NCERT Exemplar Class 9 Science Solutions Chapter 3 Atoms and Molecules
Do you know what everything around us are made of ? Like the air we breathe, the phone we are currently using to read this article, or the food we eat daily to quench our appetite. The answer to all these questions lies in NCERT Exemplar Class 9 Science Chapter 3 Atoms and Molecules. Everything in our environment, from the tiniest grain of sand to the vast universe, is made up of atoms and molecules. Atoms are the smallest unit of matter, while molecules are made up of a combination of two or more atoms, and they are so small that we cannot see them with the naked eye; to see them, we require a high-end microscope. Even a small drop of water contains countless molecules.
This Story also Contains
- NCERT Exemplar Class 9 Science Solutions Chapter 3 (MCQ)
- NCERT Exemplar Class 9 Science Solutions Chapter 3 (Short Answer)
- NCERT Exemplar Class 9 Science Solutions Chapter 3 (Long Answer)
- Important Question From NCERT Exemplar Class 9 Science Chapter 3
- Approach to Solve Class 9 Science Chapter 3 Questions
- NCERT Exemplar Class 9 Science Chapter 3 Topics:
- Advantages of Using Class 9 Science NCERT Exemplar Chapter 3 Atoms and Molecules Solutions
- NCERT Class 9 Science Exemplar Solutions Chapter-wise
- NCERT Solutions for Class 9 chapter-wise
- NCERT Notes & Solutions subject-wise
- NCERT Books and NCERT Syllabus

These NCERT Exemplar Solutions Class 9 Science provides systematic explanations of Atoms and Molecules that form the basis of chemistry. The articles feature Exemplar questions and their step-by-step explanations that help students grasp the concept, and excel in board and competitive exams. This complete set of NCERT exemplar solutions serves as a tool to improve the problem-solving abilities of students and consequently improve their academic performance. Students can also check NCERT Solutions to all questions chapter-wise.
NCERT Exemplar Class 9 Science Solutions Chapter 3 (MCQ)
At first, the MCQ questions are covered in the NCERT Exemplar Class 9 Science Chapter 3 Atoms and Molecules to enhance your knowledge. These questions help students test their conceptual understanding and prepare effectively for exams.
Question 1. Which of the following correctly represents 360 g of water?
(i) 2 moles of $H_{2}O$
(ii) 20 moles of water
(iii) $6.022 \times 10^{23}$ molecules of water
(iv) $1.2044\times10^{25}$ molecules of water
(a) (i)
(b) (i) and (iv)
(c) (ii) and (iii)
(d) (ii) and (iv)
Answer: D
The average mass of one mole of $H_{2}O$ is 18.02 grams.
The molecular weight of water is 18 g/mol. Hence, one mole of water weighs 18 g.
One mole of water contains $6.023 \times 10^{23}$ water molecules.
2 moles of $H_{2}O$ is $36 g (2 \times 18 g)$ and 20 moles of water is $360g (20 \times 18 g)$. The mass of $6.022 \times 10^{23}$ molecules of water is 18g.
Therefore, the mass of $1.2044\times10^{25}$ molecules ($= 20 \times 6.022 \times10^{23}$ molecules) of water is $20 \times 18g = 360g$.
Question 2. Which of the following statements is not true about an atom?
(a) Atoms are not able to exist independently
(b) Atoms are the basic units from which molecules and ions are formed
(c) Atoms are always neutral in nature
(d) Atoms aggregate in large numbers to form the matter that we can see, feel or touch
Answer: A
Atoms are not able to exist independently is not true, Atoms of most elements are not able to exist independently but inert gases can exists as atoms.
Question 3. The chemical symbol for nitrogen gas is
(a) Ni
(b) $N_{2}$
(c) $N^{+}$
(d) N
Answer: B
Chemical formula of Nitrogen is N but nitrogen exist as a molecule of two atoms. Therefore, the chemical symbol of nitrogen gas is $N_{2}$
Question 4. The chemical symbol for sodium is
(a) So
(b) Sd
(c) NA
(d) Na
Answer: D
Sodium word is derived from Latin word Natrium hence the chemical name of sodium is Na.
Question 5. Which of the following would weigh the highest?
(a) 0.2 mole of sucrose $(C_{12} H_{22} O_{11})$
(b) 2 moles of $CO_{2}$
(c) 2 moles of $CaCO_{3}$
(d) 10 moles of $H_{2}O$
Answer: C
From mole concept,
1 mole = molecular weight in gram.
Weight of a sample in gram = Number of moles × Molar mass
Mass of 1 mole of sucrose $(12 \times 12) + (1 \times 22) + (16 \times 11) = 342 g$
Mass of 1 mole of $CO_{2} = 12 + (16 \times 2) = 44g$
Mass of 1 mole of $CaCO_{3} = 40 + 12 + (16 \times 3) = 100g$
Mass of 1 mole of $H_{2}O = 2 + 16 = 18g$
(a) 0.2 moles of $C_{12} H_{22} O_{11} = 0.2 \times 342 = 68.4 g$
(b) 2 moles of $CO_{2}$ is $2 \times 44 = 88 g$
(c) 2 moles of $CaCO_{3} = 2 \times 100 = 200 g$
(d) 10 moles of $H_{2}O = 10 \times 18 = 180 g$
Question 6. Which of the following has maximum number of atoms?
(a) 18g of $H_{2}O$
(b) 18g of $O_{2}$
(c) 18g of $CO_{2}$
(d) 18g of $CH_{4}$
Answer: D
$N_{A} = 6.023 \times 10^{23}$
(a) 18 g of water $=18 \times \frac{3}{18}\times N_{A} = 3 N_{A}$
(b) 18 g of oxygen = $18 \times \frac{3}{32} \times N_{A} = 1.12 N _{A}$
(c) 18 g of $CO_{2} = 18 \times \frac{3}{44} \times N_{A} = 1.23 N_{A}$
(d) 18 g of $CH_{4} =18\times \frac{5}{16}\times N_{A} = 5.63 N_{A}$
Question 7. Which of the following contains maximum number of molecules?
(a) 1g $CO_{2}$
(b) 1g $N_{2}$
(c) 1g $H_{2}$
(d) 1g $CH_{4}$
Answer: C
Molar mass of other molecules are much higher than given mass, so number of molecules in them will be less than that in hydrogen.
1 g of $H_{2} = \frac{1}{2} \times N_{A}$
$= 0.5 N_{A}$
$= 0.5 \times 6.022 \times 10^{23}$
$= 3.011 \times 10^{23}$
Question 8. Mass of one atom of oxygen is
(a) $\frac{16}{6.023\times10^{23}}g$
(b) $\frac{32}{6.023\times10^{23}}g$
(c) $\frac{1}{6.023\times10^{23}}g$
(d) 8u
Answer:
Mass of one atom of oxygen $=\frac{Atomic \;mass}{N_{A}}$
$=\frac{16}{6.023\times10^{23}}g$
Question 9. 3.42 g of sucrose are dissolved in 18g of water in a beaker. The number of oxygen atoms in the solution are
(a) $6.68\times 10^{23}$
(b) $6.09\times 10^{22}$
(c) $6.022\times 10^{23}$
(d) $6.022\times 10^{21}$
Answer: A
1 mol of sucrose $( C_{12}H_{22}O_{11})$ contains $= 11\times N_{A}$ atoms of oxygen,
Here, $N_{A} = 6.023\times10^{23}$
1 mol of sucrose $( C_{12}H_{22}O_{11})$ contains $= 0.01 \times 11 \times N_{A}$ atoms of oxygen
$= 0.11\times N_{A}$$=0.11\times N_{A}$ atoms of oxygen
$=18 g/(1\times2+16)$gmol-1
= 1mol
1mol of water $(H_{2}O)$contains $1 \times N_{A}$ atom of oxygen
Total number of oxygen atoms = Number of oxygen atoms from sucrose + Number of oxygen atoms from water
$= 0.11 N_{A} + 1.0 N_{A}$
$?????=1.11 N_{A}$$=1.11 N_{A}$
Number of oxygen atoms in solution
$=1.11 \times$ Avogadro’s number
$=1.11 \times 6.022 \times 10^{23}=6.68\times 10^{23}$
Question 10. A change in the physical state can be brought about
(a) only when energy is given to the system
(b) only when energy is taken out from the system
(c) when energy is either given to, or taken out from the system
(d) without any energy change
Answer: C
A change in the physical state can be brought about when energy is either given to, or taken out from the system. When a solid change into liquid, it absorbs energy. It is because, energy change helps in changing the magnitude of attraction forces between the particles, thus helps in changing the physical states (i.e., solid, liquid, gas) of matter. When a liquid change into solid, it releases energy.
NCERT Exemplar Class 9 Science Solutions Chapter 3 (Short Answer)
Here some short answer type questions from Class 9 Science NCERT Exemplar Chapter 3 Atoms and Molecules are given for practice. This section contains important questions that are asked in the exams.Practice short answer types from the questions below.
Question 11. Which of the following represents a correct chemical formula? Name it.
(a) CaCl
(b) $BiPO_{4}$
(c) $NaSO_{4}$
(d) NaS
Answer: B
The correct formula is CaCl2 (valency of Ca = 2, valency of Cl = 1).
The correct formula is $Na_{2}SO_{4}$ (valency of Na = 1, Valency of $SO_{4} = 2$).
The correct formula is $Na_{2}S$ (valency of Na = 1, valency of sulphide = 2).
$BiPO_{4}$, is the correct formula, its name is bismuth phosphate. Bismuth phosphate is right because both ions are trivalent.
Question 12. Write the molecular formulae for the following compounds
(a) Copper (II) bromide
(b) Aluminium (III) nitrate
(c) Calcium (II) phosphate
(d) Iron (III) sulphide
(e) Mercury (II) chloride
(f) Magnesium (II) acetate
Answer:
(a) Copper(II) Bromide – $CuBr_{2}$
(b) Aluminium(III) nitrate – $Al (NO_{3})_{3}$
(c) Calcium (II) phosphate – $Ca_{3}(PO_{4})_{2}$
(d) Iron(III) sulphide – $Fe_{2}S_{3}$
(e) Mercury(II) chloride – $HgCl_{2}$
(f) Magnesium(II) acetate – $Mg(CH_{3}COO)_{2}$
Question 13. Write the molecular formulae of all the compounds that can be formed by the combination of following ions
$Cu^{2+}, Na^{+}, Fe^{3+}, Cl^{-}, SO_{4}^{2-} , PO_{4}^{3-}$
Answer:
$CuCl_{2}/ CuSO_{4} / Cu_{3} (PO_{4})_{2}$
$NaCl/ Na_{2}SO_{4}/ Na_{3} PO_{4}$
$FeCl_{3}/ Fe_{2} (SO_{4})_{3} / FePO_{4}$
Question 14. Write the cations and anions present (if any) in the following compounds
(a) $CH_{3}COONa$
(b) $NaCl$
(c) $H_{2}$
(d) $NH_{4}NO_{3}$
Answer:
Anions Cations
(a) $CH_{3} COO^{-}$ $Na^{+}$
(b) $Cl^{-}$ $Na^{+}$
(c) It is a covalent compound
(d) $NO_{3}^{-}$ $NH_{4}^{+}$
Question 15. Give the formulae of the compounds formed from the following sets of elements
(a) Calcium and fluorine
(b) Hydrogen and Sulphur
(c) Nitrogen and hydrogen
(d) Carbon and chlorine
(e) Sodium and oxygen
(f) Carbon and oxygen
Answer:
(a) Calcium and fluoride - Calcium Fluoride $(CaF_{2})$
(b) Hydrogen and sulphur - Hydrogen Sulphide $(H_{2}S)$
(c) Nitrogen and hydrogen - Ammonia $(NH_{3})$
(d) Carbon and chlorine - Carbon Tetrachloride $(CCl_{4})$
(e) Sodium and oxygen - Sodium Oxide $(Na_{2}O)$
(f) Carbon and oxygen - Carbon dioxide $(CO_{2})$; Carbon Monoxide (CO)
Question 16. Which of the following symbols of elements are incorrect? Give their correct symbols
(a) Cobalt CO
(b) Carbon c
(c) Aluminum AL
(d) Helium He
(e) Sodium So
Answer:
(a) Incorrect, the correct symbol of cobalt is Co
(b) Incorrect, the correct symbol of carbon is C
(c) Incorrect, the correct symbol of aluminum is Al
(d) Correct (He)
(e) Incorrect, the correct symbol of sodium is Na
Answer:
(a) $NH_{3}$
$N:H\times3$
$14:1\times3$
(b) $CO$
$C:O$
$12:16$
$3:4$
(c) $HCl$
$H:Cl$
$1:35.5$
$2:71$
(d) $AlF_{3}$
$Al:F\times3$
$27:19\times3$
$9:19$
(e) $MgS$
$3:4$
Question 18. State the number of atoms present in each of the following chemical species
(a) $CO_{3}^{2-}$
(b) $PO_{4}^{3-}$
(c) $P_2O_5$
(d) $CO$
Answer:
(a) $CO_{3}^{2-} = 1C + 3(O) = 4$
(b) $PO_{4}^{3-} = 1P + 4(O) = 5$
(c) $P_{2}O_{5} = 2P + 5(O) = 7$
(d) CO = 1C + 1(O) = 2
Question 19. What is the fraction of the mass of water due to neutrons?
Answer:
In water molecule $(H_{2}O)$, number of neutrons = [(number of neutrons in H) $\times2+$ (number of neutrons in O)] $= 0\times 2 + 8 = 8$(as number of
neutrons in H = 0)
Mass of 8 neutrons $= 8\times 1.00893 = 8.07$ (mass of one neutron = 1.008934)
Molar mass of water $= 1.008\times 2 + 16.0 = 18.016 u$
There are 8 neutrons in one atom of oxygen
Mass of 8 neutrons$=\frac{8}{N_{A}} g$
Fraction of mass of water due to neutrons = $\frac{8}{18}$
Question 20. Does the solubility of a substance change with temperature? Explain with the help of an example.
Answer:
Yes, the solubility of a substance depends on its temperature. The solubility of solids in liquids usually increases on increasing the temperature and decreases on decreasing the temperature. The solubility of gases in liquids usually decreases on increasing the temperature and increases on decreasing the temperature.
For example, one can dissolve more sugar in hot water than in cold water.
Question 21. Classify each of the following on the basis of their atomicity.
(a) $F_{2}$
(b) $NO_{2}$
(c) $N_{2}O$
(d) $C_{2}H_{6}$
(e) $P_{4}$
(f) $H_{2}O_{2}$
(g) $P_{4}O_{10}$
(h) $O_{3}$
(i) HCl
(j) $CH_{4}$
(k) He
(l) Ag
Answer:
(a) Diatomic (2 atoms)
(b) Triatomic (3 atoms)
(c) Triatomic (3 atoms)
(d) Polyatomic (8 atoms)
(e) Polyatomic (4 atoms)
(f) Polyatomic (4 atoms)
(g) Polyatomic (14 atoms)
(h) Triatomic (3 atoms)
(i) Diatomic (2 atoms)
(j) Polyatomic (5 atoms)
(k) Monoatomic (1 atom)
(l) Monoatomic (1 atom)
Answer.
There are many ways by which we can differentiate between sugar and salt without testing. We can identify the powder by heating. The powder will char if it is a sugar. Dissolve salt and sugar separately in alcohol, sugar will be dissolved in it while salt will not be dissolved. the powder can be dissolved in water and checked for electrical conductivity. If it conducts, then the powder is salt.
Question 23. Calculate the number of moles of magnesium present in a magnesium ribbon weighing 12 g. Molar atomic mass of
magnesium is $24\;g \;mol^{-1}$.
Answer:
Weight of a sample in grant = Number of moles × Molar mass
Atomic mass of Mg $= 24 g mol^{-1}$
24 g of Mg = 1 mol
12 g of Mg = 12/24 = 0.5 mol
NCERT Exemplar Class 9 Science Solutions Chapter 3 (Long Answer)
The following are the long-answer type questions that needs more practice. These NCERT Exemplar Class 9 Science Chapter 3 Atoms and Molecules important questions are frequently asked in the exams. Long-answer type questions are covered to improve your subject knowledge and conceptual thinking:
Question 24. Verify by calculating that
(a) 5 moles of $CO_{{2}}$ and 5 moles of $H_{2}O$ do not have the same mass.
(b) 240 g of calcium and 240 g magnesium elements have a mole ratio of $3:5$.
Answer:
(a) $CO_{2}$ has molar mass $= 44g mol^{-1}$
5 moles of $CO_{2}$ have molar mass $= 44 \times 5 = 220 g$
$H_{2}O$ has molar mass $= 18 g mol^{-1}$
5 moles of $H_{2}O$ have mass $= 18 \times 5 g = 90 g$
Hence, they do not have same mass.
(b) Number of moles in 240g Ca metal $=\frac{240}{40} = 6$
Number of moles in 240g of Mg metal $=\frac{240}{24} = 10$
Ratio $6:10 = 3: 5$
Question 25. Find the ratio by mass of the combining elements in the following compounds. (You may use Appendix-III)
(a) $CaCO_{3}$ (d) $C_{2}H_{5}OH$
(b) $MgCl_{2}$ (e) $NH_{3}$
(c) $H_{2}SO_{4}$ (f) $Ca(OH)_{2}$
Answer:
(a) $CaCO3 \rightarrow Ca: C : O = 40 : 12 : 48 = 10 : 3 : 12$
(b) $MgCl_{2} \rightarrow Mg : Cl = 24 : 2 \times 35.5 = 24 : 71$
(c) $H_{2}SO_{4} \rightarrow H : S : O = 2 \times 1 : 32 : 4 \times 16 = 2 : 32 : 64 = 1 : 16 : 32$
(d) $C_{2} H_{5} OH \rightarrow C : H : O = 2 \times 12 : 6 \times 1 : 16 = 24 : 6 : 16 = 12 : 3: 8$
(e) $NH_{3}\rightarrow N : H = 14 : 3\times 1 = 14 : 3$
(f) $Ca (OH)_{2} \rightarrow Ca : O : H = 40 : 2 \times 16 : 2 \times 1 = 40 : 32 : 2 = 20 : 16 : 1$
Question 26. Calcium chloride when dissolved in water dissociates into its ions according to the following equation.
$CaCl_{2} (aq) \rightarrow Ca^2+ (aq) + 2Cl^{-} (aq)$
Calculate the number of ions obtained from $CaCl_{2}$ when 222 g of it is dissolved in water.
Answer:
$111g(1mole)\rightarrow 1mole+2moles$
$222g(2mole)\rightarrow 2moles+2moles$
Total number of ions by 2 moles of $CaCl_{2} = 6$
Number of ions = Number of moles of ions $\times N_{A}$
$=6\times 6.023\times 10^{23}$
$=3.6132\times 10^{24}$ ions
Question 27. The difference in the mass of 100 moles each of sodium atoms and sodium ions is 5.48002 g. Compute the mass of an electron.
Answer:
Number of electrons in Na atom = 11
Number of electrons in $Na^{+} = 10$
For 1 mole of Na atom and Na+ the difference in electrons = 1 mole
For 100 moles of Na atoms and Na+ ions the difference = 100 moles of electrons
Mass of 100 moles of electrons = 5.48002 g
Mass of 1 mole of electrons $=\frac{5.48002}{100}g$
Mass of one electron $=\frac{5.48002}{100\times6.022\times10^{23}}g$
$=9.1\times10^{-28}g$
$=9.1\times 10^{-31}kg$
Answer:
Molar mass of HgS $=200.6+32=232.6gmol^{-1}$
Mass of Hg in 232.6g of HgS =200.6g
Mass of Hg in 225g of HgS $=\frac{200.6}{232.6}\times225$
$=194.04g$
One way of srews weigh $=2.475\times 10^{24}g$
$=2.475\times 10^{21}kg$
$\frac{Mass \;of\; the\; earth}{Mass\; of\; one\; mole\; of\; screws}=\frac{5.98\times 10^{24}}{2.475\times 10^{21}}=2.4\times 10^{3}$
Mass of earth is $2.4 \times 10^{3}$ times the mass of screws. The earth is 2400 times heavier than one mole of screws
Answer:
1 mole of oxygen atoms $= 6.023 \times 10^{23} atoms$
∴ Number of moles of oxygen atoms $=\frac{2.58\times 10^{24}}{6.023\times 10^{23}}$
$=4.28moles$
Question 31. Raunak took 5 moles of carbon atoms in a container and Krish also took 5 moles of sodium atoms in another container of same weight. (a) Whose container is heavier? (b) Whose container has more number of atoms?
Answer:
(a) Mass of sodium atoms carried by Krish $= (5 \times 23) g = 115 g$
While mass of carbon atom carried by Raunak $= (5 \times12) g = 60g$
Thus, Krish’s container is heavy.
(b) As the two bags have the same number of moles of atoms, both the bags will have the same number of atoms.
Question 32. Fill in the missing data in the Table
Answer.
Question 33. The visible universe is estimated to contain $10^{22}$ stars. How many moles of stars are present in the visible universe?
Answer:
1 mole of stars equals $6.023\times10^{23}$
Number of moles of stars $=\frac{10^{22}}{6.022\times 10^{23}}$
Number of moles of stars = 0.0166
Question 34. What is the SI prefix for each of the following multiples and submultiples of a unit?
(a) $10^{3}$ (b) $10^{-1}$ (c) $10^{-2}$ (d) $10^{-6}$
(e) $10^{-9}$ (f) $10^{-12}$
Answer:
(a) kilo$10^{3}$
(b) deci$10^{-1}$
(c) centri$10^{-2}$
(d) micro$10^{-6}$
(e) nano$10^{-9}$
(f) pico$10^{-12}$
Question 35. Express each of the following in kilograms
$(a) 5.84 \times 10^{-3} mg$
$(b) 58.34 g$
$(c) 0.584g$
$(d) 5.873 \times 10^{-21}g$
Answer:
$(a) 1 mg = 10^{-6} kg\: \: \:$
$5.84 \times 10^{-3} mg = 5.84 \times 10^{-3} \times10^{-6} kg = 5.84 \times10^{-9} kg$
$(b) 1 g = 10^{-3}kg$
$58.34 g = 58.34 \times 10^{-3} kg = 5.834 \times10^{-2} kg$
$(c) 1 g = 10^{-3} kg$
$0.584g = 0.584g \times 10^{-3} kg = 5.84 \times10^{-4}kg$
$(d) 1 g = 10^{-3} kg$
$5.873 \times 10^{-21} g = 5.873 \times 10^{-24} \times 10^{-3} kg = 5.873 \times 10^{-24} kg$
Question 36. Compute the difference in masses of $10^3$ moles each of magnesium atoms and magnesium ions. $(Mass \: of \: an\: electron = 9.1\times 10^{-31} kg)$
Answer:
$Mg^{2+}$ion and Mg atom differ by two electrons.
$10^3$ moles of $Mg^{2+}$ and Mg atoms would differ by $2 \times 10^3$ moles of electrons.
Mass of $2 \times 10^3$ moles of electrons.
$= 2 \times 10 ^3 \times 6.023 \times 10^{23} \times 9.1 \times 10^{-31} kg$
$\Rightarrow 2 \times 6.022 \times 9.1 \times 10^{-5} kg$
$\Rightarrow 109.6004 \times 10^{-5} kg$
$\Rightarrow 1.096 \times 10^{-3} kg$
Question 37. Which has more number of atoms?
$100g \: \: of \: \: N_2 \: \: or\: \: 100 g\: \: of \: \: NH_3$
Answer:
$(i)100g\: \: of\: \: N_2 = \frac{100}{28}moles$
$Number \: of \: molecules = \frac{100}{28} \times 6.022 \times 10 ^{23}$
$Number \: of \: atoms = \frac{2 \times 100}{28} \times 6.022 \times 10 ^{23}$
$=43.01 \times 10^{23}$
$(ii)100g\: \: of\: \: NH_3 = \frac{100}{17}moles$
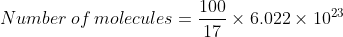 $Number \: of \: atoms = \frac{4 \times100}{28} \times 6.022 \times 10 ^{23}$
$Number \: of \: atoms = \frac{4 \times100}{28} \times 6.022 \times 10 ^{23}$
$=141.69 \times 10^{23}$
$NH_3$ will have more atoms.
Question 38. Compute the number of ions present in 5.85 g of sodium chloride.
Answer:
$5.85g$ of $NaCl=\frac{5.85}{58.5}=0.1moles$
Or, 0.1 moles of NaCl particles.
Each NaCl particle is equivalent to one $Na^{+}$ one $Cl^{-}$ i.e. 2 ions.
Total moles of ions $= 0.1 \times 2 = 0.2 moles$
No. of ions $= 0.2 \times 6.022 \times 10^{23} = 1.2042 \times 10^{23} ions$
Answer:
One gram of gold sample will contain $\frac{90}{100}=0.9g$ of gold
Number of moles of gold $=\frac{Mass\; of \;gold}{Atomic\; mass\; of\; gold}$
$=\frac{0.9}{197}$
$=0.0046$
One mole of gold contains $N_{A}$ atoms $=6.022\times 10^{23}$
$\therefore0.0046mole$ of gold will contain $=0.0046\times 6.022\times10^{23}atoms$
$=2.77\times10^{21}atoms$
Question 40. What are ionic and molecular compounds? Give examples.
Answer:
While forming some compounds, atoms may gain or lose electrons, thereby forming electrically charged particles called ions. Compounds that are formed by the attraction of cations and anions are known as ionic compounds.
Ex : $2Na + Cl_2 \rightarrow 2Na + Cl^{-} \rightarrow 2NaCl$ (sodium chloride- common salt.)
Compounds formed by the bonding of uncharged species are known as molecular compounds. The bonding is called covalent bonding. Molecular compounds are formed by sharing of electrons between the two atoms. Ex: $2C + O_{2} \rightarrow 2CO$ (Carbon monoxide)
Ionization of Al atom occurs as
$Al\rightarrow Al^{3+}+3e^-$
Therefore, $Al^{3+}$ ion is formed from Al atom by loss of 3 electrons. Difference in mass of 1 mole of Al atoms and 1 mole of $Al^{3+}$ ions
$= mass\: \: of \: \: 3 \times 6.022 \times 10^{23} electrons$
$= (3 \times 6.022 \times 10^{23}) \times (9.1 \times 10^{-28} g)$
$(as \: \: mass\: \: of\: \: an \: \: electron = 9.1 \times 10^{-28} g)$
$=164.4 \times 10^{-5}g=1.644 \times 10^{-3}g$
$1$ mole of Al atoms is heavier than 1 mole of $Al^{3+}$ ions.
It is because 1 mole of Al atom have $e^-= 13\times 6.022 \times 10^{23 }$ electrons and $1$ mole of Al ion have
$e=10\times 6.022 \times 10^{23 }$ electrons
Question 42. A silver ornament of mass’m’ gram is polished with gold equivalent to 1% of the mass of silver. Compute the ratio of the number of atoms of gold and silver in the ornament.
Answer:
Mass of silver =mg
Mass of gold deposited $=\frac{m\times1}{100}=\frac{m}{100}g$
No. of atoms of silver $=\frac{Mass}{Atomic mass}\times N_{A}$$=\frac{m}{108}\times N_{A}$
No. of atoms of gold $=\frac{Mass}{Atomic mass}\times N_{A}=\frac{m}{100\times 197}\times N_{A}$
Ratio of the number of atoms of gold to silver $=\frac{m}{100\times 197}\times N_{A}:\frac{m}{108}\times N_{A}$
$108:100\times 197=108:19700=1.182.41$
Question 43. A sample of ethane $(C_2H_6)$ gas has the same mass as $1.5 \times 10^{20}$ molecules of methane $(CH_4)$. How many$(C_2H_6)$ molecules does the sample of gas contain?
Answer:
$Mass \: of\: 1\: molecule\: of\: CH_4=\frac{16}{N_A}g$
$Mass \: of\: 1.5 \times 10^{20}\: molecule\: of\: CH_4=\frac{1.5\times 10^{20}\times 16}{N_A}g$
$Mass \: of\: 1\: molecule\: of\: C_2H_6=\frac{30}{N_A}g$
$Mass \: of\: C_2H_6\: molecules\: =\frac{1.5 \times 10^{20} \times 16}{N_A}g$
Therefore, number of molecules of $C_2H_6 = \frac{1.5 \times 10^{20} \times 16}{N_A}g \times \frac{N_A}{16}$
$=0.8 \times 10^{20}$
(a) In a chemical reaction, the sum of the masses of the reactants and products remains unchanged. This is called Law of conservation of mass.
It states that mass is neither created nor destroyed in chemical reactions.
(b) A group of atoms carrying a fixed charge on them is called ions.
An ion is a charged atom or molecule. It is charged because the number of electrons are not equal to the number of protons in the atom or molecule.
(c) The formula unit mass of $Ca_3(PO_4)_2$ is $310$.
$Ca_3 (PO_4)_2 = (40) 3 + [31 + (16) 4] 2 = 120 + 190 = 310$
(d) Formula of sodium carbonate is $Na_2CO_3$ and that of ammonium sulphate is $(NH_4)_2SO_4$
11. Ne
(b) Identify the total number of inert gases, their names and symbols from this cross word puzzle.
Answer:
(a)
(b) Six inert gases:
Helium (He); Neon ( Ne); Argon (Ar); Krypton (Kr); Xenon (Xe); Radon (Rn).
Question 47. Write the formulae for the following and calculate the molecular mass for each one of them.
(a) Caustic potash
(b) Baking powder
(c) Lime stone
(d) Caustic soda
(e) Ethanol
(f) Common salt
Answer:
(a) $KOH = 39 + 16 + 1 = 56 u$
(b) $NaHCO_{3} = 23 + 1 + 12 + 3 \times 16 = 84 u$
(c) $CaCO_3= 40 +12+ 3\times 16 = 100u$
(d) $NaOH = 23 + 16 + 1 = 40 u$
(e) $C_{2}H_{5}OH = 2 \times 12 + 6 \times 1 + 16 = 46 u$
(f) $NaCl = 23 + 35.5 = 58.5 u$
The chemical reaction involved is :
$6CO_2 + 6H_2O \xrightarrow[Sunlight (6 \times 10 g)]{Chlorophyll}C_6H_{12}O_6 \: \: (180g) + 6O_2$
To produce 18g of glucose, mass of water consumed = $108g$
To produce 18g of glucose, mass of water consumed $= \frac{(108g)}{(180g)} \times (18g) = 10.8g$
$Density \: of\: water=1\: g\: cm^{-3}$
$Volume \: of\: water = \frac{Mass\: of\: water}{Density\: of\: water}=\frac{(10.8g)}{(1gcm^{-3})}=10.8\: g\: cm^{3}$
Important Question From NCERT Exemplar Class 9 Science Chapter 3
Some Atoms and Molecules Class 9 Science Chapter 3 questions and answers are given below that will help you tackle complex problems. The questions below will help you evaluate your understanding of the concepts.
Question 1: An atom of some element Y weighs $6.644 \times 10^{-23}$ g. Calculate the number of gram-atoms in 40 kg of it.
(1) 1200
(2) 100
(3) 1000
(4) 1100
Answer:
Mass of 1 mole Y atoms
= mass of 1 atom x Avogadro constant
=$=6.644 \times 10^{-23} \times 6.022 \times 10^{23}$
= 40 g
So, the atomic mass of Y = 40
No. of gram-atoms (or moles) of X = (mass of y)/(atomic mass)
Number of gram atoms $=\frac{40 \times 1000}{40}$
Thus, the number of gram atoms = 1000 atoms
Hence, the correct answer is option (3).
Question 2: Calculate the molar mass of KAl$\left(\mathrm{SO}_4\right)_2 \cdot 12 \mathrm{H}_2 \mathrm{O}$.
(1) 462g
(2) 480g
(3) 470g
(4) 472g
Answer:
The atomic mass of the consisting elements are given below:
K = 39u
Al = 27u
S = 32u
O = 16u
H = 1u
Now, the molecular mass of KAl$\left(\mathrm{SO}_4\right)_2 \cdot 12 \mathrm{H}_2 \mathrm{O}$ is given as:
Molecular mass = 39 + 27 + 32x2 + 16x8 + 12x1 + 12x16
Molecular mass = 462u
Thus, the molar mass of KAl$\left(\mathrm{SO}_4\right)_2 \cdot 12 \mathrm{H}_2 \mathrm{O}$ = 462g
Hence, the answer is the option (1).
Question 3: How many atoms of hydrogen are there in 36 g of $\mathrm{NH}_4$?
(1) $9.635 \times 10^{23}$ atoms
(2) $96.35 \times 10^{24}$ atoms
(3) $4.81 \times 10^{23}$ atoms
(4) $48.17 \times 10^{23}$ atoms
Answer:
Molar mass (Molecular mass in gram) of $\mathrm{NH}_4$ = 14+4= 18 g
No. of moles of $\mathrm{NH}_4$ = 36/18 = 2 moles
Now, total moles of hydrogen atoms in one mole of $\mathrm{NH}_4$ = 4 moles
Thus, total Moles of Hydrogen Atoms in 2 moles of $\mathrm{NH}_4$4 = 2 x 4 = 8 moles
Now, we know that in 1 mole of hydrogen atoms, the number of hydrogen atoms = $6.022 \times 10^{23}$
Thus, in 8 moles of hydrogen atoms, the number of atoms present = 8 x $6.022 \times 10^{23}$
= $48.17 \times 10^{23}$ atoms
Hence, the answer is the option (4).
Question 4: All of these radicals have a valency of 2, except
(1) $\mathrm{SO}_4$
(2) $\mathrm{CO}_3$
(3) $\mathrm{NH}_4$
(4) $\mathrm{O}^{2-}$
Answer:
All these radicals have valencies -2 except $\mathrm{NH}_4$. The valency of $\mathrm{NH}_4$ is +1 and it is written as $\mathrm{NH}_4^{-}$. For example, $\mathrm{NH}_4 \mathrm{Cl}$.
Hence, the answer is the option (3).
Question 5: After a chemical reaction, the total mass of reactants and products
(1) is always increased
(2) is always decreased
(3) is not changed
(4) is always less or more
Answer:
The law of conservation of mass states that in a chemical reaction, the total mass of products is equal to the total mass of reactants. Thus, there is no change in mass during a chemical reaction.
Hence, the answer is the option (3).
Approach to Solve Class 9 Science Chapter 3 Questions
To solve NCERT Exemplar Class 9 Science Chapter 3 Atoms and Molecules questions, it is important to follow a systematic approach. It is recommended to strategies your study plan to solve the questions of this chapter. The following are the points that will help you build a good approach.
1). While solving questions it is very important to understand the basic concepts like Laws of chemical combinations, Daltons atomic theory, Atomic and Molecular masses, Mole concepts, Chemical equations and formulas
2). A quick reference to symbols, formulas, and valencies makes learning chemistry easier and solving problems much faster. Given below some points to keep in mind:
- Remember the symbols of every element
- Learn the valency of some common elements
- Also practice how to write chemical formulas using Criss-cross method
3). Knowing the symbols and valencies of elements is the key to forming correct chemical compounds. Practice combining symbols and valencies correctly.
4). Go through NCERT Exemplar Class 9 Science Solutions Chapter 3 Atoms and Molecules and real-life examples as they often form the basis for application questions.After studying, close the book and try recalling answers or concepts. You can also take help from Class 9 Science Chapter 3 Atoms and Molecules notes.
NCERT Exemplar Class 9 Science Chapter 3 Topics:
Atoms and Molecules Class 9 Science Chapter 3 includes the following topics :
- Definitions of atoms and molecules.
- The fundamental quantity which defines the amount of substance is called a mole.
- Molecular weight and atomic weight of any substance.
- How to find out the molecular weight of any compound if we know the chemical formula of the compound.
- NCERT exemplar Class 9 Science solutions chapter 3 discusses the Avogadro number which will tell a number of atoms or molecules in one mall of the substance.
- Study of the molecules and atoms and their molecular weight.
Advantages of Using Class 9 Science NCERT Exemplar Chapter 3 Atoms and Molecules Solutions
NCERT Exemplar Class 9 Science Solutions Chapter 3 Atoms and Molecules help students to understand the fundamental concepts of chemistry related to the structure and composition of matter. Given below some points on the advantages of these solutions:
- Students can understand concepts like atoms, molecules, laws of chemical combination, atomic mass, mole concept, molecular mass, and numerical problems based on chemical formulas.
- These NCERT Exemplar Solutions for Class 9 provide systematic explanations to help students understand how atoms combine to form molecules and compounds.
- Class 9 Science NCERT Exemplar Chapter 3 Atoms and Molecules solutions are prepared by subject experts in a clear and comprehensive manner that helps students to understand all the topics of NCERT book easily.
- They provide solved examples and practice questions that are helpful in both CBSE boards and competitive exams.
NCERT Class 9 Science Exemplar Solutions Chapter-wise
Along with atoms and Molecules Class 9 Science Chapter 3 questions and answers students can follow the links for other class 9 Science chapters:
NCERT Solutions for Class 9 chapter-wise
These solutions are prepared strictly according to the latest NCERT syllabus and CBSE guidelines. They help students understand concepts clearly, improve answer-writing skills, and prepare effectively for exams. The NCERT Solutions for all Class 9 science chapters are given below-
NCERT Notes & Solutions subject-wise
Students can refer to the links given below for the NCERT subject-wise notes and solutions:
NCERT Books and NCERT Syllabus
Students can refer to the links given below for the NCERT books and syllabus :
Frequently Asked Questions (FAQs)
An atom is the smallest unit of an element that can take part in a chemical reaction. A molecule is formed when two or more atoms are chemically bonded together.
If you react 4g of Hydrogen with 32g of Oxygen to produce water, you will get exactly 36g of water. The mass of Hydrogen + Oxygen equals the mass of Water.
A chemical formula is a symbolic representation of a molecule that shows the types of atoms present and the number of atoms of each type. For example, H₂O is the chemical formula for water, indicating two hydrogen atoms and one oxygen atom.
One atomic mass unit (amu or u) is defined as 1/12th the mass of a carbon-12 atom.
Avogadro's number (NA) is the number of entities in one mole, which is approximately 6.022 x 10²³.
Class 9 Science NCERT Exemplar Chapter 3 Atoms and Molecules solutions provide step-by-step answers to all textbook questions, helping students understand concepts like laws of chemical combination, atomic and molecular mass, and simple chemical reactions.
Atoms are considered the fundamental building blocks of matter because everything around us, including solids, liquids, and gases, is made up of atoms. The arrangement and combination of these atoms form molecules, leading to the diversity of materials we encounter in our everyday lives.
Balancing chemical equations is crucial because it ensures that the law of conservation of mass is upheld. An unbalanced equation would suggest that mass is lost or gained, which is chemically impossible.
Some common examples of molecules mentioned in this chapter include water , carbon dioxide , and glucose. These molecules are made up of various combinations of atoms and are fundamental to biological and chemical processes in nature.
In a chemical reaction, a molecule is represented by its chemical formula, which indicates the types and number of atoms involved. For example, in the reaction between hydrogen and oxygen to form water, you would write it as:2H2+O2→2H2O. This shows that two molecules of hydrogen react with one molecule of oxygen to produce two molecules of water.
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters







