NCERT Class 9 Science Chapter 3 Notes - Atoms And Molecules PDF Notes
Have you ever wondered how things around us are made? Like the air we breathe, the phone we are currently using to read this article, or the food we eat daily to quench our appetite. The answer to all those questions is NCERT Class 9 Science Chapter 3 Notes Atoms and Molecules. Atoms are the smallest unit of matter, while molecules are made up of a combination of two or more atoms, and they are so small that we cannot see them with the naked eye; to see them, we require a high-end microscope. Students can also refer to the NCERT Solutions for understanding the concepts of this chapter better with the help of solved questions.
This Story also Contains
- NCERT Notes for Class 9 Science Chapter 3: Download PDF
- NCERT Notes for Class 9 Science Chapter 3
- Previous Year Questions of Class 9 Science Chapter 3
- How to Master Class 9 Science Chapter 3 Atoms and Molecules
- Advantages of Using Class 9 Science Chapter 3 Atoms and Molecules Notes
- NCERT notes for Class 9 Science Chapter-wise
- NCERT Solutions for Class 9 Science Chapter-wise
- Subject-Wise NCERT Exemplar Solutions
The topics like laws of combinations, atomic mass, molar mass, etc., are covered in this chapter. The NCERT Notes for Class 9 Science are prepared in a systematic way to give you detailed information on the structure and behaviour of atoms and molecules. These NCERT notes will not only help you in exams but will also strengthen your fundamentals in chemistry. The important formulas and diagrams are also included to give you a clear picture.
NCERT Notes for Class 9 Science Chapter 3: Download PDF
You can download the Atoms and Molecules Class 9 Science Chapter 3 CBSE notes PDF from the download icon given below. These NCERT Notes for Class 9 are designed to simplify concepts and help students with quick and effective exam revision.
Also read
NCERT Notes for Class 9 Science Chapter 3
The Class 9 Science Chapter 3 Atoms and Molecules Notes contain all the topics in detail with appropriate examples. These notes provide a clear explanation of important concepts like laws of chemical combination, atomic mass, and molar mass in a simple manner. Students can use them as a handbook for learning and revision of the concepts. Scroll down to know more!
An Indian philosopher, Maharishi Kanad, said that if we go on dividing matter (padarth), we will get smaller and smaller particles of matter. Ultimately, we will get the smallest particle of matter, which cannot be divided any further. Based on this philosophy, Kanad was one of the first persons to propose that matter (or padarth) is made up of very small particles called ‘parmanu’. John Dalton called these particles by the name of an atom. The word ‘atom’ means ‘indivisible’; something which cannot be divided further.
Another Indian philosopher, Pakudha Katyayana, went a step further and proposed that the particles of matter (atoms or parmanu) normally exist in a combined form, and various combinations of particles give us various kinds of matter. This combined form of atoms is now called ‘molecules’.
We now know that all matter is made up of small particles called atoms and molecules. Different kinds of atoms and molecules have different properties, due to which different kinds of matter also show different properties. Thus, the properties of matter depend on the properties of atoms and molecules from which it is made. Democritus explained that if we keep on dividing matter, we will end up with its smallest unit, which won’t be divisible further, and he named it an atom. Students can understand the concepts of this chapter more clearly with the help of the NCERT Solutions for Class 9 Science Chapter 3 Atoms and Molecules.
3.1 Laws of Chemical Combination
Antoine L. Lavoisier is called the father of chemistry, for he gave two important laws of chemical combination, which laid the foundation of chemical sciences, and the laws are as follows:
3.1.1 Law of Conservation of Mass
It states that “Mass is not created and not destroyed, in a chemical reaction”. The substances that combine (or react) in a chemical reaction are known as 'reactants’, whereas the new substances formed (or produced) as a result of the chemical reaction are called ‘products’. There is no change in mass during a chemical reaction. Since there is no gain or loss in mass in a chemical reaction, the mass remains conserved.
For example,
$\underset{100 \mathrm{~g}}{\mathrm{CaCO}_3} \rightarrow \underset{56 \mathrm{~g}}{\mathrm{CaO}}+\underset{44 \mathrm{~g}}{\mathrm{CO}_2}$
3.1.2 Law of Constant Proportions
“The elements are present in definite proportions by mass in a chemical substance.” This law means that whatever be the source from which it is obtained (or the method by which it is prepared), a pure chemical compound is always made up of the same elements in the same mass percentage.
For example, water is a compound that always consists of the same two elements, hydrogen and oxygen, combined in the same constant proportion of 1:8 by mass (1 part by mass of hydrogen and 8 parts by mass of oxygen). You can also download the Atoms and Molecules Class 9 Science notes to revise such examples and understand the laws of chemical combination thoroughly.
Dalton’s Atomic Theory
The theory that ‘all matter is made up of very tiny indivisible particles (atoms)’ is called the atomic theory of matter. Dalton put forward his atomic theory of matter in 1808.
The postulates of the theory are given below:
-
“Matter is made of very small particles called atoms”.
-
“Atoms are not divisible particles, which are not created and not destroyed in a chemical reaction”.
-
“Atoms of the same element have identical mass and chemical properties”.
-
“The chemical properties of the atoms are different for different elements.”.
-
“Compounds are formed by combining atoms of different elements in ratios of small whole numbers”.
3.2 What is an Atom?
An atom is the smallest unit of matter that retains all of the chemical properties of an element. For example, a gold coin is simply a very large number of gold atoms moulded into the shape of a coin, with small amounts of other, contaminating elements. Gold atoms cannot be broken down into anything smaller while still retaining the properties of gold. A gold atom gets its properties from the tiny subatomic particles it's made up of.

An atom consists of two regions. The first is the tiny atomic nucleus, which is in the centre of the atom and contains positively charged particles called protons and neutral, uncharged particles called neutrons. The second, much larger, region of the atom is a “cloud” of electrons, negatively charged particles that orbit around the nucleus. The attraction between the positively charged protons and negatively charged electrons holds the atom together.
The size of an atom is indicated by its radius, which is called the ‘atomic radius’. Atomic radius is measured in ‘nanometres’ (which is a very small unit of measuring length). The symbol of a nanometre is nm.
$\begin{aligned} & 1 \text { nanometre }=\frac{1}{10^9} \text { metre } \\ & \text { or } 1 \mathrm{~nm}=10^{-9} \mathrm{~nm}\end{aligned}$
A hydrogen atom is the smallest atom of all. The atomic radius of a hydrogen atom is 0.037 nanometre (or 0.037 nm). If we express the radius of a hydrogen atom in metres, it will be 0.037 × 10–9 metres, which means 0.000000000037 metre! It is really very, very small. The atomic radii of some common elements are given below:
The relative size of different matters:
|
Radii (m) |
Example |
|
$10^{-10}$ |
Atoms of hydrogen |
|
$10^{-9}$ |
Molecules of water |
|
$10^{-8}$ |
Molecules of haemoglobin |
|
$10^{-4}$ |
Grain of sand |
|
$10^{-2}$ |
Ant |
|
$10^{-1}$ |
Watermelon |
3.2.1 WHAT ARE THE MODERN DAY SYMBOLS OF ATOMS OF DIFFERENT ELEMENTS?
Dalton was the first scientist to use the symbols for elements in a very specific sense.
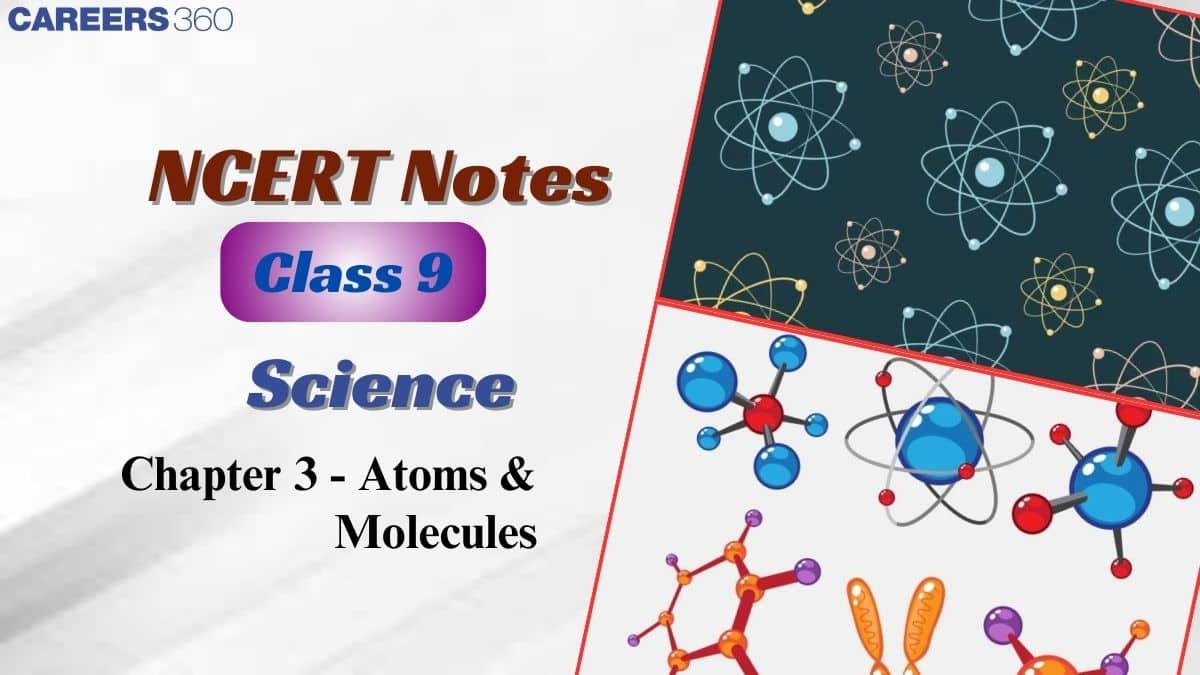
Nowadays, IUPAC (International Union of Pure and Applied Chemistry) is an international scientific organisation that approves names of elements, symbols and units. Many of the symbols are the first one or two letters of the element’s name in English. Some elements and their symbols:
|
Element |
Symbol |
|
Hydrogen |
H |
|
Helium |
He |
|
Lithium |
Li |
|
Sodium |
Na |
|
Gold |
Au |
|
Silver |
Ag |
|
Mercury |
Hg |
|
Iron |
Fe |
|
Chlorine |
Cl |
Atomic Number
It is the number of protons present in an element.
3.2.2 ATOMIC MASS
It is the number of protons and neutrons present in an element.
Carbon-12 isotope is the standard reference for calculating the atomic masses of other elements. One atomic mass unit is a mass unit equal to one-twelfth (1/12th) the mass of one atom of carbon-12.
3.2.3 HOW DO ATOMS EXIST?
Atoms of most elements are not able to exist independently. Atoms form molecules and ions.
3.3 What is a Molecule?
A molecule is an electrically neutral group of two (or more) atoms chemically bonded together. The forces that hold the atoms together in a molecule are called covalent bonds. Thus, a combination of atoms is called a molecule. A molecule is the smallest particle of a substance (element or compound) that has the properties of that substance and can exist in the free state. Molecules can be formed either by the combination of atoms of the ‘same element’ or of ‘different elements.
Depending on this, there are two types of molecules: molecules of elements and molecules of compounds. This is discussed below.
3.3.1 MOLECULES OF ELEMENTS
The molecule of an element contains two (or more) similar atoms chemically combined. For example, a molecule of hydrogen contains 2 hydrogen atoms combined, and it is written as $\mathrm{H}_2$. Some similar examples include nitrogen gas, which exists as $\mathrm{N}_2$ molecules, oxygen gas as $\mathrm{O}_2$ molecules and chlorine gas as $\mathrm{Cl}_2$ molecules.

The molecule of a compound contains two (or more) different types of atoms chemically combined. For example, hydrogen chloride(HCl) and water($\mathrm{H}_2 \mathrm{O}$).

It is a group of two or more elements held together in a chemical bond by attractive forces.
Atomicity
The number of atoms in a molecule is called the atomicity of the molecule.
- For example: argon and helium are monoatomic,
- Oxygen and chlorine are diatomic.
- Phosphorus is tetra-atomic and
- Sulphur is polyatomic.
3.3.3 WHAT IS AN ION?
An ion is a charged particle; it can be positively charged or negatively charged. Positively charged ions are called ‘cations’ and negatively charged ions are called ‘anions’.
Sodium ion ($\mathrm{Na}^{+}$) and magnesium ion ($\mathrm{Mg}^{2+}$) are cations because they are positively charged ions. A cation is formed by the loss of one or more electrons by an atom. For example, a sodium atom loses 1 electron to form a sodium ion, $\mathrm{Na}^{+}$, which is a cation :
$\underset{\text { Sodium atom }}{\mathrm{Na}} \xrightarrow{-1 \text { electron }} \underset{\begin{array}{c}\text { Sodium ion } \\ \text { (A cation) }\end{array}}{\mathrm{Na}^{+}}$
Similarly, Chloride ion ($\mathrm{Cl}^{-}$–) and oxide ion ($\mathrm{O}^{2-}$) are anions because they are negatively charged ions. An anion is formed by the gain of one or more electrons by an atom.
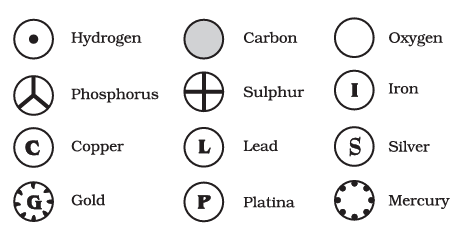
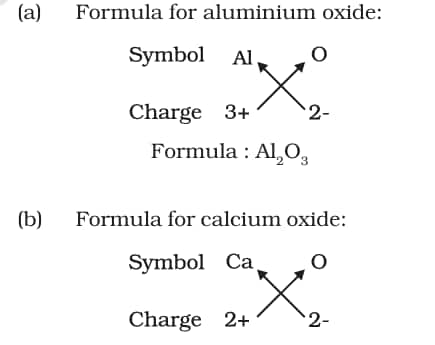
3.4 Writing Chemical Formulae
It is a symbolic representation of a compound and its composition.
Given below are the rules for writing a chemical formula:
-
The charges on the ions have to balance each other.
-
If the compound contains a metal and a nonmetal, the metal is written first.
3.4.1 FORMULAE OF SIMPLE COMPOUNDS
For example: sodium chloride (NaCl), where chlorine is a non-metal, so written on the right, and sodium is a metal, so written on the left.
-
In compounds with polyatomic ions, the ion is enclosed in a bracket, and then the number of the ion is indicated. If the number of polyatomic ions is one, the bracket is not required.
For example:
3.5 Molecular Mass
The molecular mass of a substance is equal to the sum of the atomic masses of all the atoms in that molecule.
3.5.1 FORMULA UNIT MASS
If the substance contains ions as its constituents, then the molecular mass is called Formula Unit Mass.
Formula unit mass is calculated in the same manner as we calculate the molecular mass. The only difference is that we use the word formula unit for those substances whose constituent particles are ions.
For example, sodium chloride, as discussed above, has a formula unit NaCl. Its formula unit mass can be calculated as 1 × 23 + 1 × 35.5 = 58.5 u
Previous Year Questions of Class 9 Science Chapter 3
By referring to previous year questions of this chapter, students can easily see which topics are important and often asked in exams. They can refer to the Atoms and Molecules Class 9 Science Chapter 3 CBSE notes for understanding the concepts used to solve questions.
Question 1. Which of the following is the correct representation of one mole of a substance?
(1) $6.022 \times 10^{22}$ atoms of oxygen
(2) 12 g of carbon-12
(3) 1 g of hydrogen gas
(4) 22.4 g of nitrogen gas
Answer:
12 g of carbon-12
By definition, 1 mole $=12 \mathrm{~g}$ of C-12 $=6.022 \times 10^{23}$ particles.
Hence, the correct answer is option (2).
Question 2. What is the mass of 0.5 mole of $\mathrm{O}_2$ gas?
(1) 8 g
(2) 16 g
(3) 32 g
(4) 48 g
Answer:
Molar mass of $\mathrm{O}_2=32 \mathrm{~g} / \mathrm{mol}$
So, mass $=0.5 \times 32=16 \mathrm{~g}$
Hence, the correct answer is option (2).
Question 3. Which of the following is the correct chemical formula for magnesium chloride?
(1) MgCl
(2) $\mathrm{MgCl}_2$
(3) $\mathrm{Mg}_2 \mathrm{Cl}$
(4) $\mathrm{Mg}_2 \mathrm{Cl}_3$
Answer:
Magnesium has a valency of +2, and chlorine has a valency of -1, so two chloride ions are needed to balance one magnesium ion which will give the formula $\mathbf{M g C l}_2$.
Hence, the correct answer is option (2).
Question 4: Chemical equation is balanced according to the law of:
(1) Multiple proportion
(2) Reciprocal proportion
(3) Conservation of mass
(4) Definite proportions
Answer:
When we balance an equation, we determine the ratio of reactants to products which allows for the total number of atoms of reactants to match the number of atoms of the products. Since the type of atoms does not change and the number of atoms stays the same, the total mass that goes into the chemical change will match the mass that comes out after the change. So, the law of conservation of mass is the correct answer.
Hence, the answer is the option (3).
Question 5: A water sample from a lake, ocean, rain or pond must have _____ proportions of hydrogen to oxygen.
(1) different
(2) same
(3) reciprocal
(4) none of these
Answer:
According to the law of constant proportions, "A chemical compound always consists of the same elements combined together in the same proportion by mass". This law means that whatever the source from which it is obtained (or the method by which it is prepared), a pure chemical compound is always made up of the same elements in the same mass percentage.
Hence, the answer is the option (2).
How to Master Class 9 Science Chapter 3 Atoms and Molecules
This chapter explains the concepts of atoms, molecules and fundamental laws. Students can master this chapter by referring to Class 9 Science Chapter 3 Atoms and Molecules Notes.
- Firstly, understand the basics, such as what atoms, molecules, and ions.
- Then learn about Dalton’s Atomic Theory, its main postulates and limitations
- Focus on the law of conservation of mass and the law of constant proportions
- Questions related to chemical formulas, like how to write chemical formulas using valency, are often asked in exams. Refer to the Atoms and Molecules Class 9 Science notes for a better understanding of these concepts.
- Understand the mole concept carefully, practise numerical problems on molar mass, molecular mass, and mole conversions.
- After that, solve the NCERT exercises and the previous year questions.
Advantages of Using Class 9 Science Chapter 3 Atoms and Molecules Notes
NCERT Notes for Class 9 Science Chapter 3 Atoms and Molecules help students to understand the fundamental concepts of chemistry related to the structure and composition of matter. Given below some points on the advantages of these notes:
- Students can understand concepts like atoms, molecules, laws of chemical combination, atomic mass, mole concept, molecular mass, and numerical problems based on chemical formulas.
- These notes provide systematic explanations to help students understand how atoms combine to form molecules and compounds.
- Class 9 Science Chapter 3 Atoms and Molecules Notes are prepared by subject experts in a clear and comprehensive manner that helps students to understand all the topics of NCERT book easily.
- They provide solved examples and practice questions that are helpful in both CBSE boards and competitive exams.
NCERT notes for Class 9 Science Chapter-wise
In addition to NCERT notes Class 9 Science Chapter 3 Atoms and Molecules, students can also refer to other class 9 chapter notes from the links given below :
NCERT Solutions for Class 9 Science Chapter-wise
Besides the NCERT Class 9 Science Chapter 3 Notes Atoms and Molecules, students can also follow the Class 9 chapter-wise solutions of the NCERT:
Subject-Wise NCERT Exemplar Solutions
Click on the links below to excel in your exam preparations by solving NCERT exemplar solutions.
Frequently Asked Questions (FAQs)
All compounds are molecules made from two or more different types of atoms, not all molecules are compounds. For instance, Oxygen is a molecule but not a compound, as it consists of only one type of atom. A compound, like NaCl sodium chloride, is formed from different types of atoms bonded together.
The law of conservation of mass states that matter cannot be created or destroyed in a chemical reaction. This means that the total mass of the reactants before a reaction must equal the total mass of the products after the reaction.
Chemical symbols are shorthand representations of elements, typically consisting of one or two letters, where the first letter is capitalized e.g., H for hydrogen, O for oxygen. They are used to simplify the representation of chemical substances in equations and formulas
A chemical reaction is a process in which substances undergo a chemical change to form new substances. This involves breaking and forming bonds between atoms, leading to the rearrangement of atoms.
The mole concept is crucial in chemistry as it provides a way to quantify and relate the mass of substances to the number of particles (atoms, molecules, or ions).
Atoms and molecules notes in NCERT Class 9 explain Dalton’s Atomic Theory, laws of chemical combination, concepts of atoms, molecules, ions, and valency. They also cover chemical formulas, molecular mass, and the mole concept with related numericals.
In Atoms and Molecules Class 9 Science notes, facts that matter are the key points, definitions, laws, and formulas that summarise the whole chapter in a concise way. They highlight important concepts like atomic theory, chemical laws, mole concept, equations, and numericals to help in quick revision and exam preparation.
The molecular formula is important as it provides a concise way to represent the composition of a molecule. It indicates the types and numbers of atoms present in a molecule, which helps in understanding its chemical properties and behavior.
Molecules are formed when two or more atoms chemically bond together. While an atom represents a single particle of an element, a molecule can consist of the same or different types of atoms, thereby representing a compound or a diatomic molecule.
This chapter discusses the law of conservation of mass and the law of definite proportions. The law of conservation of mass states that mass is neither created nor destroyed in a chemical reaction, while the law of definite proportions indicates that a chemical compound always contains the same proportion of elements by mass.
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters


