The NCERT Solutions for Class 9 Science Chapter 4 Structure of The Atom provide a detailed explanation of atomic structure and its components. They help students understand difficult concepts in a easy way. Apart from this, students learn many interesting facts and things, which are given below:
NCERT Solutions for Class 9 Science Chapter 4 Structure of The Atom
What makes up everything around us? If atoms are so small, how did scientists even figure out what’s inside them? Why do electrons keep spinning around the nucleus? Structure of the Atom answer all these questions. Atoms are the fundamental building blocks of everything around us, from the gold or diamonds used in jewellery to the silicon used in mobile phones. Atoms are so small that we cannot even see or feel them with the naked eye. To see them, we require a microscope. NCERT solutions consists of various important topics of Structure of atom Class 9 like atoms, subatomic particles, and the theories given by famous scientists like Rutherford, Dalton, and Thomson, who gave Thomson's model of an atom, etc.
This Story also Contains
- NCERT Solution For Class 9 Science Chapter 4: Download PDF
- NCERT Solutions for Class 9 Science Chapter 4 (In-text Exercise Questions)
- NCERT Solutions for Class 9 Science Chapter 4 (Exercise Questions with Answers)
- Practice Questions for Class 9 Science Chapter 4
- Approach to Solve Questions of Class 9 Science Chapter 4
- Topics and Subtopics Covered in the NCERT Textbook
- NCERT Class 9 Science Chapter 4: Important Formulas
- What Students Learn from NCERT Solutions for Class 9 Science Chapter 4
- Importance of Class 9 Science Chapter 4 Structure of the Atom Solutions
- NCERT Solutions for Class 9 Science Chapter-Wise
- NCERT Books and NCERT Syllabus

The NCERT Solutions for Class 9 Science will offer a very comprehensive and systematic way to present topics discussed in Structure of the Atom through a series of solved questions. Our subject matter experts prepare these structure of the atom ncert solutions, which also serve as a valuable resource for students to enhance their exam performance in board exams. The practice questions and approach for solving the questions are also included in this chapter to help students understand the concepts clearly.
NCERT Solution For Class 9 Science Chapter 4: Download PDF
Students can download the class 9 science chapter 4 structure of the atom question answer pdf for free. These solutions of NCERT are designed to help you understand the fundamental concepts and solve textbook questions. They provide clear explanations and step by step answers, making it easier for students to understand complex topics easily.
Also Read,
NCERT Solutions for Class 9 Science Chapter 4 (In-text Exercise Questions)
Detailed class 9 science chapter 4 structure of the atom question answer are given below. By referring to these questions students can strengthen their conceptual understanding. These solutions also help in improving problem solving skills.
Topic 4.1 Charged particles in the matter? (Page 39)
Question 1. What are canal rays?
Answer:
Canal rays are the positively charged radiations that consist of positively charged particles of atoms. They can pass through the perforated ( pierced ) cathode and then travel towards another cathode in a gas discharge tube.
They were given the name Canal rays by E. Goldstein in 1866, who discovered these radiations.
Question 2. If an atom contains one electron and one proton, will it carry any charge or not?
Answer:
The proton is a positively charged particle and the electron is a negatively charged particle. their magnitude is equal and hence net charge in an atom is zero.
Topic 4.2 The structure of an atom ( Page 41)
Question 1. On the basis of Thomson’s model of an atom, explain how the atom is neutral as a whole.
Answer:
According to Thomson’s model of an atom, an atom consists of a sphere of a positive charge.
The positive charge in the atom is spread all over like the red edible part of a watermelon, while the electrons are studded in the positively charged sphere, just like the seeds in the watermelon.
As negative and positive charges are equal in magnitude, they balance each other and thus the atom becomes electrically neutral as a whole.
Answer:
On the basis of Rutherford’s model of an atom, the subatomic particle which is present in the nucleus of an atom is Proton which is a positively charged particle.
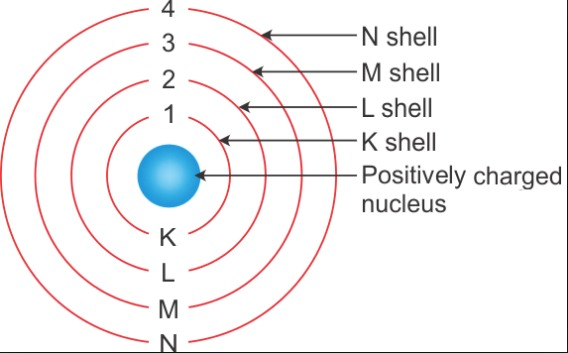
Question 3. Draw a sketch of Bohr’s model of an atom with three shells.
Answer:
The sketch of Bohr’s model of an atom with three shells:
Answer:
If a foil of a heavy metal like platinum is used, then the observations in the alpha-particle scattering experiment would be the same as that in the gold foil experiment.
If a foil of a light metal like lithium is used, then the observations in the alpha-particle scattering experiment would not be the same because these metal are not so malleable so the thin foil is difficult to obtain.
The problem with not using thin foil is that the number of the alpha particle will bounce back from the thick foil and the location of positive mass would be difficult to find.
Topic 2.4.2 Neutron ( Page 41)
Question 1. Name the three sub-atomic particles of an atom.
Answer:
The three sub-atomic particles of an atom are :
1. Electron: a negatively charged particle
2. Proton: a positively charged particle
3. Neutron: a neutral particle
Question 2. Helium atom has an atomic mass of 4 u and two protons in its nucleus. How many neutrons does it have?
Answer:
The atomic mass of Helium = 4 u
No. of protons = 2
As atomic mass = no. of protons + no. of neutrons
No. of neutrons = At. mass - no. of protons
= 4 - 2
= 2
Hence Helium atom has 2 neutrons.
Topic 4.3 How are electrons distributed in different orbits(shells)? ( Page 42)
Question 1. Write the distribution of electrons in carbon and sodium atoms.
Answer:
Number of electrons in a carbon atom = 6
Number of electrons in a sodium atom = 11
Electron Distribution:
| Element | First Orbit or K -K-shell | Second Orbit or L-shell | Third Orbit or M-shell |
| Carbon | 2 | 4 | 0 |
| Sodium | 2 | 8 | 1 |
Question 2. If $K$ and $L$ shells of an atom are full, then what would be the total number of electrons in the atom?
Answer:
Maximum Number of electrons in K-shell = 2
Maximum Number of electrons in L-shell = 8
The total no. of electrons in the atom = 2 + 8
= 10
If $K$ and $L$ shells of an atom are full, then the total number of electrons in the atom will be 10.
Topic 4.4 Valency (Page 44 )
Question 1. How will you find the valency of chlorine, sulphur and magnesium?
Answer:
Valancy is basically the minimum number of the electron we have to add or remove such that every shell in the atom is completely filled.
Mathematically,
when the outermost shell of an atom contains 4 or less than 4 electrons, its valency is equal to the number of valence electrons in the outermost shell and when the outermost shell contains more than 4 electrons, the valency of the atom is equal to 8 - no. of valence electrons in the atom.
Chlorine :
Atomic No. of Cl = 17
Its electronic configuration = 2, 8, 7
Valency of Cl = 8 - 7 = 1
Sulphur :
Atomic no. of S = 16
Its electronic configuration = 2, 8, 6
Valency of S = 8 - 6 = 2
Magnesium:
Atomic no. of Mg = 12
Its electronic configuration = 2, 8, 2
Valency of Mg = 2
Topic 4.5 Atomic number and Mass number ( Page 44)
Question 1.(i) If the number of electrons in an atom is 8 and the number of protons is also 8, then
(i) what is the atomic number of the atom?
Answer:
Given,
Number of electrons in the atom = 8
Number of proton in the atom = 8
The atomic number of an atom is equal to the number of proton in that atom. hence the atomic number of the given atom is 8.
Question 1. (ii) If the number of electrons in an atom is 8 and the number of protons is also 8, then
(ii) what is the charge on the atom?
Answer:
In the given atom, the total number of positive charges is equal to the total number of negative charge.
Number of Protons (8) = Number of electrons (8)
They both will neutralize each other. So, the atom will not possess any charge.
Question 2. With the help of Table 4.1, find out the mass number of oxygen and sulfur atom.
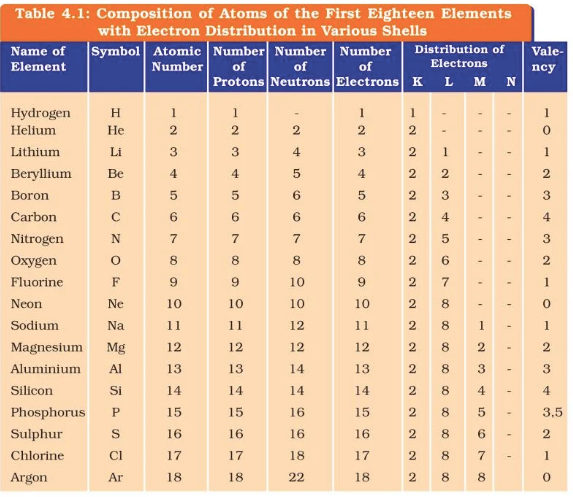
Answer:
For Oxygen:
Number of protons = 8
Number of electrons = 8
Mass number = Number of Protons + Number of neutrons
= 8 + 8
= 16
Hence mass number for Oxygen is 16.
For Sulphur:
Number of protons = 16
Number of electrons = 16
Mass number = Number of Protons + Number of neutrons
= 16 + 16
= 32
Hence Mass number for Sulphur is 32.
Topic 4.6 Isotopes (Page 45)
Question 1. For the symbol $H,D$ and $T$ tabulate three sub-atomic particles found in each of them.
Answer:
H, D, and T are the three isotopes of hydrogen with the same atomic number and different mass numbers of 1, 2 and 3 respectively.
| Element | Symbol | Number of Electrons | Number of Protons | Number of Neutrons |
| Hydrogen | H | 1 | 1 | 0 |
| Deuterium | D | 1 | 1 | 1 |
| Tritium | T | 1 | 1 | 2 |
Question 2. Write the electronic configuration of any one pair of isotopes and isobars.
Answer:
Isotopes :
Isotopes are the atoms with the same number of proton and different atomic mass. The difference in atomic mass arises due to the different number of neutrons present in the atom.
Some Examples of Isotopes are :
1. $^{12}C_6$ and $^{14}C_6$ ,
2. $^{35}Cl_{17}$ and $^{37}Cl_{17}$ .
Isobar:
Isobars are the atom with the same atomic mass and different atomic number.
Some example of Isobars are :
1. $^{40}Ca_{20}$ and $^{40}Ar_{18}$
2. $^{22}Ne_{10}$ and $^{22}Na_{11}$
NCERT Solutions for Class 9 Science Chapter 4 (Exercise Questions with Answers)
You can access the complete NCERT Solutions for Class 9 Science Chapter 4 Structure of The Atom which are given below. These answers are prepared by subject experts and follow the latest CBSE guidelines.
Question 1. Compare the properties of electrons, protons, and neutrons.
Answer:
The Comparison of Properties between Electron, Proton, and Neutron:
| Properties | Electrons | Protons | Neutrons |
| Charge | Negatively charged | Positively charged | No charge |
| Weight | Negligible | 1 a.m.u | 1 a.m.u |
| Location in atom | Outside the nucleus | Inside the nucleus | Inside the nucleus |
| Reaction with a charged particle | Attracts positive charge | Attracts negative charge | gives no reaction to any charge |
Question 2. What are the limitations of J.J. Thomson’s model of the atom?
Answer:
The limitations of J.J. Thomson’s model of the atom are:
1. Thomson's model of the atom could not explain the results of alpha particle scattering experiment carried out by Rutherford. this model failed to depict why most of the alpha particle passes through gold foil and why some of them got diverted in different angles and some of them rebounded and returned back to their paths.
2. It was solely based on the imagination and did not have any experimental evidence.
Question 3. What are the limitations of Rutherford’s model of the atom?
Answer:
The limitations of Rutherford's model of the atom is that It does not explain the stability of the atom. As we know now, when charged bodies move in a circular motion, they emit radiations.
This means that the electrons revolving around the nucleus (as suggested by Rutherford) would lose energy and come closer and closer to the nucleus, and a stage will come when they would finally merge into the nucleus.
This makes the atom unstable, which is clearly not the case. The electrons do not fall into the nucleus, atoms are very stable and do not collapse on their own.
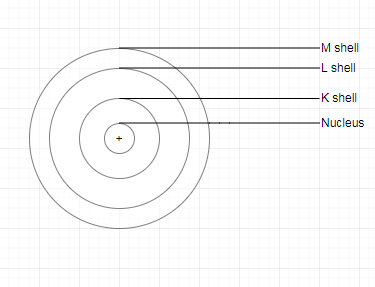
Question 4. Describe Bohr’s model of the atom.
Answer:
In order to overcome the objections raised against Rutherford's model of the atom, Neils Bohr put forward his model of the atom. According to Bohr's model of the atom,
1. An atom holds the nucleus in the center. the whole mass of the atom is concentrated at the nucleus.
2. The negatively charged particle revolves around the nucleus in definite circular paths known as orbits or which are designated as K, L, M, N, etc. or numbered as n = 1, 2, 3, 4, etc. (outward from the nucleus).
3. While revolving in discrete orbits, the electrons do not radiate energy. But when an electron jumps from one energy level to another, the energy of the atom changes.
Question 5. Compare all the proposed models of an atom given in this chapter.
Answer:
Comparison of different proposed Model:
| Feature | Thomson's Model | Rutherford's Model | Bohr's Model |
| Positive Charge | The Positive charge is distributed in Sphere | The positive charge is concentrated at the core of the atom, which is called the nucleus | The positive charge is present in the core of the atom, called nucleus. |
| Negative Charge | The electrons are embedded in the positively charged sphere of an atom, like the seeds in a watermelon. | The nucleus is surrounded by electrons, and the electrons and the nucleus are held together by the electrostatic force of attraction | The electrons move in discrete orbits, and each orbit is associated with a definite amount of energy. |
| Limitation | This model could not explain the results of an alpha particle scattering experiment | This model could not explain the stability of the atom. | This model perfectly explains the stability of an atom |
| Diagrammatic representation |  | 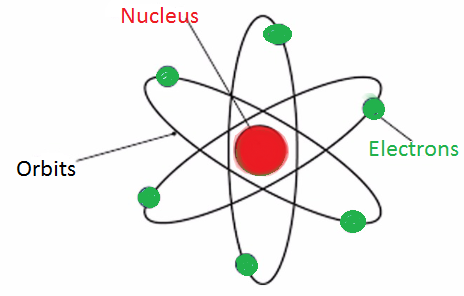 | 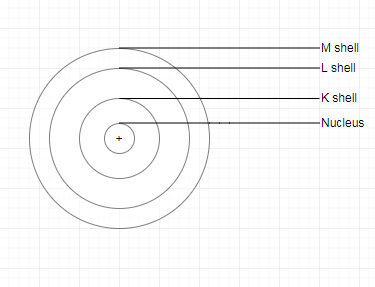 |
Answer:
The Bohr and Bury scheme for the distribution of electrons in an atom is based on the following rules :
1. The maximum number of electrons that a shell can have is represented by $2n^2$ , where n is the quantum number of that particular energy shell. Thus, the maximum number of electrons in the first four shells are :
1st (K) shell $2 \times 1^2=2$
2nd (L) shell $2 \times 2^2=8$
3rd (M) shell $2 \times 3^2=18$
4th (N) shell $2 \times 4^2=32$
2. The outermost shell, which is also called valence shell, can have a maximum of 8 electrons.
3. If permitted by rule 1, The shell inner to the outermost shell (the second last shell ) can accommodate a maximum of 18 electrons.
4. Electrons are not taken in unless the inner shells are filled, i.e., the shells are filled in a step-wise manner.
Question 7. Define valency by taking examples of silicon and oxygen.
Answer:
The definite combining capacity of an atom of an element, in which electrons are lost, gained or shared with other atoms to complete the octave in the outermost shell is defined as valency.
In other words,
Valancy is basically the minimum number of the electron we have to add or remove from or in the outermost shell such that every shell in the atom is completely filled.
And Mathematically,
when the outermost shell of an atom contains 4 or less than 4 electrons, its valency is equal to the number of valence electrons in the outermost shell and when the outermost shell contains more than 4 electrons, the valency of the atom is equal to 8 - no. of valence electrons in the atom.
The valency of Silicon:
Atomic number = 14
Distribution of electron :
K = 2
L = 8
M = 4
Number of electrons in outermost shell = 4
Valency = 8 - 4 = 4.
The valency of Oxygen:
Atomic number = 8
Distribution of electrons:
K = 2
L = 6
The number of electron in outermost shell = 6
Valency = 8 - 6 = 2
Question 8.(i) Explain with examples
(i) Atomic number,
Answer:
Atomic Number :
An atomic number of an atom is the total number of protons present within the nucleus of an atom is known as the atomic number. it is denoted by symbol Z.
Example: As the Oxygen atom has 8 protons in its nucleus, its atomic number is 8.
Question 8.(ii) Explain with examples
(ii) Mass number,
Answer:
Mass Number :
The mass number of an atom is the sum total of the masses of all the nucleons present in the nucleus of an atom, i.e.,
Mass Number = No. of Protons + No. of Neutrons
It is denoted by A.
Example: As a sodium atom has 11 protons and 12 neutrons in its nucleus,
So, it's mass number = 11 + 12 = 23.
Question 8.(iii) Explain with examples
(iii) Isotopes
Answer:
Isotopes:
Isotopes are the atoms of the same element having the same atomic number but a different mass number.
Example: Carbon molecule exists as $^{12}C_6$ and $^{14}C_6$ .
Question 8.(iv) Explain with examples
(iv) Isobars.
Answer:
Isobar:
Isobars are the atoms of different elements having the same mass number but different atomic numbers.
Example: $^{40}Ca_{20}$ and $^{40}Ar_{18}$ . Mass numbers of calcium and argon atoms have different atomic numbers (20 and 18 ) but the same mass number 40.
Two uses of isotopes are:
(i) An isotope of uranium is used as fuel in nuclear reactors.
(ii) An isotope of cobalt is used in the treatment of cancer.
Question 9. $Na^{+}$ has completely filled $K$ and $L$ shells. Explain.
Answer:
The atomic number of Na = 11
No. of electrons in Na atom = 11
In $Na^{+}$ ,the positive charge is obtained due to the loss of one electron from the M shell of Na atom.
So, No. of electrons in Na + ion = 11 -1 = 10
Hence, electronic configuration of Na + = 2, 8
In Na + , K and L shells are completely filled since K shell can have a maximum of 2 electrons and L shell can have a maximum of 8 electrons.
Answer:
Given, two isotopes $_{35}^{79}\textrm{Br}(49.7^{o}/_{o})$ and $_{35}^{81}\textrm{Br}(50.3^{o}/_{o})$ .
Average atomic mass:
$=79\times\frac{49.7}{100}+81\times\frac{50.3}{100}$
$=\frac{3926.3}{100}+\frac{4074.3}{100}$
$=\frac{8000.6}{100}$
$=80.006u$ .
Answer:
Given, the average atomic mass of a sample of an element $X$ is $16.2\; u$ .
Two isotopes of element = $_{8}^{16}\textrm{X}$ and $_{8}^{18}\textrm{X}$
Now, Let's percent of isotope $_{8}^{16}\textrm{X}$ be x and percent of $_{8}^{18}\textrm{X}$ be 100 - x
So, According to the question,
Average Atomic Mass :
$16.2=16\times\frac{x}{100}+18\times\frac{100-x}{100}$
$16.2=\frac{16x}{100}+18-\frac{18x}{100}$
$-1.8=-\frac{2x}{100}$
$2x=180$
$x=90$
Hence the percentage of isotope $_{8}^{16}\textrm{X}$ is 90 % and thepercentage isotope $_{8}^{16}\textrm{X}$ is 10%.
Question 12. If $Z=3$ , what would be the valency of the element? Also, name the element.
Answer:
Given
the Atomic number, Z = 3
Distribution of electrons :
K = 2,
L = 1
So, Valency = 1 .
The element with atomic number 3 is lithium.
Question 13. Composition of the nuclei of two atomic species $X$ and $Y$ are given as under
X Y
Protons = 6 6
Neutrons = 6 8
Give the mass numbers of $X$ and $Y$ . What is the relation between the two species?
Answer:
As we know,
the mass number of an atom = No. of protons + No. of Neutrons
So,
The mass number of X = No. of protons of X + No. of Neutrons of X
= 6 + 6
= 12
The mass number of Y = No. of protons of Y + No. of Neutrons of Y
= 6 + 8
= 14
As both X and Y have the same atomic number (6) but different numbers (i.e., 12 and 14 respectively), they are isotopes.
Question 14. For the following statements, write T for True and F for False.
(a) J.J. Thomson proposed that the nucleus of an atom contains only nucleons.
(b) A neutron is formed by an electron and a proton combining together. Therefore, it is neutral.
(c) The mass of an electron is about 1/2000 times that of a proton.
(d) An isotope of iodine is used for making tincture iodine, which is used as a medicine.
Answer:
(a) The statement is False: J.J. Thomson did not propose that the nucleus contains only nucleons. His atomic model, known as the "plum pudding model," states that an atom is a positively charged sphere and Electrons are embedded within it like plums in a pudding.
(b) The statement is False: Simply combining a proton and an electron does not result in a neutral charge, but it forms a hydrogen atom, not a neutron.
(c) The statement is True: The mass of an electron is approximately 1/2000 the mass of a proton.
(d) The statement is True: Tincture of iodine contains iodine element (not a radioactive isotope) and is used as an antiseptic, not made from an isotope.
Put a tick against correct choice and cross (×) against wrong choice in questions 15, 16 and 17
Question 15. Rutherford’s alpha-particle scattering experiment was responsible for the discovery of
(a) Atomic Nucleus
(b) Electron
(c) Proton
(d) Neutron
Answer:
Rutherford’s alpha-particle scattering experiment was responsible for the discovery of the Atomic Nucleus.
Hence, option (a) is the correct answer.
Question 16. Isotopes of an element have
(a) the same physical properties
(b) different chemical properties
(c) different number of neutrons
(d) different atomic numbers
Answer:
Isotopes are atoms of the same element that have the same number of protons but different numbers of neutrons, which leads to a different mass number.
Hence, option (c) is correct.
Question 17. A Number of valence electrons in $Cl^{-}$ ion are:
(a) 16
(b) 8
(c) 17
(d) 18
Answer:
The Electronic configuration of $Cl^{-}$ ion is :
K = 2
L = 8
M = 8
Hence Number of valance electron in $Cl^{-}$ ion = 8.
Hence, option (b) is the correct answer.
Question 18. Which one of the following is a correct electronic configuration of sodium?
(a) 2,8
(b) 8,2,1
(c) 2,1,8
(d) 2,8,1.
Answer:
The atomic number of sodium = 11
The electronic configuration of the sodium :
K = 2
L = 8
M = 1
Hence, option (d) is correct.
Question 19. Complete the following table.
Answer:
| Atomic Number | Mass Number | Number of Neutrons | Number of Protons | Number of Electrons | Number of Atomic Species |
| 9 | - | 10 | - | - | - |
| 16 | 32 | - | - | - | Sulphur |
| - | 24 | - | 12 | - | - |
| - | 2 | - | 1 | - | - |
| - | 1 | 0 | 1 | 0 | - |
First row:
atomic number = 9
so, the element is Fluorine.
Atomic no. = No. of protons = no. of electrons = 9
Mass number = no. of protons + no. of neutrons = 9 + 10 = 19
Second row:
Since atomic no. is 16 so, no. of protons = no. of electrons = 16
No. of neutrons = Mass no. - no. of protons = 32 - 16 = 16
Third row:
No. of protons = Atomic no. = 12
So, the element is Magnesium.
No. of electrons = no. of protons = 12
No. of neutrons = Mass no. - no. of protons = 24 - 12 = 12
Fourth row:
No. of protons = Atomic no. = 1
So, the element is Deuterium.
No. of electrons = no. of protons = 1
No. of neutrons = Mass no. - no. of protons = 2 - 1 = 1
Fifth row:
No. of protons = Atomic no. = 1
The element is Protium since the mass number is 1.
So the Table becomes,
| Atomic number | mass number | Number of neutrons | Number of protons | Number of electrons | Name of the element |
| 9 | 19 | 10 | 9 | 9 | Fluorine |
| 16 | 32 | 16 | 16 | 16 | Sulfur |
| 12 | 24 | 12 | 12 | 12 | Magnesium |
| 1 | 2 | 1 | 1 | 1 | Deuterium |
| 1 | 1 | 1 | 1 | 0 | Hydrogen ion |
Practice Questions for Class 9 Science Chapter 4
Some important practice questions related to the class 9 science chapter 4 structure of the atom solutions are given below. These questions have a high probability of being asked in future exams. Students can also refer to notes of this chapter for conceptual clarity.
Question 1: What are the three subatomic particles of an atom?
Answer:
The three subatomic particles are,
Protons (positively charged)
Electrons (negatively charged)
Neutrons (neutral)
Question 2: State two observations from Rutherford’s gold foil experiment.
Answer:
1. Most alpha particles passed straight through the foil, indicating that atoms are mostly empty space.
2. A few alpha particles were deflected or bounced back, suggesting that the positive charge and most of the mass are concentrated in a small, dense nucleus.
Question 3: Define atomic number and mass number.
Answer:
Atomic number (Z)- The number of protons in an atom.
Mass number (A)- The sum of protons and neutrons in an atom.
Mass number = Number of protons + Number of neutrons
Question 4: What are isotopes? Give two examples.
Answer:
Isotopes are atoms of the same element that have the same atomic number but different mass numbers.
Examples,
Hydrogen-Protium $\left({ }^{1} \mathrm{H}\right)$, Deuterium $\left({ }^2 \mathrm{H}\right)$, Tritium $\left({ }^3 \mathrm{H}\right)$
Carbon - ${ }^{12} \mathrm{C}$ and ${ }^{14} \mathrm{C}$
Question 5: Calculate the number of protons, electrons, and neutrons in ${ }^{17} \mathrm{Cl}_{35}$ .
Answer:
Atomic number = 17 $\rightarrow$ Protons = 17, Electrons = 17
Mass number = 35 $\rightarrow$ Neutrons = 35 – 17 = 18
Question 6: Why is an atom electrically neutral even though it contains charged particles?
Answer:
An atom contains positively charged protons and negatively charged electrons.
The number of protons is equal to the number of electrons in a neutral atom.
Because the total positive charge equals the total negative charge, the atom as a whole becomes electrically neutral.
Question 7: The electronic configuration of the outer most shell of the most electronegative element is
(1) $2 s^2 2 p^5$
(2) $3 s^2 3 p^5$
(3) $4 s^2 4 p^5$
(4) $5 s^2 5 p^5$
Answer:
Elements of group 17 has $n s^2 n p^5$ electronic configuration. Electronegativity decreases down the group. The elements of group 17 are the most electronegative.
Hence, the answer is the option (1).
Question 8: The correct electronic configuration of oxygen (O) is?
(1) $1 s^2, 2 s^2, 2 p x^2, 2 p y^2, 2 p z^1$
(2) $1 s^2, 2 s^2, 2 p x^1, 2 p y^2, 2 p z^2$
(3) $1 s^2, 2 s^2, 2 p x^1, 2 p y^1, 2 p z^2$
(4) $1 s^2, 2 s^1, 2 p x^2, 2 p y^2, 2 p z^2$
Answer:
The correct electronic configuration of oxygen (O) is
$1 s^2, 2 s^2, 2 p x^2, 2 p y^2, 2 p z^1$
Hence, the answer is the option (1).
Question 9: Which of the following shape is correct for the given orbital?
(1) s-orbital : Shape → spherical
(2) p-orbital : Shape → dumb-bell
(3) d-orbital : Shape → double dumb-bell
(4) All of them
Answer:
All of them are correct.
Orbitals and their shapes:
s-orbital: Shape → spherical
p-orbital: Shape → dumb-bell
d-orbital: Shape → double dumb-bell
Hence, the answer is the option (4).
Approach to Solve Questions of Class 9 Science Chapter 4
Sometimes structure of the atom class 9 question answer seem very difficult, but once we understand the basic rules and strategy, it becomes very easy to solve all the questions related to that particular topic. Students can follow the steps given below to solve questions of this chapter:
1. Before solving questions it is very important to understand the basics because a clear understanding of topics helps students to answer questions easily. Focus on subatomic particles, atomic number, mass number, isotopes, and isobars.
2. Learn the features, experiments, and limitations of Thomson’s, Rutherford’s, and Bohr’s models. You can also use the diagrams to help visualize the structure. Students can also refer structure of the atom class 9 notes.
3. Learn basic formulas because they helps in solving numerical problems quickly:
Atomic number = Number of protons
Mass number = Number of protons + neutrons
Number of neutrons = Mass number – Atomic number
4. Draw atom models and solve numerical problems that involve atomic/mass number and isotope identification. Solve examples and practice with in-text questions of class 9 science chapter 4 structure of the atom solutions. Make suitable short notes for quick revision. Timely revise your notes. And practice as many questions as asked in previous board exams, and solve mock tests.
Topics and Subtopics Covered in the NCERT Textbook
All the topics and subtopics covered in the structure of the atom class 9 question answer are given below. A basic understanding of these topics helps students to solve complex problems easily.
4.1 Charged Particles in Matter
4.2 The Structure of an Atom
4.2.1 Thomson's Model of an Atom
4.2.2 Rutherford's Model of an Atom
4.2.3 Bohr's Model of an Atom
4.2.4 Neutrons
4.3 How are Electrons Distributed in Different Orbitals(Shells)?
4.4 Valency
4.5 Atomic Number and Mass Number
4.5.1 Atomic number
4.5.2 Mass Number
4.6 Isotopes
4.6.1 Isobars
NCERT Class 9 Science Chapter 4: Important Formulas
Some important formulas used in structure of the atom class 9 question answer are given below. You can memorize and use them during solving questions.
1. The maximum number of electrons in different shells
According to the Bohr model of the atom, the maximum number of electrons that can occupy a shell is given by the formula $2 n^2$, where n represents the shell number. The first shell (n = 1) can hold a maximum of 2 electrons, the second shell (n = 2) can hold a maximum of 8 electrons, and so on.
2. Average atomic mass
Average atomic mass = (M1× P1) + (M2 × P2) + ... + (Mn × Pn)
Where:
M1, M2 ..., and Mn represent the individual atomic masses of the isotopes of the element.
P1, P2 ..., and Pn represent their respective natural abundances as decimal fractions or percentages (converted to decimal form).
What Students Learn from NCERT Solutions for Class 9 Science Chapter 4
- These solutions help students to learn about structure of the atom and its subatomic particles like electrons, protons, and neutrons through a series of solved examples.
- Here various atomic models proposed by different scientists like Thomson, Rutherford, Bohr and their limitations are explained very well. These structure of the atom ncert solutions help students to develop basic conceptual clarity of all these topics.
- Using these solutions students can understand how the modern atomic theory developed over time.
- These class 9 science structure of the atom question answer help them to gain clarity on basic topics like atomic number, mass number, isotopes, and isobars.
Importance of Class 9 Science Chapter 4 Structure of the Atom Solutions
The class 9 science chapter 4 structure of the atom solutions helps students explain how atoms combine, react, and form the world around us. Given below are some points on why these solutions are important:
- This chapter helps students to develop the base for advanced topics like atomic structure, chemical bonding, and periodic classification.
- Students can understand matter and subatomic particles like electrons, protons, and neutrons.
- The class 9 science structure of the atom question answer explains the evolution of different atomic models by scientists like Thomson, Rutherford, and Bohr.
- The arrangement of electrons helps students understand how and why chemical reactions occur.
NCERT Solutions for Class 9 Science Chapter-Wise
Besides NCERT Solutions for Class 9 Science Chapter 4 Structure of The Atom students can also follow NCERT Solutions for Class 9 chapter-wise solutions links:
NCERT Books and NCERT Syllabus
The NCERT books and syllabus links for class 9 are given below:
Frequently Asked Questions (FAQs)
The NCERT Solutions for Class 9 Science Chapter 4 Structure of The Atom include detailed answers to all in-text questions, exercise questions, and exemplar problems given in the textbook.
In a neutral atom, the number of protons equals the number of electrons, which means the positive and negative charges cancel each other out, resulting in no overall charge.
The atomic number is defined as the number of protons in the nucleus of an atom. It is significant because it determines the identity of an element.
Protons and neutrons are located in the nucleus of the atom. Electrons orbit the nucleus in specific energy levels.
Isotopes are atoms of the same element that have the same number of protons but different numbers of neutrons. This means they have different atomic masses. For example, carbon-12 and carbon-14 are both isotopes of carbon, with 6 protons and 6 or 8 neutrons, respectively.
NCERT solutions for Class 9 Science are step by step answers to all the questions given in the NCERT science textbook. They are designed to help students understand concepts clearly.
Class 9 Science Chapter 4 question answers are detailed solutions to all the textbook questions from the chapter Structure of the Atom. They include explanations of concepts, solved numerical problems, and step by step answers that help students understand the chapter thoroughly and prepare well for exams.
A proton carries a positive charge of +1, a neutron has no charge (neutral), and an electron carries a negative charge of -1. The arrangement and balance of these charges in an atom determine its overall charge and chemical stability.
No, electrons are not found in the nucleus. They exist in specific energy levels or "shells" around the nucleus. The arrangement of these electrons in different shells contributes to the chemical properties of the atom, but they remain at a distance from the nucleus due to the attraction and repulsion forces acting between them and the protons.
The atomic model has evolved through various stages, starting from John Dalton's solid sphere model to J.J. Thomson's plum pudding model, followed by Ernest Rutherford's nuclear model and Niels Bohr's planetary model. Each model built upon previous discoveries, leading to the current quantum mechanical model, which describes the behavior of electrons in terms of probabilities rather than fixed orbits.
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
