Question 3: Identify the option that represents a typical instance of 'feedback inhibition':
a. The reaction between Cyanide and cytochrome.
b. The interaction between sulpha drugs and bacteria involved in Folic acid synthesis.
c. Allosteric inhibition of Hexokinase by Glucose 6-phosphate.
d. The reaction involves Succinic dehydrogenase and Succinic acid.
Answer: The correct answer is option (c), Allosteric inhibition of Hexokinase by Glucose 6-phosphate.
Explanation: Feedback inhibition occurs when the end product of a metabolic pathway inhibits the enzyme involved in its own synthesis. In this case, Glucose 6-phosphate inhibits Hexokinase, the enzyme that catalyzes the first step of glycolysis. This regulation prevents the unnecessary breakdown of glucose when sufficient product is already formed.
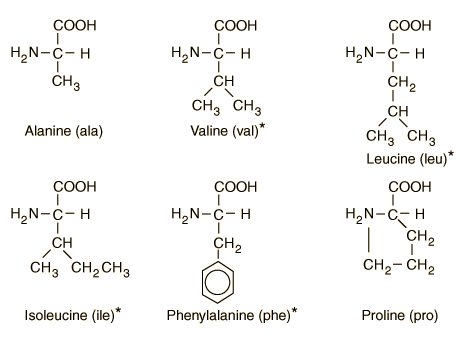
Question 4: The primary structure of a protein molecule has
a. Two ends
b. One end
c. Three ends
d. No ends
Answer: The correct answer is option (a), Two ends
Explanation: The primary structure of a protein molecule consists of a specific sequence of amino acids linked by peptide bonds. This sequence is determined by the gene encoding the protein and dictates its overall structure and function. Any change or mutation in the sequence can alter the protein's properties, potentially affecting its biological activity. The primary structure serves as the foundation for the protein's higher-order structures, including secondary, tertiary, and quaternary forms.
Question 5: Glycogen is a homopolymer made of
a. Glucose units
b. Galactose units
c. Ribose units
d. Amino acids
Answer: The correct answer is option (a), Glucose units
Explanation: Glycogen is indeed a homopolymer made of glucose units. The term "glycogen" comes from the Greek words "glyco" (meaning sweet, referring to glucose) and "gen" (meaning to produce), so it essentially means "a substance that produces glucose."
Question 6: Which of the following is an example of a nucleotide?
a. Adenosine
b. Adenine
c. Adenosine monophosphate (AMP)
d. Ribose
Answer: The correct answer is option (c), Adenosine monophosphate (AMP)
Explanation: A nucleotide is composed of a nitrogenous base, a pentose sugar, and a phosphate group. AMP contains adenine (base), ribose (sugar), and one phosphate group, fitting the definition of a complete nucleotide. Adenine alone is just a base, ribose is a sugar, and adenosine lacks the phosphate group.
Question 7: The biochemical process of converting glucose to pyruvate is called
a. Glycogenesis
b. Glycolysis
c. Gluconeogenesis
d. Glycogenolysis
Answer: The correct answer is option (b), Glycolysis
Explanation: Glycolysis is the metabolic pathway that breaks down glucose into two molecules of pyruvate, releasing energy in the form of ATP. This process occurs in the cytoplasm and is common to both aerobic and anaerobic organisms. Glycogenesis is glycogen formation, gluconeogenesis is glucose formation from non-carbohydrate sources, and glycogenolysis is glycogen breakdown.
Question 8: Which level of protein structure is stabilized by hydrogen bonds?
a. Primary structure
b. Secondary structure
c. Tertiary structure
d. Quaternary structure
Answer: The correct answer is option (b), Secondary structure
Explanation:
The secondary structure of proteins, such as α-helix and β-pleated sheet, is stabilized mainly by hydrogen bonds formed between the carbonyl oxygen and the amide hydrogen of the peptide backbone.
Question 9: Which of the following is a reducing sugar?
a. Sucrose
b. Starch
c. Lactose
d. Cellulose
Answer: The correct answer is option (c), Lactose
Explanation:
Reducing sugars have a free aldehyde or ketone group capable of reducing reagents like Benedict’s or Fehling’s solution. Lactose is a reducing disaccharide, while sucrose is non-reducing, and starch and cellulose are polysaccharides.