Make revision notes by writing key definitions, formulas, diagrams, and important concepts from each exemplar chapter in concise bullet points, and include shortcuts or examples for quick last-minute review.
NCERT Exemplar Class 9 Science Solutions Chapter 2 Is Matter Around Us Pure
Do you know everything around us is made up of matter, whether it is the air we breathe, the water we drink or the objects we use daily. Matter exists in different forms and compositions; some are in pure form and others as mixtures. The knowledge of the purity of a substance is essential in science and everyday life, as it aids in separating useful components from unwanted impurities. This chapter will detail the concept of mixtures and their types, which will help students distinguish between homogeneous and heterogeneous mixtures. NCERT Exemplar Class 9 Science Solutions will help you learn more about the topic.
This Story also Contains
- NCERT Exemplar Class 9 Science Solutions Chapter 2 (MCQ)
- NCERT Exemplar Class 9 Science Solutions Chapter 2 (Short Answer)
- NCERT Exemplar Class 9 Science Solutions Chapter 2 (Long Answer)
- Important Questions of Class 9 Science Chapter 2
- Approach To Solve Class 9 Science Chapter 2 Questions
- Topics Of NCERT Exemplar Class 9 Science Chapter 2
- Advantages of Using Is Matter Around Us Pure NCERT Exemplar Class 9 Science Chapter 2 Solutions
- NCERT Class 9 Science Exemplar Solutions Chapter-wise
- NCERT Solutions for Class 9 chapter-wise
- NCERT Notes & Solutions subject-wise
- NCERT Books and NCERT Syllabus

This chapter provides students with a straightforward understanding of the properties and composition of matter. These NCERT exemplar solutions are designed according to CBSE syllabus and are carefully prepared by expert teachers. Their concise explanations and detailed responses to crucial queries have made the learning easier. A variety of questions containing multiple-choice, short-answer, and long-answer types are provided here. Students can also check NCERT Solutions to all questions chapter-wise.
NCERT Exemplar Class 9 Science Solutions Chapter 2 (MCQ)
The MCQ questions are covered in this section of NCERT Exemplar Solutions Class 9 Science Chapter 2 Is Matter Around Us Pure to enhance your knowledge. These questions help students test their conceptual understanding and prepare effectively for exams.
Question 1. Which of the following statements are true for pure substances?
(i) Pure substances contain only one kind of particles.
(ii) Pure substances may be compounds or mixtures.
(iii) Pure substances have the same composition throughout.
(iv) Pure substances can be exemplified by all elements other than nickel.
(a) (i) and (ii)
(b) (i) and (iii)
(c) (iii) and (iv)
(d) (ii) and (iii)
Answer: B
Solution: A pure substance consists of a single type of particles. Element or compound can be considered as pure form of matter. A cylinder of oxygen gas can be considered as pure substance. Milk cannot be considered as pure substance as it is a mixture of water fat protein etc. The composition or density of pure substance will be same throughout its distribution.
Question 2. Rusting of an article made up of iron is called
(a) corrosion and it is a physical as well as chemical change
(b) dissolution and it is a physical change
(c) corrosion and it is a chemical change
(d) dissolution and it is a chemical change.
Answer: C
Solution: In a chemical change, either one of the substance changes and comes with new properties. Corrosion is a natural phenomenon, in which any metal tries to attain chemically stable form such as oxide for hydroxide. When any article made up of iron get rusted, it gets converted in iron oxide it looks reddish brown in colour. As it is a change of the substance, it is a chemical change.
Question 3. A mixture of sulphur and carbon disulphide is
(a) heterogeneous and shows Tyndall effect
(b) homogeneous and shows Tyndall effect
(c) heterogeneous and does not show Tyndall effect
(d) homogeneous and does not show Tyndall effect.
Answer: D
Solution: Solution will be homogeneous at molecular level it means if you take any part of the solution percentage of substances will be same. The Tyndall effect is shown by mixture, where mixture has tendency to scatter the light of shorter wavelength and transmit the light of larger wavelength. The Tyndall effect is shown by heterogeneous mixture such as colloid. Sulphur is soluble in carbon disulphide. A solution is formed when sulphur is mixed with carbon disulphide. Therefore, it does not show Tyndall effect.
Question 4. Tincture of iodine has antiseptic properties. This solution is made by dissolving :-
(a) iodine in potassium iodide
(b) iodine in vaseline
(c) iodine in water
(d) iodine in alcohol.
Answer: D
Solution: Tincture of iodine is also known as iodine tincture. It is a very weak solution of iodine in ethanol and water. It is used as an anti-septic. It was used since start of 20th century by surgeons.
Question 5. Which of the following are homogeneous in nature?
(i) Ice
(ii) Wood
(iii) Soil
(iv) Air
(a) (i) and (iii)
(b) (ii) and (iv)
(c) (i) and (iv)
(d) (iii) and (iv)
Answer: C
Solution: A mixture is called homogeneous, if the percentage of constituent substances will be same in any part of that mixture. The density of any sample will also be same in homogeneous mixture. If a sample is not homogeneous, it is called heterogeneous. All the pure substances will be homogeneous. Ice is a pure substance; hence it is homogeneous. Air is a homogeneous mixture of multiple gases. Soil and wood or not homogeneous.
Question 6. Which of the following are physical changes?
(i) Melting of iron metal
(ii) Rusting of iron
(iii) Bending of an iron rod
(iv) Drawing a wire of iron metal
(a) (i), (ii) and (iii)
(b) (i), (ii) and (iv)
(c) (i), (iii) and (iv)
(d) (ii), (iii) and (iv)
Answer: C
Solution: If a matter goes under a physical change its appearance might change but the substance of matter does not change. In a chemical change in a chemical change either one of the substance changes and comes with new properties. In melting, the state of metal will change but the substance will not change. In bending or drawing a wire appearance of the metal change but basic properties of substance will not change. When any article made up of iron get rusted, it gets converted in iron oxide it looks reddish brown in colour. As it is a change of the substance, it is a chemical change
$4Fe+3O_{2}\rightarrow 2Fe_{2}O_{3}.xH_{2}O$
Question 7. Which of the following are chemical changes?
(i) Decaying of wood
(ii) Burning of wood
(iii) Sawing of wood
(iv) Hammering of a nail into a piece of wood
(a) (i) and (ii)
(b) (ii) and (iii)
(c) (iii) and (iv)
(d) (i) and (iv)
Answer: A
Solution: In the decaying or burning of wood, there will be a chemical change in the composition of wood hence it is a chemical process. Sowing of wood and hammering of nail into the piece of wood does not change the properties of wood chemically, hence it is a physical change.
Question 8. Two substances, A and B were made to react to form a third substance, $A_{2}B$ according to the following reaction:$2A + B \rightarrow A_{2}B$. Which of the following statements concerning this reaction are incorrect?
(i) The product A2B shows the properties of substances A and B.
(ii) The product will always have a fixed composition.
(iii) The product so formed cannot be classified as a compound.
(iv) The product so formed is an element.
(a) (i), (ii) and (iii)
(b) (ii), (iii) and (iv)
(c) (i), (iii) and (iv)
(d) (iii) and (iv)
Answer: C
Solution: $2A + B \rightarrow A_{2}B$, A and B will combine to make a product $A_{2}B$ . The product $A_{2}B$ is called compound and it will not have any property of elements A and B. The composition of elements in product will be fixed: If N moles of element A is present that means N/2 moles of element B will be present. This product will be pure. Hence statement (i), (iii) and (iv) are wrong statements.
Question 9. Two chemical species X and Y combine together to form a product P which contains both X and Y.
$X+Y\rightarrow P$
X and Y cannot be broken down into simpler substances by simple chemical reactions. Which of the following concerning the species X, Y and P are correct?
(i) P is a compound.
(ii) X and Y are compound.
(iii) X and Y are elements.
(iv) P has a fixed composition.
(a) (i), (ii) and (iii)
(b) (i), (ii) and (iv)
(c) (ii), (iii) and (iv)
(d) (i), (iii) and (iv)
Answer: D
Solution: $X + Y\rightarrow P$
X and Y will combine to make a product P. X and Y are elements because they cannot be broken down into simpler substances. The product P is called compound and it will not have any property of elements X and Y.
The composition of elements in product will be fixed: If N moles of element X is present that means moles of element Y will be present. This product will be pure.
NCERT Exemplar Class 9 Science Solutions Chapter 2 (Short Answer)
Some short answer type questions from NCERT Exemplar Solutions Class 9 Science Chapter 2 Is Matter Around Us Pure are given for practice. This section contains important questions that are asked in the exams. Practice short answer types from the questions below.
Question 10. Suggest separation technique(s) one would need to employ to separate the following mixtures.
(a) Mercury and water
(b) Potassium chloride and ammonium chloride
(c) Common salt, water and sand
(d) Kerosene oil, water and salt
Answer:
(a) Mercury and water – Mercury and water both are liquids but they are insoluble with each other.
The density of mercury is 13.6 times higher than the density of water.
Due to this difference in density and insolubility we can separate them by separating funnel.
(b) Potassium chloride and ammonium chloride – Ammonium chloride can be sublimated that means it can convert in
vapour without having the intermediate phase of liquid. Potassium chloride does not sublime.
Hence, we can separate this mixture by the process of sublimation.
(c) Common salt, water and sand – Salt is soluble in water but sand is not, hence we can separate sand from salty water by filtration. Now by the process of evaporation we can find dry salt as the water gets evaporated but salt remain there.
(d) Kerosene oil, water and salt – Due to difference in densities of kerosene oil and salty water, we can use the method of separating funnel to separate kerosene oil from salty water. Now by the process of evaporation we can find dry salt as the water gets evaporated but salt remain there.
Question 11. Which of the tubes in fig. 2.1 (a) and (b) will be more effective as a condenser in the distillation apparatus?
Answer:
The use of condenser tube in distillation Apparatus is to liquefy separated vapour.
This liquefaction is done by continuous flow of cold water.
If the area of condenser is increased, cooling will be faster.
Due to uneven shape of tube (A), its surface area is more than tube (B).
Therefore, it is advisable to use tube (A) for condenser.
Question 12. Salt can be recovered from its solution by evaporation. Suggest some other technique for the same?
Answer:
By the process of evaporation, we can find dry salt as the water gets evaporated but salt remain there. This process is a slow process.
We can think of another method:
The name of this process is distillation. It is similar to evaporation but we will not lose water in this case.
The boiling point of salt is very large in comparison of water.
On heating, the water will start boiling and we can collect the steam and condense it back in water.
Finally in the pot, we will left with only salt.
Note: The solubility of salt in water is independent of temperature, hence we can use crystallization method.
Question 13. The ‘sea-water’ can be classified as a homogeneous as well as heterogeneous mixture. Comment.
Answer:
If we consider Sea water as salty water only then it will be homogeneous.
Salt is soluble in water and makes a solution. All solutions are homogeneous.
But due to presence of mud, sand and other insoluble compounds: Sea water is also treated as heterogeneous mixture.
Answer:
If we mix two liquids of significant different boiling points, we can separate them by process of distillation.
Generally, this technique is used if difference in boiling point is more than $25^{\circ}C \sim 30^{\circ}C$.
Here the difference in boiling point of salty water and acetone is more than $44^{\circ}C$.
On heating, the acetone will start boiling and we can collect the vapour and condense it back in acetone liquid.
Finally in the pot, we will left with only salty water.
Question 15. What would you observe, when
(a) a saturated solution of potassium chloride prepared at $60^{\circ}C$ is allowed to cool to room temperature
(b) an aqueous sugar solution is heated to dryness
(c) a mixture of iron filings and sulphur powder is heated strongly?
Answer:
(a) The solubility of potassium chloride decrease on decreasing temperature. Therefore on cooling, Crystals of potassium chloride will separate out.
(b) On heating, the water will start boiling and completely evaporate to dryness.
Finally in the pot, we will left with only black sugar.
(c) At High temperature, they will react and Iron sulphide is formed when a mixture of iron filings and sulphur is heated strongly.
Question 16. Explain why particles of a colloidal solution do not settle down when left undisturbed, while in the case of a suspension they do.
Answer:
The size of colloidal particles are small, apparently they look like homogeneous solution but they are heterogeneous at molecular level.
These particle move randomly (Brownian motion) within the solution and do not settle down.
The particles in suspension are heavy and can be seen by naked eye.
When it is left undisturbed, due to gravity, these particles settle down.
Question 17. Smoke and fog both are aerosols. In what way are they different?
Answer:
A colloidal solution of particle dispersed in air or gas is called aerosol.
Therefore, Smoke and Fog; both are aerosol as both of them are dispersed in air.
The difference in both of them is on the basis of solute particle.
In Smoke: solid carbon is the solute.
In Fog: liquid water is the solute.
Question 18. Classify the following as physical or chemical properties.
(a) The composition of a sample of steel is: $98\%$ iron, $1.5\%$ carbon and $0.5\%$ other elements.
(b) Zinc dissolves in hydrochloric acid with the evolution of hydrogen gas.
(c) Metallic sodium is soft enough to be cut with a knife.
(d) Most metal oxides form alkalis on interacting with water.
Answer:
Chemical property: When two substance combine to make a new compound, which does not show the properties of any reacting substances.
Physical property: Appearance, composition, state, melting point, elasticity, malleability etc.
(a) Physical property
(b) Chemical property
(c) Physical property
(d) Chemical property
Answer:
Mass by volume: X% mass by volume solution means X grams of a solute dissolved in 100 mL of solution.
Mass by volume $(\%)=\frac{mass\; of\; solute \times100}{Volume\; of\; solution}$
Student A: dissolved 50 g of NaOH in 100 mL of water.
Therefore, volume of solution will be more than 100 ml as only solvent is 100 mL.
This solution will have less than $50\%$ (mass by volume) solution
Student B: dissolved 50 g of NaOH in 100 g of water. (100 gram water =100mL of water)
Therefore, volume of solution will be more than 100 ml as only solvent is 100 mL.
This solution will have less than $50\%$ (mass by volume) solution.
Student C: dissolved 50 g of NaOH in water to make 100 mL.
It means, Volume of solution is 100mL.
C has made the desired solution by dissolving 50 g NaOH in water to make the volume of the solution 100 mL
Question 20. Name the process associated with the following:
(a) Dry ice is kept at room temperature and at one atmospheric pressure.
(b) A drop of ink placed on the surface of water contained in a glass spreads throughout the water.
(c) A potassium permanganate crystal is in a beaker and water is poured into the beaker with stirring.
(d) An acetone bottle is left open and the bottle becomes empty.
(e) Milk is churned to separate cream from it.
(f) Settling of sand when a mixture of sand and water is left undisturbed for some time.
(g) Fine beam of light entering through a small hole in a dark room, illuminates the particles in its paths.
Answer:
(a) Sublimation of dry ice (solid) to CO2 (gas): Dry Ice will convert in gas without going through intermediate liquid state.
(b) Diffusion of ink into water: The ink flow from higher concentration to lower concentration.
(c) Dissolution of solid into liquid: Slowly potassium permanganate crystal will dissolve in water to make a solution.
(d) Evaporation of acetone in air : As the boiling point of acetone is very small , it get evaporated and diffused in the atmosphere.
(e) Centrifugation of cream: In the process of churning, a centrifugal force acts away from the central line. Thus, cream get collected on the walls of vessel.
(f) Sedimentation
(g) Tyndall effect – Heterogeneity of air causes bending and scattering of light.
Answer:
When any non-volatile solute (impurities) is added in water, It causes elevation in boiling point as well as depression in freezing point.
Therefore impure water will have boiling point more than $100^{\circ}C$ and freezing point will be less than $0^{\circ}C$,
Sample ‘B’ which boils at $102^{\circ}C$ contains impurities. It will not freeze at $0^{\circ}C$.
Answer:
If we use pure gold (24 carat), it is difficult to make ornaments.
Pure Gold is highly malleable and soft in nature, which will not give any strength to the ornament and it will be de-shaped easily.
When Pure is alloyed with copper or silver (less than 24 carat), it becomes hard and strong. Now it can be used to make ornaments.
Question 23. An element is sonorous and highly ductile. Under which category would you classify this element? What other characteristics do you expect the element to possess?
Answer:
Sonorous: It means, substance makes ringing sound when we strike it.
Ductile: We can create wire, as it can be deformed without losing strength.
These two properties indicate that the element must be metal.
The metal is expected to be lustrous, malleable and good conductor of heat and electricity
Question 24. Give an example each for the mixture having the following characteristics. Suggest a suitable method
to separate the components of these mixtures.
(a) A volatile and a non-volatile component.
(b) Two volatile components with appreciable difference in boiling points.
(c) Two immiscible liquids.
(d) One of the components changes directly from solid to gaseous state.
(e) Two or more coloured constituents soluble in some solvent.
Answer:
(a) A volatile (can be easily boiled) and a non-volatile (cannot be boiled) component.–
We can use process of distillation or evaporation
Example: Common salt solution
(b) Two volatile components with appreciable difference in boiling points.
We can use process of distillation.
Example: Acetone (boiling point = $56^{\circ}C$) and water (boiling point = $100^{\circ}C$)
(c) Two immiscible liquids (of different densities)
We can use process of Separation using separating funnel
Example: Oil Water mixture
(d) One of the components changes directly from solid to gaseous state.
We can use process of Sublimation
Example: Ammonium chloride (sublimate) and potassium chloride (will not sublimate)
(e) Two or more coloured constituents soluble in some solvent.
We can use process of chromatography in which mixture is dissolved in solvent to form mobile phase and on passing through a fixed phase they separate
Example: ink or plant dyes.
Question 25. Fill in the blanks.
(a) A colloid is a _______ mixture and its components can be separated by the technique known as _______.
(b) Ice, water and water vapour look different and display different _______ properties but they are _______ the same.
(c) A mixture of chloroform and water taken in a separating funnel is mixed and left undisturbed for some time. The upper layer in the separating funnel will be of _______ and the lower layer will be that of _______.
(d) A mixture of two or more miscible liquids, for which the difference in the boiling points is less than 25 K can be separated by the process called _______.
(e) When light is passed through water containing a few drops of milk, it shows a bluish tinge. This is due to the _______ of light by milk and the phenomenon is called _______. This indicates that milk is a _______ solution.
Answer:
(a) heterogeneous; centrifugation
Colloid: It is heterogeneous mixture of tiny particles which perform random motion and cannot be separated by gravity.
Centrifugation: on rotating container particle are repelled towards wall.
(b) physical, chemically
Physical Property: appearance, state etc.
Chemical Property: capability to react with some other substance.
(c) water, chloroform
Density of water (1 gram per cc) is less than density of chloroform ( 1.49 gram per cc)
(d) fractional distillation
Example: Acetone (boiling point = $56^{\circ}C$) and water (boiling point = $100^{\circ}C$)
(e) scattering, Tyndall effect, colloidal
Milk is a heterogeneous colloidal of water, fat and protein etc.
Question 26. Sucrose (sugar) crystals obtained from sugarcane and beetroot are mixed together. Will it be a pure substance or a mixture? Give reasons for the same.
Answer:
The composition of the sugar (sucrose – which is a compound) will remain constant irrespective of the source of its preparation.
It means sugar is pure substance and its composition will be same.
Question 27. Give some examples of Tyndall effect observed in your surroundings?
Answer:
Scattering of light through heterogeneous mixture is called Tyndall effect.
Examples of Tyndall effect:
(i) In Fog, Beam of head light of car is visible.
(ii) Sunlight passing through dust particle will be visible.
(iii) Path of light rays seen in front of the projector in a cinema hall.
(iv) Shining of flash light when pass through milk.
Question 28. Can we separate alcohol dissolved in water by using a separating funnel? If yes, then describe the procedure. If not, explain.
Answer:
We can separate immiscible liquids of different densities by using a separating funnel. Mixture of water and alcohol cannot be separated, since both are miscible and they form a solution.
Question 29. On heating, calcium carbonate gets converted into calcium oxide and carbon dioxide.
(a) Is this a physical or a chemical change?
(b) Can you prepare one acidic and one basic solution by using the products formed in the above process? If so, write the chemical equation involved.
Answer:
If a matter goes under a physical change its appearance might change but the substance of matter does not change.
In a chemical change in a chemical change either one of the substance changes and comes with new properties.
$CaCO_{3}\rightarrow CaO+CO_{2}$
(a) It is a chemical change as new substances are formed.
(b) Calcium oxide when dissolved in water, forms a basic solution.
$CaO + H_{2}O \rightarrow Ca(OH)_{2}$
Carbon dioxide when dissolved in water, forms an acidic solution
$CO_{2}+H_{2}O\rightarrow H_{2}CO_{3}$
Question 30. Non-metals are usually poor conductors of heat and electricity. They are non-lustrous, non- sonorous, non-malleable and are coloured.
(a) Name a lustrous non-metal.
(b) Name a non-metal which exists as a liquid at room temperature.
(c) The allotropic form of a non-metal is a good conductor of electricity. Name the allotrope.
(d) Name a non-metal which is known to form the largest number of compounds.
(e) Name a non-metal other than carbon which shows allotropy.
(f) Name a non-metal which is required for combustion.
Answer:
(a) Iodine and diamond: They shine as they are lustrous
(b) Bromine: fuming red brown liquid at room temperature
(c) Graphite: Allotrope of carbon (non-metal) good electric conductor
(d) Carbon: each carbon can combine with four different atoms
(e) Sulphur: Yellow rhombic sulphur and monoclinic sulphur are allotropic form
(f) Oxygen: it is required for combustion.
Question 31. Classify the substances given in Fig. 2.2 into elements and compounds.
Answer:
Elements – pure substance which cannot be broken down in simpler substance
$Cu (metal), Zn (metal), F_{2} (gas), O_{2} (gas)$, diamond (Carbon-non-metal), Hg (metal)
Compounds – They are also pure, but can be broken down in constituents.
$CaCO_{3}, H_{2}O$, NaCl(aq), Wood and Sand
Question 32. Which of the following are not compounds?
(a) Chlorine gas
(b) Potassium chloride
(c) Iron
(d) Iron sulphide
(e) Aluminium
(f) Iodine
(g) Carbon
(h) Carbon monoxide
(i) Sulphur powder
Answer:
Elements – pure substance which cannot be broken down in simpler substance.
Chlorine, Iron, Aluminium, Iodine, Carbon and Sulphur Powder
Compounds – They are also pure, but can be broken down in constituents.
Potassium Chloride (KCl)
Iron Sulphide (FeS)
Carbon monoxide (CO)
NCERT Exemplar Class 9 Science Solutions Chapter 2 (Long Answer)
Here some long-answer type questions from Is Matter Around Us Pure NCERT Exemplar Class 9 Science Chapter 2 are given that needs more practice. These questions are frequently asked in the exams. Long-answer type questions are covered to improve your subject knowledge and conceptual thinking:
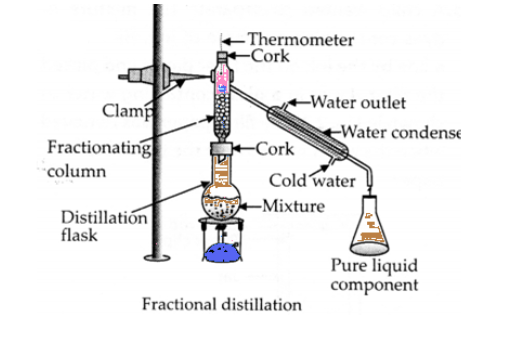
Question 33. Fractional distillation is suitable for separation of miscible liquids with a boiling point difference of about 25 K or less. What part of fractional distillation apparatus makes it efficient and possess an advantage over a simple distillation process? Explain using a diagram.
Answer:
If we mix two liquids of significant different boiling points, we can separate them by process of distillation.
Generally, this technique is used if difference in boiling point is more than $25^{\circ}C \sim 30^{\circ}C$.
But Fractional Distillation is effective even the boiling point difference is less than $25^{\circ}C$
In fractional distillation Method, a fractionating column is used and it makes it efficient. This column is packed with glass beads or small plates. It increases the surface area. The increase in surface area will increase the rate of condensation for the vapours. Vapour quickly loose energy, when they come in contact with beads or plates and can be condensed easily.
The length of the column would increase the efficiency of the process.
Answer:
Any Homogeneous mixture of two substances is called Solution.
The substance in large quantity is called solvent and substance in small quatity is called Solute.
Solution can be gaseous (For example; Air), Liquid (For example; Salt solution with water) and Solid (For example; Alloy
(a) Alloys they have uniform composition throughout hence they are homogeneous solid solution.
(b) No, Any Solution can be gaseous (For example; Air), Liquid (For example; Salt solution with water) and Solid (For example; Alloy)
(c) No, a solution is a homogeneous mixture and does not show Tyndall Effect.
Question 35. Iron filings and sulphur were mixed together and divided into two parts, ‘A’ and ‘B’. Part ‘A’ was heated strongly while Part ‘B’ was not heated. Dilute hydrochloric acid was added to both the Parts and evolution of gas was seen in both the cases. How will you identify the gases evolved?
Answer:
Part A –
When iron and sulphur are heated they react with each other and form iron-sulphide.
$Fe+S\rightarrow FeS$
This iron sulphide can react with acid Hydrochloride and hydrogen Sulphide gas will come out from the mixture.
$FeS+2HCl\rightarrow FeCl_{2}+H_{2}S(gas)$
Hydrogen Sulphide has smell like rotten egg and can be identified easily.
This gas can be chemically tested by reaction with lead Salts.
Part B –
When iron and sulphur are heated they react with each other and form iron-sulphide but without heat they will remain unaffected.
Sulphur does not react with hydrochloride but iron will react and hydrogen gas will come out.
$Fe+2HCl\rightarrow FeCl_{2}H_{2}(gas)$
Hydrogen gas is highly inflammable and produces sound on burning.
If we bring any spark near the mixture, we can identify the hydrogen gas.
(i) What would you expect to see, if the ink contains three different coloured components?
(ii) Name the technique used by the child.
(iii) Suggest one more application of this technique.
Answer:
Chromatography is a method, which is used to separate components of any mixture.
In this process different component travels with different speed when dissolved in solution.
Due to different speed, all the components get separated from each other
(i) The components of the ink will travel with water with different speeds and we would see three bands on the filter paper at different levels.
(ii) Chromatography.
(iii) This technique is also used for separating pigments present in chlorophyll.
(a) Explain why the milk sample was illuminated. Name the phenomenon involved.
(b) Same results were not observed with a salt solution. Explain.
(c) Can you suggest two more solutions which would show the same effect as shown by the milk solution?
Answer:
Scattering of light through heterogeneous mixture is called Tyndall effect.
Examples of Tyndall effect:
(i) In Fog, Beam of head light of car is visible.
(ii) Sunlight passing through dust particle will be visible.
(iii) Path of light rays seen in front of the projector in a cinema hall.
The Tyndall effect is shown by mixture, where mixture has tendency to scatter the light of shorter wavelength and transmit the light of larger wavelength.
The Tyndall effect is shown by heterogeneous mixture such as colloid.
(a) Milk is a colloidal solution hence shows Tyndall effect.
(b) Salt solution is homogeneous therefore do not show Tyndall effect and they do not scatter light.
(c) Detergent mixture and air with dust will show Tyndall effect.
Question 38. Classify each of the following, as a physical or a chemical change. Give reasons.
(a) Drying of a shirt in the sun.
(b) Rising of hot air over a radiator.
(c) Burning of kerosene in a lantern.
(d) Change in the colour of black tea on adding lemon juice to it.
(e) Churning of milk cream to get butter.
Answer:
If a matter goes under a physical change its appearance might change but the substance of matter does not change.
In a chemical change in a chemical change either one of the substance changes and comes with new properties.
a) Drying of a shirt in the sun $\Rightarrow$Substance of shirt does not change$\Rightarrow$- Physical change
(b) Rising of hot air over a radiator$\Rightarrow$Substance in air does not change$\Rightarrow$- Physical change
(c) Burning of kerosene in a lantern$\Rightarrow$ Kerosene goes under chemical reaction with oxygen$\Rightarrow$- Chemical change
(d) Change in the colour of black tea on adding lemon juice to it$\Rightarrow$Black tea goes under chemical reaction$\Rightarrow$-Chemical change
(e) Churning of milk cream to get butter$\Rightarrow$Substance in milk does not change$\Rightarrow$-Physical change
(a, b, e) : Physical changes because there is no change in chemical composition, (c), (d) : Chemical changes because new substances are formed.
Question 39. During an experiment the students were asked to prepare a $10\%$ (Mass/Mass) solution of sugar in water. Ramesh dissolved 10 g of sugar in 100 g of water while Sarika prepared it by dissolving 10 g of sugar in water to make 100 g of the solution
(a) Are the two solutions of the same concentration?
(b) Compare the mass $\%$ of the two solutions.
Answer:
Mass Percent: amount of solute in 100 gram of solution.
Mass percent(%) $=\frac{massof Solute\times100}{massofSolution}$
Ramesh’s Solution:
Mass percent(%) $=\frac{10gram\times100}{10gram+100gram}=9.0909\%$
Sarika’s Solution:
Mass percent(%) $=\frac{10gram\times100}{100gram}=10\%$
Part a: Sarika has higher mass percentage than Ramesh.
Part b: The mass percentage ratio of Sarika and Ramesh is 10: 9.0909
Question 40. You are provided with a mixture containing sand, iron filings, ammonium chloride and sodium chloride. Describe the procedures you would use to separate these constituents from the mixture?
Answer:
Given Mixture – Sand + Iron filings + Ammonium chloride +Sodium chloride
Iron filing can be separated by using magnet which will attract all the iron filings.
Now by the process of sublimation we can separate ammonium chloride from the mixture as it is the only constituent which get sublimate.
Now we mix the remaining salt and sand in water. The sodium chloride will dissolve in water and we can collect the sand which will settle in the base.
Now we are left with salt solution. By the process of evaporation water will vaporise and we are left with salt .
Question 41. Arun has prepared $0.01\%$ (by mass) solution of sodium chloride in water. Which of the following correctly represents the composition of the solutions?
(a) $1.00 g \;of\; NaCl + 100g \;of\; water$
(b) $0.11 g \;of \;NaCl + 100 g\; of \;water$
(c) $0.01 g \;of\; NaCl + 99.99 g \;of\; water$
(d) $0.10 g \;of \;NaCl + 99.90 g \;of\; water$
Answer: C
Mass Percent: amount of solute in 100 gram of solution.
Mass percent (%) $=\frac{massofsolute\times100}{massofSolution}$
Option a:
Mass percent (%) $=\frac{1gram\times100}{1gram+100gram}=0.99$
Option b:
Mass percent (%) $=\frac{0.11gram\times100}{0.11gram+100gram}=0.1099$
Option c:
Mass percent (%) $=\frac{0.1gram\times100}{0.1gram+99.99gram}=0.01$
Option d:
Mass percent (%) $=\frac{0.1gram\times100}{0.1gram+99.90gram}=0.1$
Hence the correct answer is C.
Question 42. Calculate the mass of sodium sulphate required to prepare its 20% (mass percent) solution in 100 g of water?
Answer:
Mass Percent: amount of solute in 100 gram of solution.
Mass Percent (%) $=\frac{massofsolute\times100}{massofSolution}$
Let the mass of sodium sulphate required be = m gram
Mass of the solvent (water) = 100 gram
The mass of solution = (m + 100) gram (sum of mass of solute and solvent)
$20=\frac{m\times100}{m+100}$
$\Rightarrow 20m+2000=100m$
$\Rightarrow 2000=80m$
$\Rightarrow m=25g$
Important Questions of Class 9 Science Chapter 2
Some Is Matter Around Us Pure NCERT Exemplar Class 9 Science Chapter 2 questions and answers are given below that will help you tackle complex problems. The questions below will help you evaluate your understanding of the concepts.
Question 1: Why does the temperature remain constant as the heated liquid gets converted to its gaseous state at its boiling point?
(1) because change in state of matter is associated with increase in potential energy
(2) because change in state of matter is associated with increase in kinetic energy
(3) because change in state of matter is associated with increase in internal energy
(4) because change in state of matter is associated with increase in elastic energy
Solution: When the heated liquid is converted to its gaseous state at its boiling point, the temperature remains constant because the change in the state of matter is associated with an increase in potential energy, and the kinetic energy remains constant.
Hence, the answer is the option (1).
Question 2: An inflated balloon full of air becomes smaller and smaller slowly even though the knot at the mouth of the balloon is airtight. And after a week all the air has escaped from the balloon. Explain how the air particles got out of the balloon.
(1) Through the hole of the balloon
(2) Knot at the mouth of the balloon loosen after some days
(3) Diffusion through the rubber sheet of the balloon
(4) None of these
Solution:
The fast-moving molecules of air trapped in the inflated balloon exert continuous pressure on the thin, stretched rubber sheet of the balloon and keep on diffusing out gradually
through it.
Hence, the answer is the option (3).
Question 3: According to ancient philosphers matter consists of
(1) three constituents
(2) four constituents
(3) five constituents
(4) six constituents
Solution:
Matter can be classified in a number of ways. Ancient Indian philosophers said that all the matter (padarth), living or non-living was made up of five basic elements, air, earth, sky, fire, and water.
Hence, the answer is the option (3).
Question 4: Which of the following is a homogeneous mixture?
(1) Soil
(2) Air
(3) Sand and iron filings
(4) Oil and water
Answer:
Air is a uniform mixture of gases like nitrogen, oxygen, carbon dioxide, etc., and has the same composition throughout, so it is a homogeneous mixture.
Hence, the correct answer is option (2)
Question 5: A substance that cannot be broken down into simpler substances by chemical methods is called:
(1) Compound
(2) Mixture
(3) Element
(4) Solution
Answer:
An element consists of only one type of atom and cannot be decomposed into simpler substances by chemical reactions.
Hence, the correct answer is option (3).
Approach To Solve Class 9 Science Chapter 2 Questions
To solve Class 9 Science Chapter 2 NCERT Exemplar Is Matter Around Us Pure questions, it is important to follow a systematic approach. It is recommended to strategies your study plan to solve the questions of this chapter. The following are the points that will help you build a good approach.
1). While solving questions it is very important to understand the basic concepts, start by understanding about pure substance, the difference between the elements, compounds and mixtures, various separation techniques of mixtures.
2). Question relatied to differentiations are ferquently asked in exams, so it is very important to learn the key points of differences between
- Homogeneous and Heterogeneous mixtures
- Compound and Mixture
3). Always use examples in your solution to make it more attractive:
- Use examples related to everyday activities
- Add real-life applications as well
4). Learn about the Classification of substances based on their composition and properties for better understanding. The Class 9 Science Chapter Chapter 2 Is Matter Around Us Pure notes will help you revise the topics.
- Pure or mixture
- Element or compound
- Homogeneous or heterogeneous
5). Go through NCERT Exemplar Solutions Class 9 Science Chapter 2 Is Matter Around Us Pure and real-life examples as they often form the basis for application questions. After studying, close the book and try recalling answers or concepts.
Topics Of NCERT Exemplar Class 9 Science Chapter 2
NCERT exemplar Class 9 Science solutions chapter 2 explores the following topics:
- Compounds and compositions of matter.
- Different types of mixtures whether it is homogeneous or heterogeneous.
- The Tyndall effect will be used to check the heterogeneity of the mixture.
- Different methods of filtration of constituents from a mixture are discussed.
- Class 9 Science Chapter 2 NCERT Exemplar Is Matter Around Us Pure discusses the following methods of filtration of constituents from a mixture:
- Method of evaporation
- Method of distillation
- Method of fractional distillation
- Method of sublimation
- Method of chromatography
Advantages of Using Is Matter Around Us Pure NCERT Exemplar Class 9 Science Chapter 2 Solutions
NCERT Exemplar Solutions Class 9 Science Chapter 2 Is Matter Around Us Pure help students to understand the classification and properties of substances and mixtures. Given below some points on the advantages of these solutions:
- Students can use these solutions to understand the topics like elements, compounds, mixtures, types of mixtures, methods of separation, and physical and chemical changes.
- These NCERT Exemplar Solutions for Class 9 provide systematic explanations that make it easier to understand the composition and purification of substances.
- Class 9 Science Chapter 2 NCERT Exemplar Is Matter Around Us Pure solutions are prepared by experts in a very clear and comprehensive manner.
- They are well structured solutions that cover all the topics from NCERT that are helpful for boards and competitive exams.
NCERT Class 9 Science Exemplar Solutions Chapter-wise
Along with NCERT Exemplar Class 9 Science Chapter 2 Is Matter Around Us Pure, students can follow the links for other class 9 solutions
NCERT Solutions for Class 9 chapter-wise
The NCERT Solutions for all Class 9 science chapters are given below-
NCERT Notes & Solutions subject-wise
Students can refer to the links given below for the NCERT subject-wise solutions:
NCERT Books and NCERT Syllabus
Students can refer to the links given below for the NCERT books and syllabus :
Frequently Asked Questions (FAQs)
A mixture is a combination of two or more substances that are physically combined, but not chemically bonded. This means each substance retains its individual properties.
Pure substances have a fixed composition and definite properties, while mixtures have variable composition and their properties are a combination of the properties of the substances that make them up. Pure substances can be elements or compounds. Mixtures can be homogeneous or heterogeneous.
A homogeneous mixture has a uniform composition throughout; you can't see the different components easily. Examples include saltwater or air. A heterogeneous mixture has a non-uniform composition; you can easily see the different components. Examples include a salad, gravel, or oil and water.
It is a heterogeneous mixture because you can distinguish the different components (tea, ice cubes).
The Tyndall effect is the scattering of light by tiny particles in a colloid or a fine suspension. This makes the light beam visible as it passes through the mixture. A common example is the way sunlight becomes visible when it passes through dust in a dark room or through fog.
NCERT Exemplar Solutions for Class 9 Science are important because they offer advanced questions and detailed explanations that strengthen conceptual understanding.
Yes, the NCERT Exemplar for Is Matter Around Us Pure is important because it provides higher-level questions that help students deeply understand mixtures, solutions, and separation techniques, improving their conceptual clarity and exam preparation.
Chapter 2 of NCERT Exemplar Solutions for Class 9 Science covers concepts like mixtures, types of solutions, concentration, separation techniques, and differences between elements, compounds, and mixtures.
A mixture is physically combined and can be separated by physical methods, while a compound is chemically combined and cannot be separated physically.
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters




