NCERT Class 9 Science Chapter 2 Notes Is Matter Around Us Pure- Download PDF Notes
It is very crucial to understand whether the matter around us is pure or not. Have you ever wondered why oil does not dissolve in water, while salt dissolves in water, or sugar completely dissolves in water, while sand settles at the bottom? All these observations are linked to the concept of the purity of matter. Objects around us are categorised as either pure or a mixture. We can understand these concepts better with the help of NCERT Solutions, which explains these concepts with the help of solved questions.
This Story also Contains
- NCERT Notes Class 9 Chapter 2: Download PDF
- NCERT Class 9 Science Chapter 2 Class Notes
- Previous Year Questions of Class 9 Science Chapter 2
- How to Master Class 9 Science Chapter 2 Is Matter Around Us Pure
- Advantages of Using Class 9 Science Chapter 2 Is Matter Around Us Pure Notes
- NCERT notes for Class 9 Science Chapter-wise
- NCERT Solutions for Class 9 Science Chapter-wise
- Subject-Wise NCERT Exemplar Solutions

Everything around us is matter, whether it is the air we breathe, the water we drink or the food we eat. The NCERT Notes for Class 9 Science will help you understand matter, mixture, solutions and their properties. These NCERT Class 9 Science Chapter 2 Notes Is Matter Around Us Pure will provide the basic and detailed knowledge needed to know if the matter around us is pure or not. The NCERT notes are designed by our subject experts to help the students tackle easy to complex questions in the exam. The important formulas and diagrams are included to help you grasp the concepts easily.
NCERT Notes Class 9 Chapter 2: Download PDF
You can download the Is Matter Around Us Pure Class 9 Science notes PDF to access a clear explanation of all the concepts from the Download PDF icon given below. NCERT notes for Class 9 help students build strong conceptual understanding and prepare effectively for exams.
Also read
NCERT Class 9 Science Chapter 2 Class Notes
These notes provide a clear explanation of concepts like pure substances, mixtures, and solutions, making revision easier for students. Is Matter Around Us Pure Class 9 Science Chapter 2 CBSE notes are designed to strengthen understanding. The notes contain all the topics in detail, which will help you understand it deeply. You can use these notes for quick revision and to attempt the questions effectively. Scroll down to know more!
Matter around us
If we observe some sugar and some soil placed on two different sheets of paper with a magnifying glass, we will find that the colour, shape and size of all the particles of sugar are the same, but the soil contains particles of different colours, shapes and sizes. For example, the soil contains clay particles, some grass particles and even some dead insects, etc. Now, sugar, which contains particles of only one kind, is called a pure substance, whereas soil, which contains particles of different kinds, is called an impure substance (or mixture). From this, we conclude that all the matter around us is not pure. The matter around us is of two types: pure substances and mixtures.
2.1 What is the mixture?
A mixture is a substance that consists of two or more elements or compounds not chemically combined. For example, air is a mixture of gases like oxygen, nitrogen, argon, carbon dioxide, water vapour, etc. The various gases of the air are not chemically combined with one another. Similarly, gunpowder is a mixture of potassium nitrate, sulphur and charcoal (charcoal is a form of carbon), whereas brass is a mixture of copper and zinc. All the solutions are mixtures. For example, salt solution (brine) is a mixture of common salt (sodium chloride) in water. Along with simple explanations and exercises, NCERT Solutions for Class 9 Science Chapter 2 Is Matter Around Us Pure help students link real-life examples of mixtures to the scientific ideas discussed in the chapter.
Types of Mixtures
- A homogeneous mixture
- A heterogeneous mixture
Those mixtures in which the substances are completely mixed together and are indistinguishable from one another are called homogeneous mixtures. A homogeneous mixture has a uniform composition throughout its mass. It has no visible boundaries of separation between the various constituents. A mixture of sugar in water (called a sugar solution) is a homogeneous mixture because all the parts of the sugar solution have the same sugar-water composition and appear to be equally sweet! There is no visible boundary of separation between sugar and water particles in a sugar solution.
Those mixtures in which the substances remain separate and one substance is spread throughout the other substance as small particles, droplets or bubbles, are called heterogeneous mixtures. A heterogeneous mixture does not have a uniform composition throughout its mass. It has visible boundaries of separation between the various constituents. The mixture of sugar and sand is a heterogeneous mixture because different parts of this mixture will have different sugar-sand compositions.
The suspensions of solids in liquids are also heterogeneous mixtures. For example, a suspension of chalk in water is a heterogeneous mixture. A mixture containing two (or more) immiscible liquids is also a heterogeneous mixture. For example, a mixture of petrol and water is a heterogeneous mixture.
2.2 What is a Solution?
A solution is a homogeneous mixture of two or more substances. A homogeneous mixture means that the mixture is just the same throughout. Some common examples of solutions are: Salt solution, Sugar solution, Vinegar, Metal alloys (such as Brass) and Air. Salt solution is a homogeneous mixture of two substances, salt and water, whereas sugar solution is a homogeneous mixture of two substances, sugar and water. Some more examples of the solutions are : Sea-water, Copper sulphate solution, Alcohol and water mixture, etc.

Properties of a Solution
The important characteristic properties of a solution (or true solution) are as follows :
- A solution is a homogeneous mixture.
- The size of solute particles in a solution is extremely small. It is less than 1 nm in diameter (1 nanometre = 10-9 metre).
- The particles of a solution cannot be seen even with a microscope.
- The particles of a solution pass through the filter paper. So, a solution cannot be separated by filtration.
- The solutions are very stable. The particles of solute present in a solution do not separate on standing.
- A true solution does not scatter light (This is because its particles are very, very small).
2.2.1 CONCENTRATION OF A SOLUTION
Based on the concentration of the solution, there are three types of solutions: diluted, concentrated and saturated.
- A dilute solution is one in which the amount of solute in a given mass is fairly minimal compared to the amount of solvent. A concentrated solution contains a large amount of solute, and a saturated solution has dissolved all of the solute it is capable of dissolving at a given temperature. In other terms, a saturated solution is one in which no more solute can be dissolved in a solution at a given temperature.
- The solubility of a solute is the amount of the solute present in a saturated solution at this temperature.
- An unsaturated solution is one in which the amount of solute in the solution is less than the saturation level.
A solution's concentration can be stated in a variety of ways-
(i) Mass by mass percentage of a solution
$
=\frac{\text { Mass of solute }}{\text { Mass of solution }} \times 100
$
(ii) Mass by volume percentage of a solution
$
=\frac{\text { Mass of solute }}{\text { Volume of solution }} \times 100
$
(iii) Volume by volume percentage of a solution
$
=\frac{\text { Volume of solute }}{\text { Volume of solution }} \times 100
$
2.2.2 WHAT IS A SUSPENSION?
A suspension is a heterogeneous combination in which the solute particles do not dissolve but stay suspended in the medium's bulk.
Properties of a Suspension
-
Suspension is a heterogeneous mixture. A suspension's particles can be seen with the naked eye.
-
The particles in a suspension scatter a light beam travelling across it, revealing its path.
-
When a suspension is left undisturbed, the solute particles settle down, making the suspension unstable. Filtration can be used to separate them from the rest of the mixture. The suspension breaks as the particles settle down, and light is no longer scattered.
2.2.3 WHAT IS A COLLOIDAL SOLUTION?
A colloid is a type of solution in which the size of solute particles lies between that of genuine solutions and that of suspensions. A colloid is a heterogeneous combination of particles that are too tiny to be seen individually with the naked eye. Colloids are large enough to scatter a light beam passing through them, revealing their path. Students can also download NCERT Class 9 Science Chapter 2 Notes Is Matter Around Us Pure for quick revision and easy understanding of important concepts like colloids and their properties.
The scattering of light by particles in a colloid or by particles in a very fine suspension is known as the Tyndall effect.
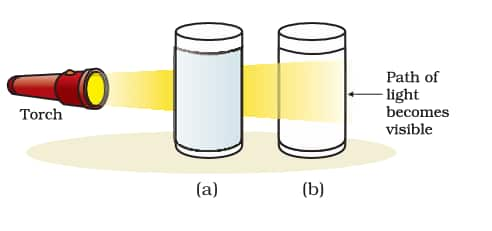
Here, in the above figure, (a) the Solution of copper sulphate does not show the Tyndall effect, and (b) a mixture of water and milk shows the Tyndall effect.
Dispersed phase- The dispersed phase of a colloid is the solute-like component of dispersed particles.
Dispersion medium- The dispersion medium is the component in which the dispersed phase is suspended.
Tyndall effect can be observed when sunlight passes through the canopy of a dense forest. In the forest, mist contains tiny droplets of water, which act as particles of colloid dispersed in the air.

Aerosol- Aerosol is a colloidal solution containing a solid/liquid dispersed phase and a dispersing medium, such as a gas, such as clouds.
Foam- Foam, for example, is a colloidal solution with a dispersed phase gas and a dispersing medium, solid/liquid.
Sol- Sol is a colloidal solution including a solid dispersed phase and a liquid dispersing medium. Magnesia milk and mud
|
Dispersed Phase |
Dispersing Medium |
Type |
Example |
|
Liquid |
Gas |
Aerosol |
Fog, clouds, mist |
|
Solid |
Gas |
Aerosol |
Smoke, automobile exhaust |
|
Gas |
Liquid |
Foam |
Shaving cream |
|
Liquid |
Liquid |
Emulsion |
Milk, face cream |
|
Solid |
Liquid |
Sol |
Milk of magnesia, mud |
|
Gas |
Solid |
Foam |
Foam, rubber, sponge, pumice |
|
Liquid |
Solid |
Gel |
Jelly, cheese, butter |
|
Solid |
Solid |
Solid Sol |
Coloured gemstone, milky glass |
2.3 Physical and Chemical Changes
There are some changes during which no new substances are formed. On the other hand, there are some other changes during which new substances are formed. So, on the basis of whether new substances are formed or not, we can classify all the changes into two groups: physical changes and chemical changes.
Physical Changes
Those changes in which no new substances are formed are called physical changes. In a physical change, the substances involved do not change their identity. They can be easily returned to their original form by some physical process. This means that physical changes can be easily reversed. The changes in physical state, size and shape of a substance are physical.
Some common examples of physical changes are: Melting of ice (to form water); Freezing of water (to form ice); Boiling of water (to form steam); Condensation of steam (to form water).
Chemical Changes
Those changes in which new substances are formed are called chemical changes. In a chemical change, the substances involved change their identity. They get converted into entirely new substances. The new substances usually cannot be returned to their original form. This means that chemical changes are usually irreversible.
Some common examples of chemical changes are: Burning of a magnesium wire, burning of paper, rusting of iron, ripening of fruits and cooking of food.
2.4 What are the Types of Pure Substances?
A pure substance is made up of only one kind of particle. These particles may be atoms or molecules. So, we can also say that a pure substance is made up of only one kind of atom or molecule. For example, the sulphur element is made up of only one kind of particle (called sulphur atoms); therefore, sulphur is a pure substance.
2.4.1 ELEMENTS
Robert Boyle was the first scientist to use the term element in 1661. Antoine Laurent Lavoisier (1743–94), a French chemist, was the first to establish an experimentally useful definition of an element. He defined an element as a basic form of matter that cannot be broken down into simpler substances by chemical reactions. Elements can be normally divided into metals, non-metals and metalloids.
Metals usually show some or all of the following properties:
- They have a lustre (shine).
- They have a silvery-grey or golden-yellow colour.
- They conduct heat and electricity.
- They are ductile (can be drawn into wires).
- They are malleable (can be hammered into thin sheets).
- They are sonorous (make a ringing sound when hit).
Examples of metals are gold, silver, copper, iron, sodium, potassium, etc. Mercury is the only metal that is liquid at room temperature.
Non-metals usually show some or all of the following properties:
- They display a variety of colours.
- They are poor conductors of heat and electricity.
- They are not lustrous, sonorous or malleable.
- Examples of non-metals are hydrogen, oxygen, iodine, carbon (coal, coke), and bromine.
Metalloids are elements that have properties that are halfway between those of metals and non-metals; examples include boron, silicon, and germanium.
2.4.2 COMPOUNDS
A compound is a substance composed of two or more elements, chemically combined in a fixed proportion.
.png)
Separating the Components of a Mixture
- To separate distinct components from a mixture, many separation procedures are used.
- Separation enables the study and use of distinct components of a mixture.
- Simple physical procedures like handpicking, sieving, and filtering that we employ in our daily lives can be used to separate heterogeneous mixtures into their constituents.
Previous Year Questions of Class 9 Science Chapter 2
By referring to previous year questions of this chapter, students can easily see which topics are important and often asked in exams. They can refer to the Is Matter Around Us Pure Class 9 Science Chapter 2 CBSE notes for understanding the concepts used to solve questions.
Question 1: Which of the following mixtures shows the Tyndall effect?
(1) Salt solution
(2) Copper sulphate solution
(3) Milk
(4) Air
Answer: Tyndall effect is the scattering of light by particles in a colloid. Milk is a colloid (liquid in liquid), so it scatters light.
Hence, the correct answer is option (3).
Question 2: Which of the following is a homogeneous mixture?
(1) Air
(2) Oil and water
(3) Sand and iron filings
(4) Water and chalk powder
Answer:
Air is a homogeneous mixture of gases, where the components are uniformly mixed and not visibly separate.
Hence, the correct answer is option (1).
Question 3: A mixture of ammonium chloride and common salt is best separated by:
(1) Filtration
(2) Sublimation
(3) Distillation
(4) Crystallisation
Answer: Ammonium chloride sublimes, while common salt does not. Sublimation is the only technique that separates a sublimable component from a non-sublimable one.
Hence, the correct answer is option (2).
Question 4: Which method is used to separate cream from milk?
(1) Filtration
(2) Evaporation
(3) Centrifugation
(4) Sublimation
Answer:
Centrifugation works by spinning milk rapidly so that lighter cream separates from heavier milk due to density difference.
Hence, the correct answer is option (3)
Question 5: Which of the following is an element?
(1) Water
(2) Oxygen
(3) Carbon dioxide
(4) Common salt
Answer:
Oxygen consists of only one type of atom, so it is an element.
Hence, the correct answer is option (2).
How to Master Class 9 Science Chapter 2 Is Matter Around Us Pure
This chapter explains how substances are classified as pure or mixtures and introduces methods to separate mixtures. Students can master this chapter by referring to Class 9 Science Chapter 2 Is Matter Around Us Pure Notes
- Firstly, understand what pure substances and mixtures are and the differences between them.
- Then learn about solutions, suspensions, and colloids.
- Also understand different separation techniques like filtration, evaporation, distillation, chromatography, centrifugation, etc. Refer Is Matter Around Us Pure Class 9 Science notes for better learning.
- Remember these topics by making tables comparing types of mixtures and their properties.
- After that, solve NCERT exercises and previous year questions.
Advantages of Using Class 9 Science Chapter 2 Is Matter Around Us Pure Notes
NCERT Class 9 Science Chapter 2 Notes Is Matter Around Us Pure help students to understand the classification and properties of substances and mixtures. Given below some points on the advantages of these notes:
- Students can use these notes to understand the topics like elements, compounds, mixtures, types of mixtures, methods of separation, and physical and chemical changes.
- These notes provide systematic explanations that make it easier to understand the composition and purification of substances.
- Class 9 Science Chapter 2 Is Matter Around Us Pure Notes are prepared by experts in a very clear and comprehensive manner.
- They are well structured notes that cover all the topics from NCERT books that are helpful for boards and competitive exams.
NCERT notes for Class 9 Science Chapter-wise
In addition to the NCERT notes Class 9 Science Chapter 2 Is Matter Around Us Pure, students can also refer to other Class 9 chapter notes from the links given below
NCERT Solutions for Class 9 Science Chapter-wise
Besides Class 9 Science Chapter 2 Is Matter Around Us Pure Notes , students can also follow Class 9 chapter-wise solutions of NCERT:
Subject-Wise NCERT Exemplar Solutions
Ace your exam preparations by solving NCERT exemplar solutions.
Frequently Asked Questions (FAQs)
A solution is a homogeneous mixture formed when a solute dissolves completely in a solvent. For instance, when salt is added to water and dissolves, it forms a saline solution, which is uniform throughout.
The physical and chemical properties of substances can indicate their purity. For instance, pure substances have distinct boiling and melting points, while mixtures do not. Chemical properties also help determine how a substance reacts, revealing its purity based on predictable reactions.
Air is considered a mixture because it consists of various gases, primarily nitrogen and oxygen, along with smaller amounts of other gases like carbon dioxide and water vapor. These components can exist in different proportions, making air a heterogeneous mixture.
A technique used to separate components of a mixture based on their solubility and movement on a medium.
Matter around us is mostly a mixture rather than a pure substance. Pure substances have a fixed composition, while mixtures contain two or more substances physically combined, like air, seawater, and soil.
A pure substance is a type of matter that contains only one kind of particle and has a definite composition and properties. Examples include elements like oxygen and compounds like water.
Filtration, evaporation, distillation, crystallisation, centrifugation, and chromatography.
Is Matter Around Us Pure Class 9 Science notes cover the distinction between pure substances and mixtures, types of mixtures, and common methods to separate mixtures, like filtration, distillation, and chromatography.
The Two Types of Matter:
Pure Substances: Made of only one type of particle with a fixed composition.
Mixtures: Made of two or more substances physically combined, with variable composition.
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
