Class 9 Science Chapter 4 Structure of the Atom Notes cover the discovery of electrons, protons, and neutrons, along with various atomic models like Thomson, Rutherford, and Bohr. They also explain concepts of atomic number, mass number, isotopes, isobars, and electron distribution.
NCERT Class 9 Science Chapter 4 Notes - Structure Of The Atom PDF Notes
What makes up everything around us, from the air we breathe to the buildings and technology we use? The simple answer to this question is atoms. They are the fundamental building blocks of everything around us, from the gold or diamonds used in jewellery to the silicon used in mobile phones. Have you ever picked a grain of sand that is very small in size, and that grain is made up of millions of atoms.
This Story also Contains
- NCERT Notes for Class 9 Science Chapter 4: Download PDF
- NCERT Notes for Class 9 Science Chapter 4
- Previous Year Questions of Class 9 Science Chapter 4
- How to Master Class 9 Science Chapter 4 Structure of the Atom
- Advantages of Using Class 9 Science Chapter 4 Structure of the Atom Notes
- NCERT notes for Class 9 Science Chapter-wise
- NCERT Solutions for Class 9 Science Chapter-wise
- Subject-Wise NCERT Exemplar Solutions
NCERT Class 9 Science Chapter 4 Notes Structure of the Atom covers many important topics like atomic models, isotopes, isobars, etc., that will form the basis for the concepts in the upcoming classes. The NCERT Notes for Class 9 Science is designed to help you memorise the concepts in the easiest way possible. These notes break down complex atomic theory in a simple and engaging way, which makes learning enjoyable and effective. These NCERT notes will help students in their exams and will improve their understanding of the concepts. The formulas and diagrams are also provided for effective learning. Students can also refer to the NCERT Solutions for understanding the concepts of this chapter better with the help of solved questions.
NCERT Notes for Class 9 Science Chapter 4: Download PDF
Download the Structure of the Atom Class 9 Science Chapter 4 CBSE notes PDF to access a clear explanation of this chapter. These NCERT notes for Class 9 cover all the key concepts of this chapter. You can download the PDF from the button given below:
Also read
NCERT Notes for Class 9 Science Chapter 4
These notes will work as a key guide for understanding the concepts deeply. Here, we will look into various experiments explaining the characteristic features of an atom. Class 9 Science Chapter 4 Structure of the Atom Notes will help you build a strong command of the topics.
Atoms and molecules are the building blocks of matter. The existence of different kinds of matter around us is due to the different types of atoms and molecules present in them. For a long time, it was thought that the atoms were indivisible, so they did not have an inner structure. But now we know that atoms are divisible and they do have an inner structure. Atoms have smaller particles in them which are called subatomic particles.
4.1 Charged Particles in Matter
Initially, atoms were considered indivisible, but later on, it was formulated that they are made up of subatomic particles that are negatively charged, and they were called electrons. Those were discovered by J.J. Thomson. Detailed explanations of such discoveries are given in the Structure of the Atom Class 9 Science notes, helping students understand the structure of atoms.
- E. Goldstein in 1886 found out that there were some radiations present in atoms that were termed as canal rays, and it was concluded later that two kinds of sub-atomic particles exist: electrons and protons.
- Protons have the same magnitude but opposite charge as compared to electrons, and protons have 2000 times more mass than electrons.
4.2 The Structure of an Atom
After the discovery of electrons and protons, the scientists started thinking of arranging these particles in an atom; therefore, different models were proposed to explain the distribution of subatomic particles in an atom.
The first simple model was proposed by JJ Thomson, which is known as the Thomson atomic model.
4.2.1 THOMSON’S MODEL OF AN ATOM
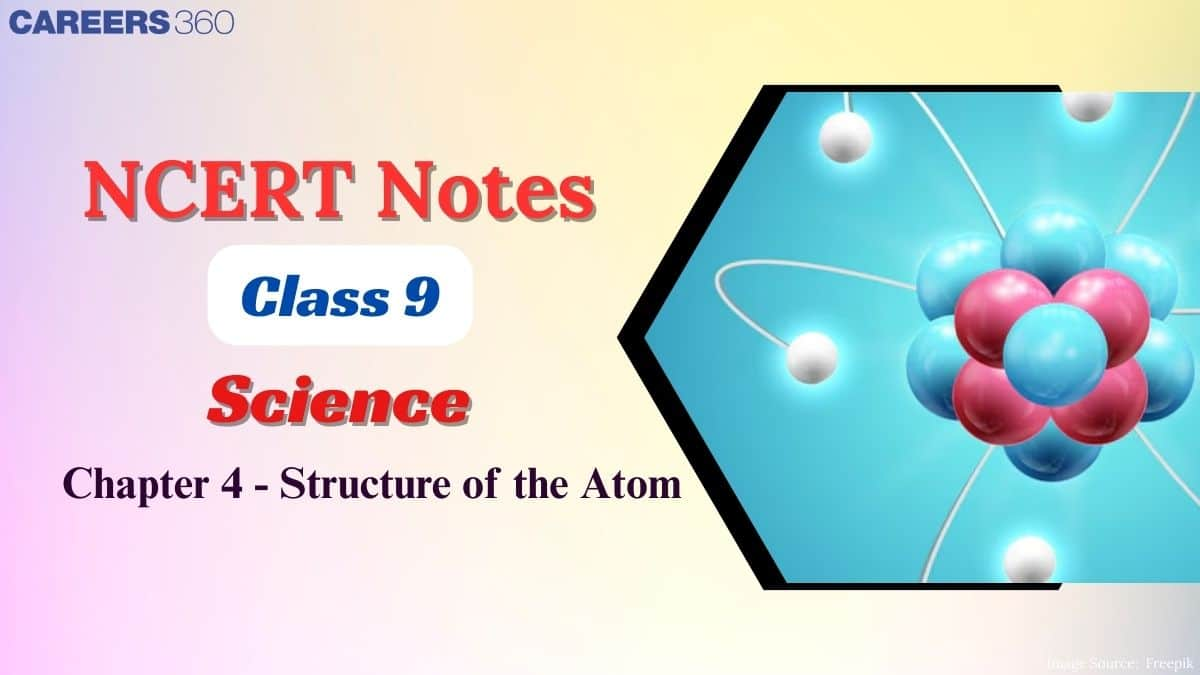
JJ Thomson proposed that an atom consists of a uniform sphere in which positive charge is uniformly distributed.
- The electrons are embedded into it in such a way as to give the most stable atom.
- This model was much like pudding or cake because he assumed a pudding as a positive charge and the resins inside as electrons embedded into it.
- This model was also compared with the watermelon model, which has a positive charge in which seeds (electrons) are embedded.
- An important feature of this model is that the mass of the atom is considered to be evenly spread over the atom.
This model explains the neutrality of the atom however, it was soon discarded when other scientists' experimental results raised some questions that were not answered by this model.
4.2.2 RUTHERFORD’S MODEL OF AN ATOM
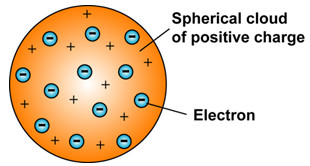
In 1909, Rutherford and a student conducted a series of experiments to better understand how electrons and protons are arranged in an atom.
To detect alpha particles, a high-energy particle beam was aimed at a thin gold foil with a thickness of around 100 nm.
Observation
-
Through the gold foil, the majority of the alpha particles remained undeflected.
-
A small percentage of alpha particles were discovered to have been deflected at modest angles.
-
A small percentage of particles did not travel through the foil at all, but instead suffered significant deflection or even returned after 180° deflection.
Because the majority of the alpha particles went through the gold foil undeflected, Rutherford concluded that there must be a lot of free space within the atom. Since some of the alpha particles deflected to certain angles, it means that there is a heavily positively charged mass present in the atom, and because just a few particles experienced large deflections, this mass must be occupying a very limited space within the atom.
Main Features of Rutherford's Model
The following were the main features of the model of the Rutherford
-
The whole mass and positive charge of an atom are concentrated in a very tiny region at the core known as the nucleus; nonetheless, the volume occupied by the nucleus is negligibly small when compared to the overall volume of the atom.
-
Protons are responsible for the nucleus's positive charge.
-
The mass of the nucleus comes from protons and other neutral particles, each of which has a mass that is almost equivalent to that of a proton.
-
The structure is electrically neutral because the nucleus is surrounded by negatively charged electrons that balance the positive charge on the nucleus.
-
Electrons are not static; they spin around the nucleus at a rapid rate, similar to how planets orbit the sun.
-
The electrostatic force of attraction holds electrons and the nucleus together.
This atomic model failed to explain the stability of atoms.
Drawbacks of the Rutherford Model
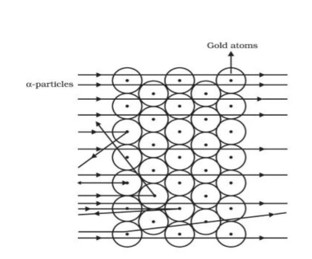
Electrons revolve around the positively charged nucleus, according to the Rutherford atom model. However, in the long run, it is not conceivable since atoms are stable, whereas any particle in a circular orbit will accelerate.
Charged particles would radiate energy as they accelerated. As a revolving electron loses energy and eventually falls into the nucleus, this model fails to explain atomic stability.
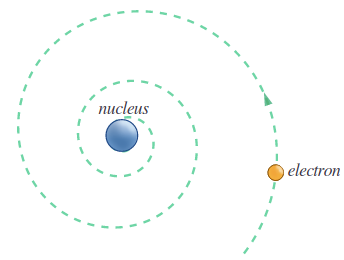
4.2.3 BOHR’S MODEL OF ATOM
To correct the drawbacks of Rutherford, Bohr came up with another model of an atom. The following were the postulates of Bohr’s atomic model
1. Only certain orbits known as discrete orbits are allowed inside the atom
2. While revolving in these discrete orbits, electrons do not radiate energy.
These orbits or shells are known as energy levels.These are names as K(n=1), M(n=2), O(n=3), P(n=4)...
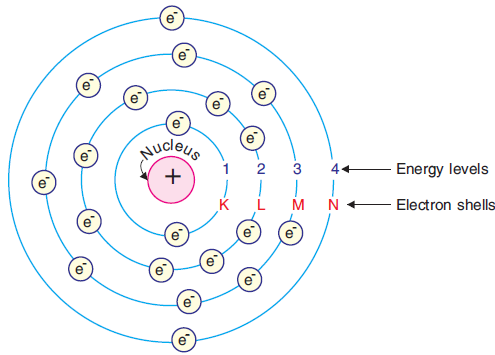
4.2.4 NEUTRONS
In 1932, J. Chadwick discovered another subatomic particle called a neutron. A neutron does not have any charge, and its mass is equal to that of a proton. It was suspected that the nucleus has protons and neutrons, and as neutrons are chargeless, that’s why the charge of the nucleus was concluded to be positive. Neutron is represented by 'n', and the mass of an atom is given by the sum of neutrons and protons. You can also download the Class 9 Science Chapter 4 Notes to revise such concepts anytime and anywhere.
4.3 How are Electrons Distributed in Different Orbits (Shells)?
Lets find out how electrons are filled in different orbits;
The dispersion of electrons into different orbits of an atom was proposed by Bohr and Bury. They proposed a set of principles that must be observed when filling a shell with electrons. The number of electrons in different energy levels or shells is written using the following rules:
-
The formula $2 n^2$ calculates the maximum number of electrons in a shell, where 'n' is the orbit number or energy level index. The values of n are 1,2,3,...
As a result, the maximum number of electrons that can be present in each shell is:
-
The first orbit, often known as the K-shell, will be = 2 x 1 = 2. The second orbit, often known as the L-shell, will be = 2*4=8. The third orbit, often known as the M-shell, will be 2 * 9= 18. The fourth orbit, often known as the N-shell, will be 2*16 = 32, and so on.
-
In the outermost orbit, a maximum of eight electrons can be supported.
-
The inner shells must be filled before electrons may be accommodated in a given shell. That is to say, the shells are filled one at a time.
4.4 Valency
The electrons that are present in the outermost shell are known as valence electrons.
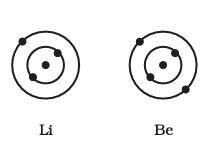
If you take the example of magnesium, which has an atomic number of 12, its configuration is 2,8,2 it will be easier for magnesium to lose two electrons to gain stability.
All the elements in which the number of electrons in the outermost shell is less than 8 are chemically reactive. They share, gain, or lose an electron to complete their octet and completing their octet provides them stability. This combining capacity of the elements to complete their octet is known as their valency.
4.5 Atomic Number and Mass Number
4.5.1 ATOMIC NUMBER
The atomic number of an element is described by the total number of protons present in its nucleus. It is represented by Z, the number of protons(p) is equal to the number of electrons(e) in an atom.
4.5.2 Mass number
The sum of the neutrons and protons is known as the mass number. The total mass of the atom is equal to the mass of the nucleus, and neutrons and protons are present in the nucleus; both these subatomic particles together are known as nucleons. The mass number is represented by A.
Generally, an atom is represented by its symbol, for the element's atomic number is written on the lower side of the symbol and the mass number is written on the upper side. Students can understand the concepts of this chapter more clearly with the help of the NCERT Solutions for Class 9 Science Chapter 4 Structure of Atom.
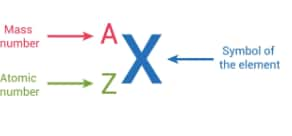
4.6 Isotopes
Atoms of the same element that have the same atomic number but different mass numbers are called isotopes.
Example- (i) carbon, ${ }_6^{12} \mathrm{C}$ and ${ }_6^{14} \mathrm{C}$, (ii) chlorine, ${ }_{17}^{35} \mathrm{Cl}$ and ${ }_{17}^{37} \mathrm{Cl}$, etc.
Similarly, chlorine has 2 isotopes having the same atomic number Z = 17, whereas their mass numbers are 35 and 37. When we take the mass number of chlorine, then we take an average of these 2 numbers.
The isotopes of a given element will show the same chemical properties.
4.6.1 ISOBARS
Atoms of different elements having the same mass number but different atomic numbers are called isobars.
These atoms differ in their atomic number and therefore they have a different number of protons or electrons, and also a different number of neutrons.
For example, if you take the case of argon, potassium, and calcium, all of them have mass numbers 40, but their atomic numbers are 18, 19, and 20, respectively. Isobars are atoms of different elements and hence they have different properties.
Previous Year Questions of Class 9 Science Chapter 4
By practising previous year questions on the structure of the atom, students can easily identify the important topics and commonly asked concepts, which makes their revision more effective. They can refer to the NCERT Class 9 Science Chapter 4 Notes Structure of the Atom for understanding the concepts used to solve questions.
Question 1: Which of the following statements about the atom is correct according to Bohr's model?
(1) Electrons radiate energy while revolving in orbits
(2) Electrons move in random paths
(3) Electrons revolve in fixed energy levels without radiating energy
(4) Nucleus revolves around electrons
Answer:
According to Bohr, electrons move in specific circular orbits (energy levels) where they do not lose energy. Energy is absorbed or emitted only when an electron jumps between orbits.
Hence, the correct answer is option (3).
Question 2: The mass of an electron is approximately:
(1) Equal to that of a proton
(2) 1/1837 times the mass of a proton
(3) Twice the mass of a proton
(4) Zero
Answer:
An electron is much lighter than a proton. Its mass is nearly 1/1837 of a proton, which is why the mass of an atom is concentrated mostly in its nucleus.
Hence, the correct answer is option (2).
Question 3: Which of the following particles is not present in the nucleus of an atom?
(1) Proton
(2) Neutron
(3) Electron
(4) Both A and B
Answer:
Electrons revolve around the nucleus in shells; only protons and neutrons are present inside the nucleus.
Hence, the correct answer is option (3).
Question 4: Which of the following is correct about orbit?
(1) The maximum no. of electrons in an orbit is 2n2 where n stands for no. of orbit.
(2) Orbits are circular in shape.
(3) It represents planar motion of electron
(4) All of them
Answer:
All of them are correct.
Orbits:
The maximum no. of electrons in an orbit is 2n2 where n stands for no. of orbit.
Orbits are circular in shape.
It represents the planar motion of the electron
Hence, the answer is the option (4).
Question 5: d - orbital can accommodate a maximum no. of ____ electrons.
(1) 12
(2) 5
(3) 14
(4) 10
Answer:
d - orbital can accommodate a maximum no. of 10 electrons.
Hence, the answer is the option (4).
How to Master Class 9 Science Chapter 4 Structure of the Atom
Structure of atoms explains how atoms are structured, the discovery of subatomic particles, and the evolution of different atomic models. Students can master this chapter by referring to the NCERT notes Class 9 Science Chapter 4 Structure of the Atom.
- Start this chapter by understanding the atomic models and theories like Dalton's atomic theory, Thomson's model, Rutherford's experiment and Bohr’s model.
- Then learn about subatomic particles and the properties of electrons, protons, and neutrons.
- Try to learn the relative charge, mass, and position in the atom.
- Questions related to Atomic Number, Mass Number and Isotopes are often asked in exams. Refer to the Structure of the Atom Class 9 Science Chapter 4 CBSE notes for better understanding.
- Then learn about the rules of electron distribution and how to write valence based on outer shell electrons.
- Solve numerical problems on atomic number, mass number, and isotopes.
Advantages of Using Class 9 Science Chapter 4 Structure of the Atom Notes
NCERT notes Class 9 Science Chapter 4 Structure of the Atom help students understand the basic models and composition of atoms. Given below some points on advantages of these notes:
- Students can use these notes to understand the concepts like atomic models by Thomson, Rutherford, Bohr, atomic number, mass number, valency, isotopes, and isobars.
- These notes are prepared in a systematic manner that help students understand the structure and behaviour of subatomic particles.
- The Structure of the Atom Class 9 Science Chapter 4 CBSE notes are prepared by subject experts in a very clear and comprehensive manner that are helpful for both boards and competitive exams.
- These notes cover all the topics from the NCERT book that helps students to understand the concepts easily.
NCERT notes for Class 9 Science Chapter-wise
Along with the Class 9 Science Chapter 4 Structure of the Atom Notes, students can also refer to other Class 9 chapter notes from the links given below
NCERT Solutions for Class 9 Science Chapter-wise
Besides NCERT notes Class 9 Science Chapter 4 Structure of the Atom students can also follow Class 9 chapter-wise solutions of NCERT:
Subject-Wise NCERT Exemplar Solutions
The links below will give you NCERT exemplar solutions that can help you excel in your exam preparations.
Frequently Asked Questions (FAQs)
Understanding the Structure of the Atom Class 9 Science Chapter 4 CBSE notes is essential for students because it lays the foundation for concepts in chemistry and physics. It helps in comprehending how different elements interact, the nature of chemical reactions, and the basis for the periodic table of elements.
According to classical electromagnetic theory, any charged particle (like an electron) in accelerated motion (like orbiting the nucleus) should radiate energy and continuously lose it. This would cause the electron to spiral inwards and eventually fall into the nucleus, making the atom unstable. But atoms are known to be stable.
The main components of an atom are protons, neutrons, and electrons. Protons carry a positive charge and are found in the nucleus, neutrons are neutral particles also located in the nucleus, and electrons are negatively charged particles that orbit around the nucleus.
The atomic number of an element is the number of protons in its nucleus. It determines the element's identity and its position in the periodic table. For example, hydrogen has an atomic number of 1, meaning it has one proton.
Isotopes are variants of the same element that have the same number of protons but different numbers of neutrons. This difference in neutrons results in different atomic masses. For example, carbon-12 and carbon-14 are isotopes of carbon.
Electronic configuration refers to the distribution of electrons in the various energy levels or shells of an atom. It can be written using the Aufbau principle, Hund's rule, and the Pauli exclusion principle to describe how electrons fill the orbitals.
Neutrons play a crucial role in stabilizing the nucleus of an atom. They help mitigate the repulsive forces between positively charged protons. Without neutrons, the protons would repel each other due to their like charges.
Elements consist of a single type of atom and cannot be broken down into simpler substances. Compounds are formed when two or more different elements chemically combine in fixed proportions. Mixtures are combinations of two or more substances that retain their individual properties and can be separated physically.
The atomic number of carbon is 6 and the atomic number of sodium is 11. So the distribution of electrons in the carbon atom is 6 = 2,4 Distribution of electrons in the sodium atom is 11 = 2,8,1.
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
