NCERT Class 10 Science Chapter 5 Notes Periodic Classification of Elements- Download PDF Notes
Class 10 Science Chapter 5 notes are revision notes on the Class 10 NCERT chapter Periodic Classification of Elements: Careers360 provides CBSE Class 10 notes for other subjects as well. The Class 10 Periodic Classification of Elements revision notes are provided to revise all the important concepts given in Chapter 5. CBSE Notes for Class 10 Science Chapter 5 are short ideas on periodic classification of elements, with an explanation of each topic and formula. The derivations in the NCERT chapters are not included in the NCERT Notes Class 10 Science chapter 5. Students can utilise the Class 10 science chapter 5 CBSE notes for revision of major concepts while preparing for the CBSE Class 10 exam. The revision notes for periodic classification of elements can be downloaded, and the Class 10 NCERT notes PDF can be used offline. NCERT solutions and CBSE notes for Class 10 are helpful for CBSE board exam preparation.
This Story also Contains
- Periodic Classification of Elements Class 10 Notes
- NCERT Class 10 Chapter 5 Class notes Periodic Classification of Elements
- Early Attempts AT The Classification of Elements
- Mendeleev’s Periodic Table
- The Modern Periodic Table
Periodic Classification of Elements Class 10 Notes
Periodic classification of elements is a very important chapter of chemistry in Class 10 from an exam point of view. The NCERT Class 10 Science Chapter 5 notes give you a basic idea of the organisation and periodic classification of elements. The topics covered in NCERT Class 10 Science notes are: definitions, making order out of chaos—early attempts at the classification of elements, Döbereiner’s Triads, Newlands' law of octaves, Mendeleev's periodic table: achievements and limitations, The modern periodic table: position and trends of elements. Download the CBSE Notes for Class 10 Chemistry, Chapter 5, Periodic Classification of Elements, PDF to use offline anywhere. Students must go through each topic in the periodic classification of elements class 10 notes in the easiest and most effective way possible with the help of NCERT Notes for Class 10.
Also, scholars can refer,
NCERT Class 10 Chapter 5 Class notes Periodic Classification of Elements
Till now, 118 elements have been discovered in the world, and only 98 of them are naturally occurring in nature.
Early Attempts AT The Classification of Elements
Firstly, the scientists have classified the elements into metals and non-metals.
Döbereiner’s Triads:
- In 1817, Dobereiner arranged some elements into groups that had similar properties. He called these groups triads.
- When these elements were arranged in the increasing sequence of increasing atomic masses, the atomic mass of the middle element was found to be approximately equal to the average of the atomic mass of the other two elements.
Example:
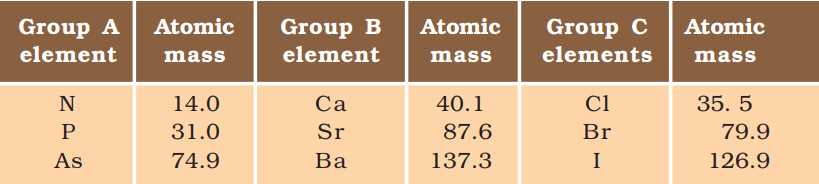
Fig. 1: Dobereiner’s Triads
It is clearly seen that the group B and C form dobereiner triads.
Drawbacks:
He could only find three triads from all the elements which were known at that time. So this system of classification was unsuccessful and useless.
Newlands’ Law of Octaves:
- In the year 1866, Newlands’ also made the arrangement of elements known at that time in the order of their increasing atomic masses.
- The element with the lowest atomic mass known at that time was hydrogen and the element with the highest atomic mass was thorium which was the 56th element.
- He started with hydrogen and ended at thorium and found that every eight elements had properties similar to that of the first element.
- This law is known as Newland's law of Octaves. The eight elements resemble the octaves found in the music.
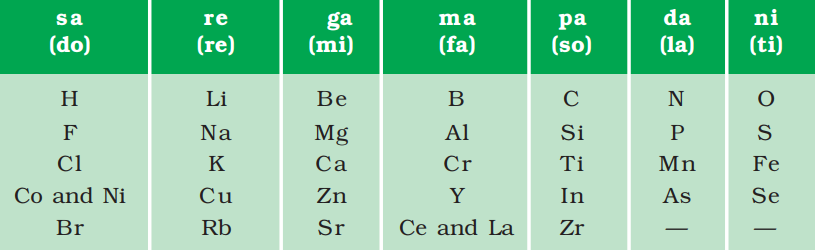
Fig. 2: Newlands’ octaves
In this example the properties of beryllium and magnesium resemble each other and the properties of sodium and lithium resemble each other.
Drawbacks:
1. This law holds true only for the elements up to calcium.
2. When the law was proposed there were only 56 elements but later on several new elements were discovered which did not obey the law of Octaves.
3. Newland wanted to fit all his elements in his periodic table. So, he adjusted to elements in the same slot and put some different elements under the same.
Mendeleev’s Periodic Table
- Mendeleev regarded atomic mass as the fundamental property of the elements. So Mendeleev arranged the elements of the periodic table on the basis of the atomic mass of the element and their chemical properties.
- When Mendeleev came into action, there were 63 elements that were known to the world.
- He studied the relationship between the atomic mass of an element and its physical and chemical properties.
- He chose hydrogen and oxygen because they are highly reactive elements.
- He took 63 cards and wrote down the properties of each element on each card.
- He selected the elements that had similar properties and observed that most of the elements in his periodic table were arranged in the order of their increasing atomic masses.
- He also observed the recurrence of elements with similar physical and chemical properties.
- On the basis of his results, he formulated the periodic law, which states that "the properties of elements are the periodic function of their atomic masses."
The periodic table formulated by Mendeleev contained vertical columns called groups and horizontal rows called periods.
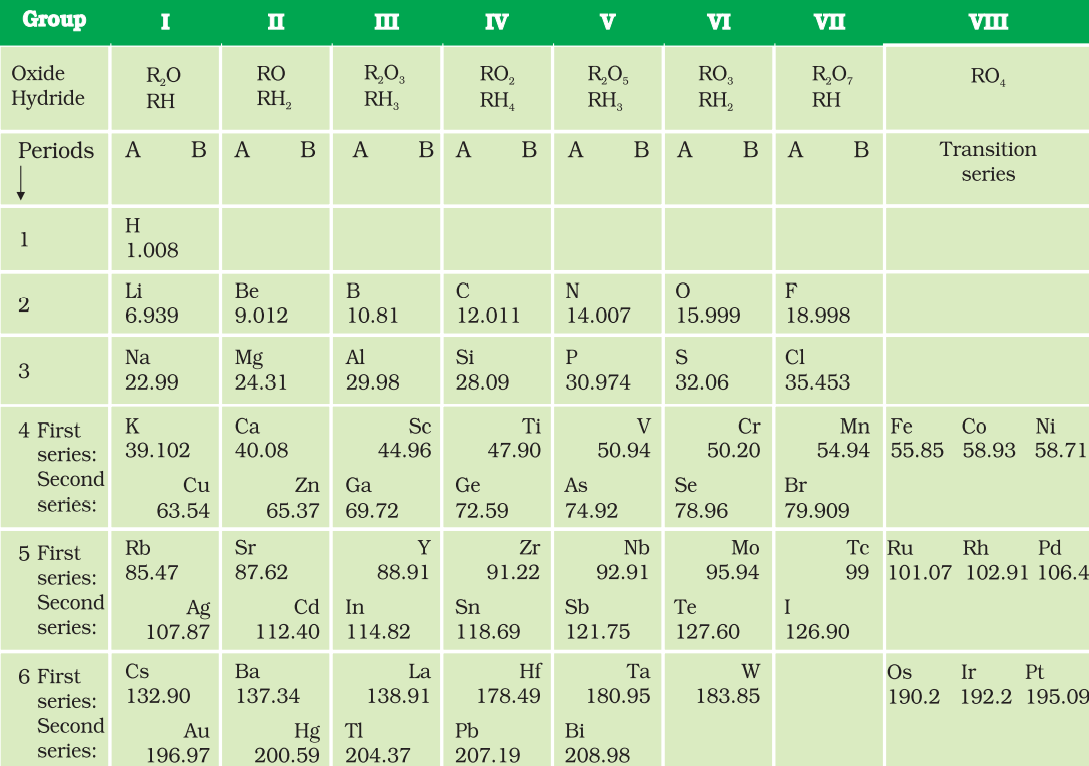
Fig. 3: Mendeleev’s Periodic Table
Achievements of Mendeléev’s Periodic Table:
Sometimes, Mendeleev had to place an element with greater atomic mass before an element with a lower atomic mass To group the elements with similar properties.
Mendeleev left some spaces in his periodic table and predicted the presence of some elements, which would be discovered later.
When the noble gases were discovered, these elements could be placed in a new group without disturbing the existing order of the periodic table.
Limitations of Mendeléev’s Periodic Table:
The element hydrogen was not given a fixed place in the periodic table developed by Mendeleev.
Isotopes were not given any place in his periodic table because they were discovered much later than the discovery of Mendeleev periodic table.
The atomic masses were not arranged in a regular manner, going from one element to the next, so it was impossible to know how many elements could be found between two elements.
The Modern Periodic Table
- In 1913, Mosley stated that instead of atomic mass, atomic number is the fundamental property of an element. He modified the Mendeleev periodic table and discovered the Modern Periodic table on the basis of atomic number.
- The Modern Periodic Law states that ‘Properties of elements are a periodic function of their atomic number.’
- All three limitations of the Mendeleev Periodic table were overcome in the Modern Periodic table.
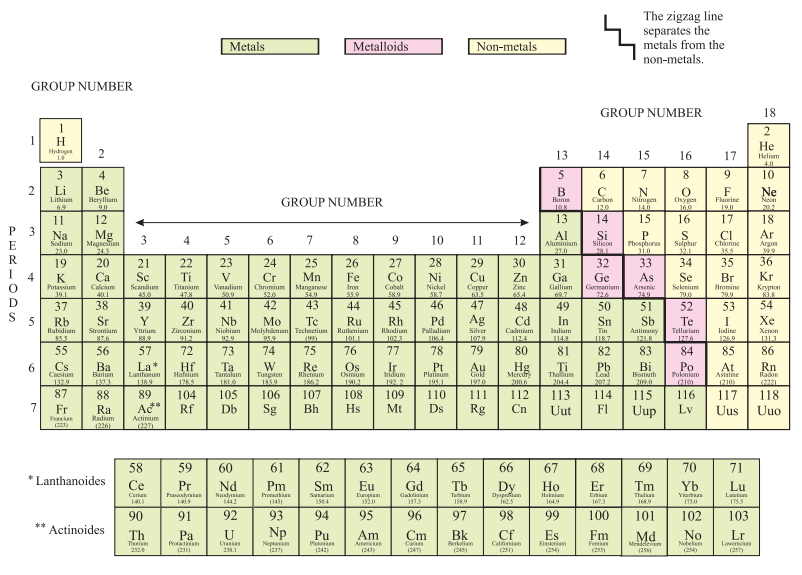
Fig. 4: The Modern Periodic Table
Position of Elements in the Modern Periodic Table:
- There are 18 vertical columns called groups and 7 horizontal rows called periods in the Modern Periodic Table.
- All the elements of a group in the Modern Periodic Table contain the same number of valence electrons. E.g., fluorine (F) and chlorine (C) belong to Group 17 and contain 7 electrons in the valence shells.
- It is concluded that the groups signify valence shell electronic configuration.
- The number of shells increases when we go down the group. The atoms of different elements with similar numbers of shells are placed in the same period.
- The number of outermost electrons increases by 1 as the atomic number increases by 1 on moving from left to right in a period.
Calculation of elements in a period:
- It is based on the number of electrons filled into various shells.
- The maximum number of electrons which can be adjusted in a shell depends on 2n2 where,
n= no of shell from nucleus
For example,
K Shell – 2 × (12) = 2, hence the first period has 2 elements.
L Shell – 2 × (22) = 8, hence the second period has 8 elements.
M Shell – 2 × (32) = 18, but the outermost shell can have only 8 electrons, so the third period also has only 8 elements.
Trends in the Modern Periodic Table:
Valency: It is the number of outermost electrons in the outermost shell of an atom.
Atomic Size: Also known as the radius of an atom, it is the distance between the centre of the nucleus and the valence shell of an atom.
- The atomic radii decrease when we move left to right along a period because, when nuclear charge increases, it pulls the electrons nearer to the nucleus, and hence, the size of the atom reduces.
- The atomic size of the elements increases down the group in the periodic table because the new shells are added when we go down the group; thereby, the distance between the valence electrons and the nucleus reduces.
Metallic and non-metallic properties:
- The metals are found on the left-hand side portion of the periodic table, while the non-metals are found on the right-hand side portion of the periodic table. The middle portion of the periodic table contains metalloids and semimetals. Eg-Boron.
- Metals are electropositive in nature, and they tend to lose electrons. When the effective nuclear charge increases over a period, the tendency to lose electrons will decrease.
- When we go down a group, the effective nuclear charge decreases, and hence the outermost electrons can be removed easily. Hence, it is clear that the metallic character decreases across a period and increases down a group.
- The non-metals are electronegative in nature, and they gain electrons and form bonds. As the effective nuclear charge increases across a period, the tendency to gain electrons increases, and the outermost electrons come closer to the nucleus. So, the non-metallic character increases across a period and decreases down a group.
Class 10 Science: Chapter Wise CBSE Notes
Class 10 NCERT Solutions Subject Wise
NCERT Class 10 Exemplar Solutions:
CBSE Class 10 Books and Syllabus
Frequently Asked Questions (FAQs)
Ans: decreases, increases
Ans: Silicon
Ans: +2
Ans: 118
Ans: The Modern Periodic Law given by Moseley states that the ‘Properties of elements are a periodic function of their atomic number.’
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
