm for the best results.
CBSE Chemistry Marking Scheme 2025
Both theoretical and practical examinations are part of the CBSE 12th Chemistry marking scheme; theory exams are worth 70 marks, while practical exams are for 30. Students must understand the grading scheme, key subjects, and test structure in order to do well on the assessment.
Detailed Section-Wise Pattern
| Section | Question Type | Number of Questions | Marks per Question | Total Marks |
|---|---|---|---|---|
Section A | MCQs (Objective) | 16 | 1 | 16 |
Section B | Short Answer Type I | 5 | 2 | 10 |
Section C | Short Answer Type II | 7 | 3 | 21 |
Section D | Long Answer Type | 3 | 5 | 15 |
Section E | Case-Based Questions | 2 | 4 | 8 |
Total | Complete Paper | 33 Questions | - | 70 Marks |
A major shift from earlier years is a perceptible rise in the weightage of competency-based questions, such as MCQs, which now account for approximately 50% of the marks, whereas the weightage of short and long answer questions has been marginally decreased to approximately 30% - in line with the emphasis on application-based knowledge encouraged by the NEP 2020; the theory section continues to have 70 marks and the practical section continues to have 30 marks.
Important points regarding the changes:
- Greater emphasis on competency-based questions:
- There are more multiple-choice and application-based questions in the exam pattern.
- Less weightage of conventional long answer questions:
- The number of long descriptive questions has been reduced to give greater emphasis on a wider evaluation of concepts.
- Greater emphasis on practical application:
- The practical exam still retains a high 30% weightage, prompting students to implement theoretical knowledge in laboratory conditions.
Also Check:
Questions related to CBSE Class 12th
On Question asked by student community
Dear Student,
Please go through the link to check 12th CBSE Chemistry question paper: https://school.careers360.com/boards/cbse/cbse-previous-year-question-papers-class-12-chemistry
The Second Language English paper for the 2025-26 academic session (Summative Assessment 2 or SA-2 phase) focused on comprehension, grammar, and literature.
Exam Pattern & Marking Scheme (Class 8 English)
The second language English paper is divided into four main sections, totalling 80 Marks (for most state boards like Karnataka)
The Class 9th Social Science (SST) annual exam 2025-26 follows a standardised structure across CBSE and most State Boards. The exams for most of the boards are being held in February- March 2026. Check the marking scheme here for the SST exams
|
Section |
Type of Questions |
Number of Questions |
Marks |
Dear Student,
You can check Class 12 Physics CBSE Hindi medium PYQs here:
CBSE Class 12 Previous Year Question Papers With Solutions PDF Download
I am assuming the CBSE Board Physics Exam 2026 question paper. Download it here .
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
