These class 9 science chapter 1 matter in our surroundings solutions help students understand the fundamental concepts of matter and its different states in a simple language. They also make it easier to understand how matter changes from one state to another in everyday life.
NCERT Solutions For Class 9 Science Chapter 1 Matter in Our Surroundings
Ever wondered what makes up everything you see, touch, or feel? It's all about matter! Everything around us is made up of matter, whether it is the food we eat, the water we drink, the notebook we use to write, and the chair or table we use to sit. Matter is defined as anything that has mass and occupies space. These matter in our surroundings ncert solutions are important as they form the basis of topics that students will learn later in their session. The concept of matter provides reasons for everyday phenomena, such as the melting of ice or the spreading of perfume fragrance.
This Story also Contains
- Download PDF for NCERT Solutions Class 9 Science Chapter 1
- NCERT Solutions for Class 9 Science Chapter 1 (In-text Questions)
- NCERT Solutions for Class 9 Science Chapter 1 Matter in Our Surroundings (Exercise Questions)
- Practice Questions for Class 9 Science Matter in Our Surroundings
- Approach to Solve Questions of Class 9 Science Chapter 1
- Topics of NCERT Class 9 Science Matter in Our Surroundings
- What Students Learn from NCERT Solutions for Class 9 Science Chapter 1
- Importance of Class 9 Science Chapter 1 Matter in Our Surroundings Solutions
- NCERT Solutions for Class 9 Science - Chapter Wise
- NCERT Books and NCERT Syllabus

The topics like states of matter, evaporation, condensation, etc. are discussed in class 9 science chapter 1 matter in our surroundings solutions. These topics are well explained in the NCERT Solutions for Class 9 Science through a series of solved questions. These solutions contain step by step solutions with detailed explanations to help you understand the fundamental concepts. Some practice questions are added in this article to improve your critical thinking. The approach to attempt the questions is also mentioned to help you improve your accuracy and speed.
Download PDF for NCERT Solutions Class 9 Science Chapter 1
Students can download the NCERT Solutions for Class 9 Science Chapter 1 Matter in Our Surroundings pdf from the link below and can revise the concepts anytime. These solutions of NCERT make it easier for students to understand the properties and states of matter, helping them understand the concepts in a simple and effective way.
Download PDFAlso Read
NCERT Solutions for Class 9 Science Chapter 1 (In-text Questions)
Below are the class 9 science chapter 1 matter in our surroundings question answer. These questions and answers help students revise key concepts quickly and prepare effectively for exams. These NCERT solutions follow the CBSE curriculum and provide clarity on every topic covered in Matter in Our Surroundings.
Topic 1.2 - Characteristics of particles of matter (Page 3)
Question 1. Which of the following is matter?
Chair, air, love, smell, hate, almonds, thought, cold, lemon water, the smell of perfume.
Answer:
Anything that has mass and occupies space is called matter. It is made up of particles.
In the above question, chair, air, almonds, and lemon water are matters.
And love, smell, hate, thought, cold and smell of perfume are not in the category of matters because they are feelings and emotions of human beings and do not acquire any space.
Question 2. Give reasons for the following observation:
The smell of hot sizzling food reaches you several meters away, but to get the smell of cold food, you have to go close. The smell of hot sizzling food reaches you several meters away
Answer:
The smell of hot sizzling food reaches us from several metres away, as the particles of hot food have more kinetic energy than the cold food.
So, the rate of diffusion is higher in hot food compared to cold food.
Answer:
A diver is able to cut through water in a swimming pool. It shows that the particles of matter (Water) have space between them and have less intermolecular forces of attraction.
Question 4. What are the characteristics of the particles of matter?
Answer:
There are three characteristics of the particles of matter -
- The particle of matter has space between them.
- Particles of matter are continuously moving.
- The particle of matter attracts each other.
Topic 1.3 State of Matter (Page 6)
Question 1. The mass per unit volume of a substance is called density. (density = mass/volume).
Arrange the following in order of increasing density: air, exhaust from chimneys, honey, water, chalk, cotton, and iron
Answer:
Increasing order of the density-
Air < exhaust from chimneys < cotton < water < honey < chalk < iron
Question 2. (a) Tabulate the differences in the characteristics of states of matter.
Answer:
The difference in the characteristics of the three states of matter-
| Characterstics | Solid | Liquid | Gases |
| Shape | Solid has a fixed form | Liquid has no fixed shape | Gas has no fixed shape |
| Volume | Solid has fixed volume | The fluid has a fixed volume | Gas has no fixed volume |
| Rigidity/fluidity | Solids are rigid and cannot flow | Liquid can flow and have no any rigidity | Gas can flow and have no rigidity |
| Intermolecular force and space | Solids have a high intermolecular force and less space | Liquid has an intermediate intermolecular force and has more space than solids | Gas has a very less intermolecular force and has a high space |
Question 2.(b) Comment upon the following: rigidity, compressibility, fluidity, filling a gas container, shape, kinetic energy and density.
Answer:
Rigidity- The tendency of a substance to maintain its shape when subjected to an external force.
Compressibility - It means the contraction in the volume of a substance under the applied pressure. Liquid and gas are compressible because they have empty space, but solid does not.
Fluidity- The tendency of a particle to flow is known as fluidity. Liquids and gases can flow.
Filling of a gas container- The gases can fill the container with a large amount when we apply external pressure.
Shape- Solids have fixed shapes and boundaries. Liquid and gases have no fixed shape and definite boundaries.
Kinetic energy- The energy possessed by particles due to their motion is known as kinetic energy. Gas has maximum kinetic energy because they have more random motion.
Question 3.(a) Give reasons
- A gas fills the vessel completely in which it is kept.
Answer:
A gas fills the vessel completely in which it is kept.
The molecules of gas can have the tendency to move in any random direction due to their high kinetic energy.
Question 3. (b) Give reasons
- A gas exerts pressure on the walls of the container.
Answer:
A gas exerts pressure on the walls of the container because the molecules of the gas are continuously in random motion because of their high kinetic energy.
So, the molecules of gas are vibrating and hitting the walls of the container, and as a result exert pressure on the walls.
Question 3.(c) Give reasons
- A wooden table should be called a solid.
Answer:
A wooden table should be called a solid because it has a definite shape, fixed volume and definite boundaries. Also, it cannot flow and is incompressible.
Question 3.(d) Give reasons
- We can easily move our hand in the air but to do the same through a solid block of wood we need a karate expert.
Answer:
We can easily move our hand in the air but to do the same through a solid block of wood we need a karate expert because, in air, there is less force of attraction between the particles. So, a very small amount of external force can break it.
But in the case of solids, the force of attraction is very strong and the molecular space is so high. Henc,e a large amount of force is required to break it.
Answer
Ice is solid but its density is lower than that of water due to its network structure and forms a cage-like structure with a lot of vacant space. So that's why ice floats on water.
Topic 1.4 Can matter change its state? (Page-9)
Question 1. Convert the following temperature to Celsius scale:
a. 300 K
b. 573 K.
Answer:
It is known that,
Tk = 273 + Tc
Tk = Temperature in Kelvin and
Tc = temperature in Celsius.
Therefore,
(i) Temperature(C) = 300 - 273 = 27 ºC
(ii) Temperature (C) = 573 - 273 = 300 ºC
Question 2. What is the physical state of water at:
a. 250 ºC
b. 100 ºC
Answer:
The primary state of water is liquid at room temperature. It changes to the gaseous state above 100 ºC. At 100 ºC, water (liquid) can be in equilibrium with the gaseous state.
Hence, (i) At 250 ºC physical state of water is gas. and,
(ii) At 100 ºC physical state of water can be gas as well as liquid.
Question 3 For any substance, why does the temperature remain constant during the change of state?
Answer:
The temperature of the substance does not change because the heat is used to overcome the forces of attraction.
This heat energy is known as latent heat.
That is why for any substance, the temperature remains constant during the change of state.
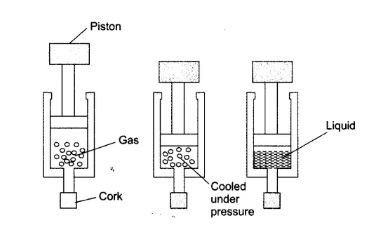
Question 4. Suggest a method to liquefy atmospheric gases.
Answer
The atmospheric gases are transferred into a cylinder with a piston attached to it. By cooling and applying external pressure(by pushing the piston) on them. This way gases can be liquified.
Topic 1.5 - Evaporation (Page 10)
Question 1. Why does a desert cooler cool better on a hot dry day?
Answer:
A desert cooler cools better on a hot and dry day. It is because the inner walls of the cooler get sprinkled by the water continuously and due to warm, dry weather, this water gets evaporated.
Evaporation causes cooling of the present air inside of the cooler. This cold air is sent into the room by a fan.
Question 2. How does the water kept in an earthen pot (matka) become cool during summer?
Answer:
The water inside the earthen pot becomes cold during the summer because the earthen pot is porous with a lot of pores. So, water comes out on the surface of the earthen pot, and this water gets evaporated.
And thus the temperature of water present inside the pot is much lower than outside, and hence the water becomes cold.
Question 3. Why does our palm feel cold when we put some acetone or petrol or perfume on it?
Answer:
When we put some acetone, petrol or perfume evaporate in our palm, they come into contact with the air and hence evaporation causes a cooling effect on our palm.
Question 4. Why are we able to sip hot tea or milk faster from a saucer rather than a cup?
Answer:
As we know, the evaporation rate is faster with the increased surface area. Tea in a saucer has a larger surface area than in a cup.
Therefore, the cooling of tea is more rapid in the saucer, and thus, we can sip hot tea or milk faster from a saucer rather than a cup.
Question 5. What type of clothes should we wear in summer?
Answer:
We should wear light coloured cotton clothes because the light colours reflect the solar radiation and cotton clothes have more porosity so that they allow sweat through it to evaporate faster, thereby causing a cooling effect.
NCERT Solutions for Class 9 Science Chapter 1 Matter in Our Surroundings (Exercise Questions)
The class 9 science matter in our surroundings question answer have been in this section. They provide clear explanations and examples to help students understand the properties and behaviour of matter in different states.
Question 1 Convert the following temperatures to the Celsius scale.
(a) 293 K
(b) 470 K.
Answer:
It is known that,
Tk = 273 + Tc
Tk = Temperature in Kelvin and
Tc = temperature in Celsius.
Therefore,
(i) Temperature (ºC) = (293 - 273) = 20 ºC
(ii) Temperature (ºC) = 470 -273 = 197 ºC
Question 2. Convert the following temperatures to the kelvin scale.
(a) 25 ºC
(b) 373 ºC
Answer:
It is known that,
TK=273+Tc
TK = Temperature in Kelvin and
TC = Temperature in Celsius.
Therefore,
(i) Temperature (K) = 273 + 25 = 298 K
(ii) Temperature (K) = 273 + 373 = 646 K
Question 3.(a) Give reason for the following observations.
- Naphthalene balls disappear with time without leaving any solid.
Answer:
Naphthalene balls disappear with time without leaving any solid because they can sublimate and directly convert into the gaseous state without leaving any solid.
Question 3.(b) Give reason for the following observations.
- We can get the smell of perfume sitting several metres away
Answer:
Perfumes contain a volatile solvent which diffuses very quickly and due to this, we can get the smell of perfume sitting several meters away.
Answer:
The general increasing order of forces of attraction in three states of matter is gas < liquid < solid.
Therefore, increasing order for the above-given substance is-
Oxygen < water < sugar
Question 5. What is the physical state of water at-
(a) 25 ºC
(b) 0 ºC
(c) 100 ºC?
Answer:
The primary physical state of water is liquid. It converts into a gaseous state at a temperature above 100 ºC but at this temperature, it (liquid water) is in equilibrium with the gaseous state.
And below 0 oC it changes into solid and also at this temperature it (liquid water) is in equilibrium with the solid state.
Therefore;
(i) At 25 ºC water is in the liquid state
(ii) At 0 ºC it can be liquid or solid and
(iii) At 100 ºC it can be liquid or the gaseous state.
Question 6. (a) Give two reasons to justify-
- water at room temperature is a liquid.
Answer:
Water at room temperature is a liquid because-
- Below 0 ºC it converts to ice (solid)
- Above 100 ºC it converts into the gaseous state.
Question 6.(b) Give two reasons to justify-
- An iron almirah is a solid at room temperature.
Answer:
An iron almirah is a solid at room temperature because-
- At room temperature, it has a definite shape and boundaries. Also, it has a fixed volume.
- The melting point of iron is much higher than room temperature.
Question 7. Why is ice at 273 K more effective in cooling than water at the same temperature?
Answer:
At 273K, ice can easily absorb more heat energy from the surroundings in the form of latent heat and overcome the fusion to become water.
Water does not absorb this extra heat from the medium.
Thus, the cooling effect of ice is more than water at the same temperature.
Question 8. What produces more severe burns, boiling water or steam?
Answer:
Steam at 100 ºC produces more severe burns.
It is because extra heat is hidden in it, called latent heat, whereas the boiling water doesn't have any hidden heat.
Question 9. Name A,B,C,D,E and F in the following diagram showing change in its state
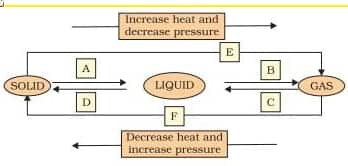
Answer:
In the above flow chart-
A = fusion/ melting/ liquefication
B = Vaporisation/ evaporation
C = Condensation (transformation of water from a gaseous state to a liquid state)
D = Solidification (the conversion of liquid into solid is known as solidification)
E = F = Sublimation
Direct conversion of solid into gas or gas into solid without attaining the liquid phase is called sublimation.
Practice Questions for Class 9 Science Matter in Our Surroundings
These class 9 science chapter 1 matter in our surroundings question answer help students test their understanding of the concepts. By solving these NCERT Solutions for Class 9 students can strengthen their knowledge of the states of matter, properties of substances, and changes in matter. Regular practice of class 9 science matter in our surroundings question answer also prepares them for exams effectively.
Question 1: Define matter. List the characteristics of particles of matter.
Answer:
Matter is anything that has mass and occupies space. Characteristics of particles of matter are
- Particles of matter are very small.
- They have space between them.
- They are constantly moving.
- They attract each other.
Question 2: Explain how evaporation causes cooling. Give two examples from daily life.
Answer:
Evaporation causes cooling because particles on the surface of a liquid absorb energy from their surroundings to change into the vapor state. This absorption of heat lowers the temperature of the surroundings.
Examples-
We feel cool when sweat evaporates from our skin.
Water kept in an earthen pot stays cool due to evaporation from the pot’s surface.
Question 3: What is sublimation? Name two substances that undergo sublimation.
Answer:
Sublimation is the process in which a solid directly changes into a gas without becoming a liquid.
Examples- Camphor and ammonium chloride.
Question 4: Select the correct statement:
(1) a small raindrop (water drop) contains about 1021 particles of water in it.
(2) The particles which make up matter are so small that we cannot see them even with a high power microscope.
(3) The evidence also shows that the particles which make up the matter are constantly ‘moving’ (they are in motion).
(4) all the above
Answer:
According to the definition of matter as given in the concept, all the given options are correct.
Hence, the answer is the option (4).
Question 5: When a glass of ice-cold water is kept open at room temperature, what observation is found?
(1) water starts to evaporate
(2) water droplets are observed on the outer walls of the glass
(3) temperature of water remains same
(4) volume of the ice cold water increases
Answer:
When a glass of water is kept at room temperature, water droplets are observed on the outer walls of the glass. This is because the water vapor present in the air when coming in contact with glass containing ice-cold water loses the heat energy, and thus, the water vapor present in the air gets condensed.
Hence, the answer is the option (2).
Question 6: Which of the following will respond to sublimation?
(1) Common salt
(2) Sugar
(3) Camphor
(4) Potassium nitrate
Answer:
Sublimation is the transition of a substance directly from the solid to the gas phase without passing through the intermediate liquid phase. So, all solids that possess an appreciable vapor pressure at a certain temperature usually can sublime in air (e.g. water ice just below 0°C). Thus, out of the given options, camphor is the substance that undergoes sublimation.
Hence, the answer is the option (3).
Question 7: An inflated balloon full of air becomes smaller and smaller slowly even though the knot at the mouth of the balloon is airtight. And after a week all the air has escaped from the balloon. Explain how the air particles got out of the balloon.
(1) Through the hole of the balloon
(2) Knot at the mouth of the balloon loosen after some days
(3) Diffusion through the rubber sheet of the balloon
(4) None of these
Answer:
The fast-moving molecules of air trapped in the inflated balloon exert continuous pressure on the thin, stretched rubber sheet of the balloon and keep on diffusing out gradually
through it.
Hence, the answer is the option (3).
Question 8: Take two beakers of same size and pour equal volumes of water in each. Now place first beaker where the atmosperic pressure is 1 atm and place second beaker where the atmospheric pressure is 5 atm. In which of these beakers the water will evaporate faster?
(1) First beaker
(2) Second beaker
(3) Both beakers will have the same rate of evaporation
(4) No evaporation will occur in both of them
Answer:
Since the first beaker is placed under lower atmospheric pressure that's why its rate of evaporation will occur faster.
Hence, the answer is the option (1).
Approach to Solve Questions of Class 9 Science Chapter 1
To solve matter in our surroundings class 9 question answer effectively, one should build a good approach. Follow the points below to make a good strategy for solving questions from this chapter:
1. Try to learn the main ideas like the states of matter, properties of solids, liquids and gases. Also, learn the terms like diffusion, evaporation, and factors affecting the change of state.
2. It is advisable to practice drawing diagrams such as the states of matter chart, change of state by heating or cooling and cooling curve of water. This will add up to the beauty of your answer.
3. Know how to define terms like sublimation and latent heat and write clear differences between boiling and evaporation. Refer these class 9 science chapter 1 matter in our surroundings solutions for understanding these concepts better with the help of solved examples.
4. Attempt all questions from the book including in-text questions. Learn to write answers in simple, clear sentences based on textbook explanations. The matter in our surroundings ncert solutions will help you revise the topics.
5. Go through NCERT activities and real-life examples e.g., camphor sublimation, perfume diffusion as they often form the basis for application questions. After studying, close the book and try recalling answers or concepts. You can also take help from Matter in our surroundings class 9 notes.
Topics of NCERT Class 9 Science Matter in Our Surroundings
The topics covered in matter in our surroundings ncert solutions are given below. Learn the concepts through the NCERT solutions:
1.1 Physical Nature of Matter
- 1.1.1 Matter Is Made up of Particles
- 1.1.2 How Small Are These Particles of Matter?
1.2 Characteristics of Particles of Matter
- 1.2.1 Particles of Matter Have Space Between Them
- 1.2.2 Particles of Matter Are Continuously Moving
- 1.2.3 Particles of matter attracts each other
1.3 States of Matter
- 1.3.1 The Solid State
- 1.3.2 The Liquid State
- 1.3.3 The Gaseous State
1.4 Can Matter Change Its State?
- 1.4.1 Effect of Change of Temperature
- 1.4.2 Effect of Change of Pressure
1.5 Evaporation
- 1.5.1 Factors Affecting Evaporation
- 1.5.2 How Does Evaporation Cause Cooling?
What Students Learn from NCERT Solutions for Class 9 Science Chapter 1
-
These solutions explain what matter is , its basic characteristics, and the difference between substances, mixtures, and compounds.
-
Using these solutions states of matter, the arrangement and movement of particles are explained well.
-
Here they learn about physical changes such as melting, freezing, evaporation, condensation, and sublimation and how energy affects these changes.
-
In these matter in our surroundings ncert solutions properties of matter like density, compressibility, and elasticity are explained very well with the help of solved examples.
Importance of Class 9 Science Chapter 1 Matter in Our Surroundings Solutions
The matter in our surroundings class 9 question answer are important because they help students to understand the basic concepts of matter, such as its states, properties, and behaviour under different conditions. Given below some points on why these solutions are important:
1. These solutions help students to understand the basic concept of matter and its different states.
2. They explain key terms like melting, evaporation, condensation, and sublimation.
3. The class 9 science matter in our surroundings question answer helps in understanding how matter behaves under different conditions of temperature and pressure.
4. Here detailed explanations are given that help in writing structured answers in exams.
5. Practising these solutions ensures better performance as most exam questions are based on NCERT.
NCERT Solutions for Class 9 Science - Chapter Wise
Students can also explore the NCERT Solutions for other Class 8 Science chapters along with NCERT Solutions for Class 9 Science Chapter 1 Matter in Our Surroundings to build a thorough understanding of all topics.
|
NCERT Solutions for Class 9 Chapter 1 Matter in Our Surroundings |
|
NCERT Solutions for Class 9 Chapter 2 Is Matter Around Us Pure |
|
NCERT Solutions for Class 9 Chapter 5 The Fundamental Unit of Life |
|
NCERT Solutions for Class 9 Chapter 8 Force and Laws of Motion |
|
NCERT Solutions for Class 9 Chapter 12 Improvement in Food Resources |
NCERT Books and NCERT Syllabus
Follow the links below to get the syllabus and prescribed books. Learn more from the Matter in our surroundings class 9 science NCERT notes.
Frequently Asked Questions (FAQs)
NCERT Solutions for Class 9 Science Chapter 1 provide detailed answers to all the questions in the textbook. They help students understand the concepts of matter, its states, and properties clearly.
Matter exists in three primary states: solid, liquid, and gas. Solids have a definite shape and volume, liquids have a definite volume but take the shape of their container, and gases have neither a definite shape nor volume and expand to fill their container.
In solids, particles are closely packed and vibrate in fixed positions, giving them a definite shape. In liquids, particles are close but can move past each other, allowing them to flow. In gases, particles are far apart, moving freely and at high speeds, resulting in a lack of fixed shape or volume.
Melting point is the temperature at which a solid changes into a liquid at atmospheric pressure. The melting point of ice is 0°C (or 273.15 K).
Wind blows away the water vapor formed due to evaporation that allows more water to escape from the clothes. This speeds up the evaporation process, so clothes dry faster.
In Class 9 Science, students learn the basics of Physics, Chemistry, and Biology. The subject helps build analytical thinking, problem-solving skills, and a strong foundation for higher level science studies.
The states of matter differ in their properties. Solids have a definite shape and volume, liquids have a definite volume but take the shape of their container, and gases have neither definite shape nor volume.
Matter is important in science because it makes up everything around us and helps us understand the physical and chemical properties of substances. Studying matter allows scientists to explain how materials behave, interact, and change, forming the foundation for physics, chemistry, and biology.
The kinetic theory of matter states that all matter is composed of tiny particles that are in constant motion. The speed and energy of these particles vary depending on the state of the matter.
The class 9 science chapter 1 matter in our surroundings question answer discusses several changes in states of matter, including melting, freezing, evaporation, and condensation.
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
