These notes summarise the key points of the chapter, including the definition of matter, its states, properties of particles, and changes of state such as melting, freezing, evaporation, condensation, and sublimation. They help in quick revision and understanding of important concepts for exams.
NCERT Class 9 Science Chapter 1 Notes - Matter In Our Surroundings (PDF Download)
Have you ever wondered how water turns into steam? Why does ice melt into water? Why does a balloon expand when heated? The answer to all these questions lies in Matter in Our Surroundings. Everything around us is made up of matter. This includes the air we breathe, the water we drink, and all of the products we use daily. Matter is anything that has weight and occupies space. This chapter will teach us about matter, its numerous forms, and how it evolves in different contexts. Understanding matter is very important because it is the basis of everything we see and use in the world.
This Story also Contains
- NCERT Notes Class 9 Chapter 1: Download PDF
- NCERT Notes Class 9 Chapter 1
- Matter in Our Surroundings: Previous Years' Questions and Answers
- How to Master Class 9 Science Chapter 1 Matter In Our Surroundings
- Advantages of Using Class 9 Science Chapter 1 Matter in Our Surroundings Notes
- NCERT Notes Class 9 Chapter-Wise
- NCERT Solutions for Class 9 Science Chapter-wise
- NCERT Exemplar Solutions Subject-Wise

NCERT Notes for Class 9 are provided to revise all the important concepts given in this chapter. These notes are designed by our experienced subject matter experts, which ensures the credibility of the content provided and also includes an explanation of each topic and formula. It becomes difficult and time-consuming for students to read the NCERT textbooks point-to-point. So, to solve this problem, we are providing these NCERT notes that cover all the topics and concepts provided in the NCERT textbook in a very clear and comprehensive way. Also, check the NCERT Solutions for all the chapters.
NCERT Notes Class 9 Chapter 1: Download PDF
You can download Class 9 Science Chapter 1 Matter in Our Surroundings Notes PDF to access a clear explanation of all the concepts from the Download PDF icon given below. These NCERT Notes for Class 9 Science help students build strong conceptual understanding and prepare effectively for exams.
Also Read
NCERT Notes Class 9 Chapter 1
Matter in Our Surroundings Class 9 Science notes provide a comprehensive explanation of the topic as per the CBSE syllabus, making it easier for students to understand the concepts of Chemistry better. The matter we see around us generally comes in three forms: solid, liquid, and gas. Take water as an example, it is ice when it is cold, water when it is warm, and steam when it is hot. Matter can switch between these forms, which is important in everyday life. Think about melting ice caps because of climate change or how fridges and air conditioners work. In this chapter, students will learn about the different properties of matter, how it acts in different situations, and why all of this matters in real life. Read on to learn more.
Matter in Our Surroundings
Definition of Matter: Anything that occupies space and has mass is referred to as matter.
- Early Greek philosophers classified matter in the form of 5 elements: "panch tattva" - air, earth, fire, sky and water.
- Modern scientists classify matter in two ways: physically and chemically.
- Everything around us, be it living forms like plants or animals or non-living forms like tables, chairs, pencils, etc., is made of some material that is referred to by scientists as matter.
Let's discuss its classification on the basis of physical properties.
Physical Nature of Matter
The physical nature of matter explains that everything around us is made up of tiny particles. Students can learn these concepts better with the help of NCERT Solutions for Class 9 Science Chapter 1 Matter in Our Surroundings.
Matter is Made Up of Particles
- When the question came about the nature of matter, there were two thoughts.
- The first said that matter is continuous, and the second said that matter is made up of small particles.
- At last, the second thought was proved right with the help of various experiments, for example.
How small are these particles?
- If we add potassium permanganate to a solution, then a very small amount can change the colour of the whole solution, proving that it is made up of very small particles.
- Also, if we add Dettol to water, it will give the same smell after repeated dilution. This small experiment also supports the very small size of these particles.
Characteristics of Particles of Matter
Understanding the characteristics of particles of matter is essential, as it explains how matter behaves in different states. These characteristics form the foundation of Class 9 Science Chapter 1 Notes.
Particles of matter have spaces between them
- It can be seen that one kind of matter can get dissolved in another kind of water, e.g., salt in water, lemonade, etc, which proves that in reality, particles of one matter get into the spaces of the other.
Particles of matter are continuously moving
- Particles of matter are proven by experiments to be continuously moving, and thus they possess kinetic energy.
- As the temperature rises, their kinetic energy also rises, and these particles start moving faster.
- The process where a particle of one kind of matter goes into spaces between another kind of matter is known as diffusion.
- It is observed that when heated, this process becomes faster.
Particles of matter attract each other
It has been observed that a force of attraction is present between particles of matter, and the strength of this force of attraction depends upon the kind of matter.
States of Matter
Matter exists in three different states, viz Solid, liquid and gas.
These different states of matter exist due to variations in their characteristics and properties discussed above.
The Solid State
The following are the characteristic features of solids :
-
They possess a definite size (Volume) and a definite shape
-
The shape of a solid can be changed, but it usually requires considerable force
-
They are generally hard and rigid
-
They have negligible compressibility
-
E.g. Iron, silver, common salt, etc.
The Liquid State
The following are the characteristic features of Liquid :
-
They possess a definite volume but no definite shape
-
It takes up the shape of the container in which it is placed
-
They also have a tendency to flow.
-
They are fluid and have a very high diffusion capacity
-
Particles move freely and have greater spaces between them
-
For example, Water, alcohol, milk, oil, etc.
The Gaseous State
The following are the characteristic features of Gases :
-
They neither possess a definite volume nor a definite shape.
-
They are having very high compressibility
-
Particles of gases have the highest diffusion rate.
-
Particles move randomly and very fast.
-
For Eg, air, Carbon dioxide, oxygen, hydrogen, etc.
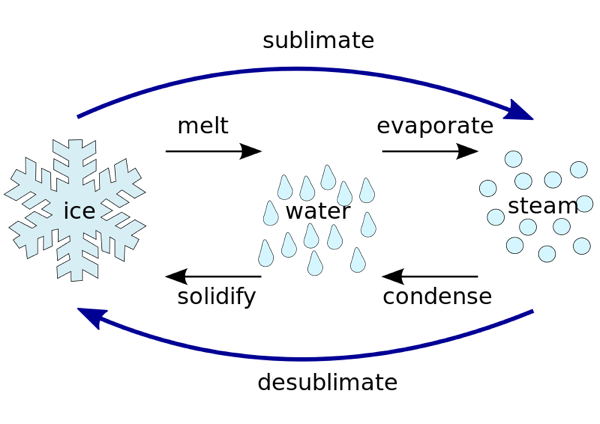
Can Matter Change Its State?
We can take the example of water, as we know that it exists in all 3 states, i.e.
-
Solid as ice
-
Liquid, as it's a familiar water state
-
Gas as a vapour
Now we need to look into the reasons that are responsible for bringing out these changes in the state of matter.
Effect of Change of Temperature
- When the temperature is increased, particles of the solid are seen with more kinetic energy.
- The particles start vibrating more, and the heat energy that is provided helps overcome the strong forces of attraction that are present between the particles.
- In this process, a particular stage is reached when the solid is melted into a liquid.
- The temperature at which a solid is converted into a liquid at atmospheric pressure is known as the melting point.
- The process of melting, which is the conversion of a solid state into a liquid state, is also known as fusion.
- We can see that after the ice is completely converted into a liquid, which is done by using the heat energy to overcome the force of attraction that is present between particles, there is no increase in the temperature of the ice.
- It is believed that the heat gets hidden in the beaker and is known as latent heat.
- The amount of heat energy that is required to convert one kilogram of solid into one kilogram of liquid at its melting point at atmospheric pressure is known as the latent heat of fusion.
- In a similar way, when we provide heat to liquid water, its molecules get heated up and can overcome the force of attraction.
- Liquid starts changing into vapour.
- The temperature at which the liquid is converted into its vapour form at one atmospheric pressure is known as its boiling point.
- It can be seen that with increasing temperatures, solids can be converted into liquids, which can further be converted into gases.
- There's also a process by which we can directly convert solids into gases without converting them into liquids, and the process is sublimation.
Effect of Change of Pressure
- The state of matter can also be changed by changing its pressure, as we know that the characteristic feature of matter is the space present between the particles.
- By increasing the pressure, space can be removed, and by compressing the gas, we can change its state.
- On decreasing pressure and increasing temperature, we can liquefy a gas
- A very common example of this process is carbon dioxide.
- Solid carbon dioxide can be directly converted into its gaseous form by decreasing its pressure to one atm, and as it's converted from its solid state to a gaseous state without coming into its liquid state, that’s why it's also known as dry ice.
Evaporation
- A change in the state of matter can also take place without a change in temperature or pressure.
- The particles of matter are always in motion and have kinetic energy.
- In the case of liquids, the kinetic energy of the molecules at the surface is higher than that of those present inside.
- Surface molecules can break the force of attraction and are converted into their vapour form.
- This conversion of liquid into its gaseous state at a temperature below its boiling point is known as evaporation.
Factors Affecting Evaporation
The following are the factors that affect evaporation (e.g., drying of wet clothes)
-
Surface Area: As we know, evaporation is a surface phenomenon, and with an increase in temperature, the rate of evaporation also increases
-
Temperature: With the increase in temperature, the particles can get more heat energy and thereby more kinetic energy, and they are able to escape into the atmosphere more easily.
-
Decrease in Humidity: It helps in increasing evaporation, as when humidity is higher than the number of water particles in the surrounding area, fewer water particles will be able to get into the area.
-
Increase in Wind Speed: This helps in increasing the evaporation as the water vapours can move away from the cloth with the help of the wind. Also, the surrounding air has a smaller number of water particles, so that more water vapour can evaporate there
How Does Evaporation Cause Cooling?
- When the process of evaporation goes on, then the particles of that liquid absorb the energy from their surroundings to regain the energy that they lost during evaporation, which makes the surroundings cooler.
- Thus, it can be concluded that the process of evaporation results in cooling.
- We must prefer cotton clothes during the summer because, in summer, when we sweat, that sweat evaporates.
- The energy equal to the latent heat of vaporisation is absorbed from our body, resulting in cooling our body, as cotton helps in the absorption of water and its evaporation in a much easier way.
- That’s why we must prefer cotton clothes in the summer.
- The presence of water droplets is seen around a cold water glass, and that happens because water vapours that are present in the air lose energy upon coming in contact with a cold surface and are then converted into their liquid state.
Matter in Our Surroundings: Previous Years' Questions and Answers
Students can refer to previous year questions of this chapter, which helps students understand important questions and frequently asked concepts, making revision more effective. Also refer to Matter in Our Surroundings Class 9 Science Chapter 1 CBSE notes for understanding the concepts used to solve questions.
Question 1. In which of the following conditions does the distance between the molecules of hydrogen gas increase?
(i) Increasing pressure on the hydrogen contained in a closed container
(ii) Some hydrogen gas is leaking out of the container
(iii) Increasing the volume of the container of hydrogen gas
(iv) Adding more hydrogen gas to the container without increasing the volume of the container
(1) (i) and (iii)
(2) (i) and (iv)
(3) (ii) and (iii)
(4) (ii) and (iv)
Answer:
Molecules of gas occupy the complete volume of the container. These molecules move randomly inside the container; therefore, the average molecular separation can be calculated by assuming the given number of molecules in a given volume.
It is easy to understand that the average molecular separation will increase by either increasing the volume, keeping the number of molecules constant or by decreasing the number of molecules, keeping volume constant.
Increasing pressure will lead to an increase in the temperature of a gas in a closed container. This will increase the kinetic energy of molecules. Therefore, it will not help in increasing the distance between molecules.
Hence, statement numbers (i) and (iii) are correct
Hence, the correct answer is option (3).
Question 2. Which condition out of the following will increase the evaporation of water?
(1) Increase in the temperature of water
(2) Decrease in the temperature of water
(3) Less exposed surface area of water
(4) Adding common salt to water
Answer:
In the case of evaporation, some liquid molecules take energy from the rest of the liquid molecules and get converted into vapour.
If we increase the temperature of any liquid, it would be easy for some molecules to take the energy from the rest of the molecules. At higher temperatures, all of them have a higher amount of energy, which will help the process of evaporation.
The process of evaporation will be supported by exposure of the surface to the surroundings; hence, a higher surface area leads to higher evaporation.
When we add common salt to water, some part of this salt covers the surface area along with water molecules, which will create obstacles in evaporation.
Hence, the correct answer is option (1).
Question 3. Which process directly converts a solid to a gas?
(1) Melting
(2) Condensation
(3) Deposition
(4) Sublimation
Answer: Sublimation is when a solid changes directly into a vapour without becoming a liquid.
Hence, the correct answer is option (4).
Question 4: Select the correct statement:
(1) Everything around us is made of tiny pieces or particles
(2) Our body is made of particles
(3) The number of particles in everything is, however, very, very large
(4) all the above
Answer:
According to the definition, matter is composed of a large number of tiny particles. Thus everything around us including our own body is made up of matter and hence tiny particles.
Hence, the answer is the option (4).
Question 5: According to ancient philosphers matter consists of
(1) three constituents
(2) four constituents
(3) five constituents
(4) six constituents
Answer:
Matter can be classified in a number of ways. Ancient Indian philosophers said that all the matter (padarth), living or non-living was made up of five basic elements, air, earth, sky, fire, and water.
Hence, the answer is the option (3).
How to Master Class 9 Science Chapter 1 Matter In Our Surroundings
This chapter introduces the concept of matter, its various states, and the physical changes it undergoes. Students can refer to Class 9 Science Chapter 1 Matter in Our Surroundings Notes for understanding many topics in science.
- First learn what matter is and its states: solid, liquid, gas.
- Then understand the properties of matter like shape, volume, compressibility, and density and how matter changes state with temperature
- After that, focus on concepts like the Kinetic theory of matter, effects of temperature and pressure on states of matter. Students can refer to the NCERT notes Class 9 Science Chapter 1 Matter in Our Surroundings for understanding these concepts better.
- Lastly, solve NCERT exercises and previous year questions.
Advantages of Using Class 9 Science Chapter 1 Matter in Our Surroundings Notes
NCERT notes Class 9 Science Chapter 1 Matter in Our Surroundings help students to understand the basic concepts of matter and its states effectively. Given below some points on the advantages of these solutions:
- Students can understand the topics like states of matter, intermolecular forces, changes of state, characteristics of solids, liquids, and gases
- These notes provide systematic explanations that help students understand the behaviour of matter under different physical conditions.
- The Matter in Our Surroundings Class 9 Science notes are prepared by subject experts in a clear and concise manner. These notes cover all the NCERT topics
- They are well organised and are very helpful for CBSE boards and competitive exams.
NCERT Notes Class 9 Chapter-Wise
Along with the Matter in Our Surroundings Class 9 Science Chapter 1 CBSE notes, students can refer to notes of other Class 9 chapters from the links provided below.
NCERT Solutions for Class 9 Science Chapter-wise
Besides Class 9 Science Chapter 1 Matter in Our Surroundings Notes , students can also follow Class 9 chapter-wise solutions of NCERT:
NCERT Exemplar Solutions Subject-Wise
Subject-wise NCERT exemplar solutions for class 9 are given below:
Frequently Asked Questions (FAQs)
The three states of matter discussed in this chapter are solids, liquids, and gases. Each state has distinct characteristics: solids have a fixed shape and volume, liquids have a definite volume but take the shape of their container, and gases have neither fixed shape nor volume, expanding to fill their container.
Physical changes are changes that affect the form of a substance but not its chemical composition. For instance, melting ice changes from solid to liquid but is still water. Chemical changes, on the other hand, result in the formation of new substances with different properties, such as rust forming on iron.
Temperature and pressure significantly influence gas behavior. According to the gas laws, increasing the temperature of a gas at constant pressure leads to an increase in volume. Conversely, if the temperature is kept constant and pressure increases, the volume decreases .
The state of matter is primarily determined by temperature and pressure. Increasing temperature usually causes matter to change from a solid to a liquid and from a liquid to a gas. Conversely, decreasing temperature can lead to condensation and freezing, changing matter from gas to liquid, and from liquid to solid.
Intermolecular forces are the attractive forces between molecules. These forces vary in strength depending on the state of matter. In solids, these forces are strong, keeping the particles closely packed. In liquids, the forces are weaker, allowing for flow. In gases, the intermolecular forces are negligible, enabling particles to move freely.
Matter is anything that has mass and occupies space.
No, energy is not matter. Energy is the ability to do work. While energy can affect matter (e.g., heat can change the state of matter), it doesn't have mass and doesn't occupy space in the same way as matter. Examples of energy include heat, light, sound, and electricity.
The three common states of matter are solid, liquid, and gas. There's also a fourth state called plasma, but it's less commonly encountered in everyday life.
- Solids: Particles are tightly packed in a fixed arrangement, giving them a definite shape and volume. They vibrate in place but don't move around freely.
- Liquids: Particles are close together but can move around and slide past each other. They have a definite volume but take the shape of their container.
- Gases: Particles are far apart and move randomly and rapidly. They have no definite shape or volume and can be easily compressed.
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
