Aakash Repeater Courses
ApplyTake Aakash iACST and get instant scholarship on coaching programs.
Ever wondered what makes atoms stick together to build molecules, how the shape of a molecule can be predicted using hybridisation and how we can determine whether the bond is polar or non-polar? The answer to all these questions lies in NCERT Class 11 Chemistry Chapter 4 Chemical Bonding and Molecular Structure. This chapter explains the properties of chemical bonds, their types, and how they are formed. Chemical bonding is the force that holds atoms together in molecules or crystals. We can relate this chapter to our day-to-day life. For example, the water we drink is a molecule formed by the bonding of hydrogen and oxygen atoms.
JEE Main Scholarship Test Kit (Class 11): Narayana | Physics Wallah | Aakash | Unacademy
NEET Scholarship Test Kit (Class 11): Narayana | Physics Wallah | Aakash | ALLEN
The topics like valence bond theory, hybridisation and overlapping of orbitals are well explained in this chapter. The NCERT solutions for Class 11 Chemistry are designed in a systematic way to help you understand these complex topics discussed in Chemistry Chapter 4 Chemical Bonding and Molecular Structure. These NCERT solutions will help you strengthen your basics through detailed solutions and simple explanations. Various theories have also been discussed in the solutions to give you a clear idea of bonding interactions. We have also included higher-order thinking skills (HOTS) to improve your critical thinking.
Also read
You can download the NCERT solutions for class 11 chemistry chapter 4 PDF from the icon below. These Solutions are designed to help you understand the fundamental concepts and solve questions with ease.
The detailed NCERT solutions for class 11 chemistry chapter 4 to the exercise questions are given below. This is a very important chapter from the point of view of boards and competitive exams.
Question 4.1:Explain the formation of a chemical bond.
Answer :
The attractive force that holds various constituents (atoms, ions, etc.) together in different chemical species is called a chemical bond. Different theories and concepts have been put forward from time to time to analyze the formation of the bond. These are the Kössel-Lewis approach, Valence Shell Electron Pair Repulsion (VSEPR) Theory, Valence Bond (VB) Theory, and Molecular Orbital (MO) Theory.
And every system tends to be more stable, and bonding is nature’s way of lowering the energy of the system to attain stability.
Atoms, therefore, combine with each other and complete their respective octets or duplets to attain a stable configuration of the nearest noble gases. It was seen that the noble gases are very stable and inert and do not react with others.
So, there is a sharing of electrons or transferring one or more electrons from one atom to another; as a result a chemical bond is formed known as a covalent bond or ionic bond.
Question 4.2(a) Write Lewis dot symbols for atoms of the following elements :
Mg
Answer :
There are two valence electrons in the Mg atom as it belongs to the second group (alkaline earth metals). So we will show two dots (electrons) on Mg.
Hence, the Lewis dot symbol for Mg is: Mg¨.
Question 4.2(b) Write Lewis dot symbols for atoms of the following elements :
Na
Answer:
The electronic configuration of Na is 2,8,1. So there is only one electron in the valence shell.
Hence, the Lewis dot structure is Na˙.
Question 4.2(c) Write Lewis dot symbols for atoms of the following elements :
B
Answer :
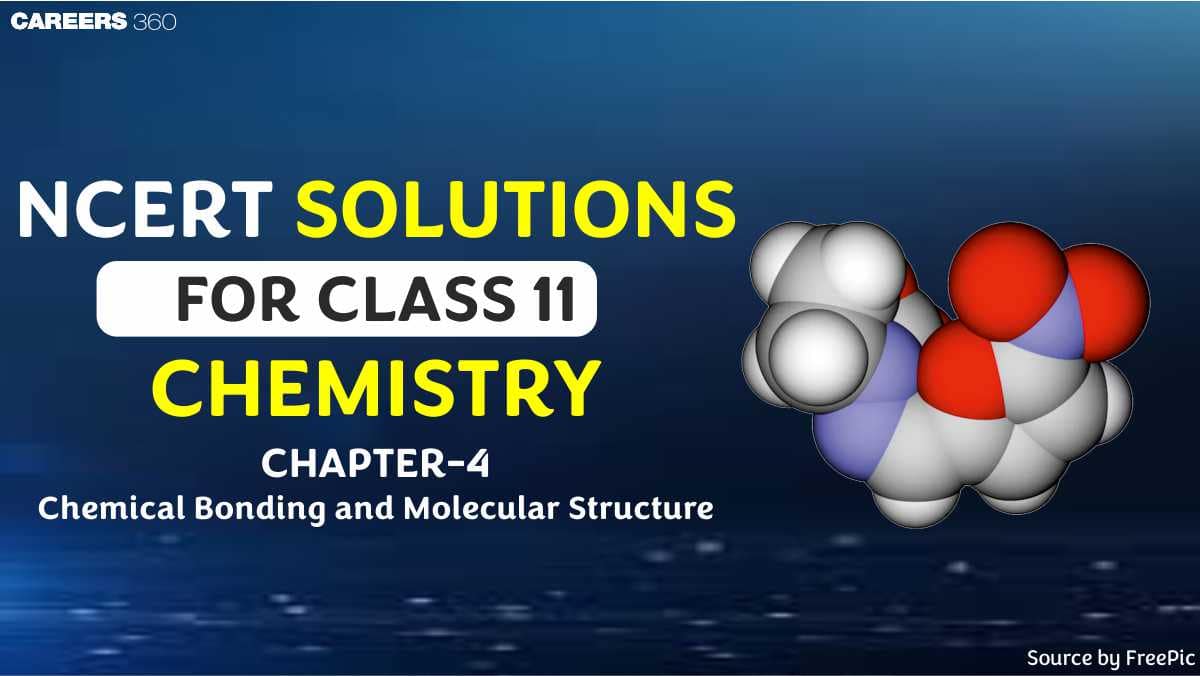
Boron belongs to the 13th group of the periodic table (p-block). So, there are three valence electrons in B atom. Hence, the Lewis dot structure is
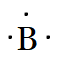
Question 4.2(d) Write Lewis dot symbols for atoms of the following elements :
O ,
Answer :
The electronic configuration oxygen is 2,8,6. so, there are six valence electrons in an atom of O. Hence, the Lewis dot structure is
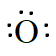
Question 4.2(e) Write Lewis dot symbols for atoms of the following elements :
N,
Answer :
As there are five valence electrons in an atom of N,. Hence, the Lewis dot structure is
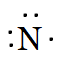
Question 4.2(f) Write Lewis dot symbols for atoms of the following elements :
Answer :
Bromine belongs to halogen family (17th group). So, there are seven valence electrons in an atom of Br. Hence, the Lewis dot structure is
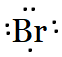
Question 4.3(a) Write Lewis symbols for the following atoms and ions:
Answer :
Sulphur belongs to the oxygen family. So the number of valence electrons in sulphur is six. Therefore, its Lewis dot symbol of sulphur(S) is
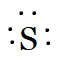
And of S2− is, if it has two electrons more because of its dinegative charge.
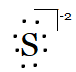
Question 4.3(b) Write Lewis symbols for the following atoms and ions:
Answer :
Al belongs to the boron family, so the number of valence electrons in aluminium is three.
Therefore, the Lewis dot symbol of aluminium(Al) is
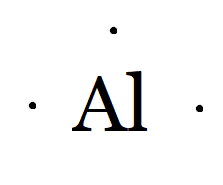
And of Al3+, it means it has donated three electrons and acquires a tripositive charge. Hence, the Lewis symbol is

Question 4.3(c) Write Lewis symbols for the following atoms and ions:
Answer :
As the number of valence electrons in hydrogen is one.
Therefore, its Lewis dot symbol of hydrogen (H) is
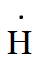
And of H−, it has one more electron because of its negative charge. Hence, the Lewis symbol is
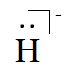
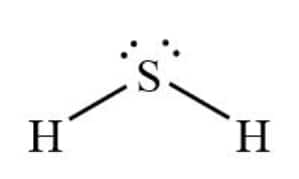
Question 4.4(a) Draw the Lewis structures for the following molecules and ions :
Answer :
Hydrogen atoms each form a single bond with sulphur. As S has six electrons, of which two are bonded to hydrogen, it is left with two lone pairs, giving it a bent shape.
Here, hydrogen completed its duplet and Sulphur its octet
The Lewis structure of H2S is:
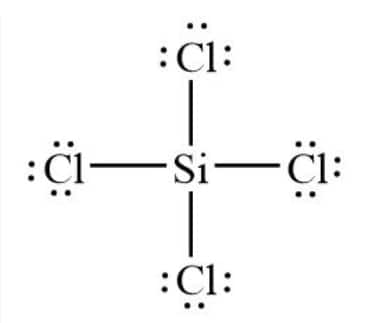
Question 4.4(b) Draw the Lewis structures for the following molecules and ions :
Answer :
Silicon forms four single bonds with four chlorine atoms as Si has 4 valence electrons and chlorine has one. The Lewis structure of SiCl4 is:
Question 4.4(c) Draw the Lewis structures for the following molecules and ions :
Answer :
Be has two valence electrons, with which it forms two single bonds with each F to complete its octet. F has seven electrons in its valence shell. So after bonding, there will be three lone pairs on each F. Beryllium forms two single bonds with two fluorine atoms and has no lone pairs, resulting in a linear shape.
The Lewis structure of BeF2 is:
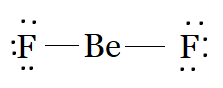
Question 4.4(d) Draw the Lewis structures for the following molecules and ions :
Answer :
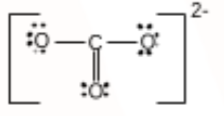
C has 4 valence electrons, and O has 6. So to complete its octet, C needs four more electrons. Carbon forms one double bond and two single bonds with oxygen atoms with a -2 charge delocalized over the three oxygens.
The Lewis structure of CO32− is:
Question 4.4(e) Draw the Lewis structures for the following molecules and ions :
Answer :
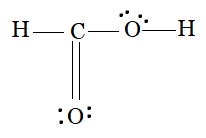
The carbon atom forms a single bond with an oxygen (bonded with a hydrogen), a double bond with one oxygen atom (=O), and a single bond with a hydrogen atom. So, both the oxygens have 2 lone pairs on them.
The Lewis structure of HCOOH is:
Question 4.5 Define octet rule. Write its significance and limitations.
Answer:
Atoms can combine either by transfer of valence electrons from one atom to another (gaining or losing) or by sharing of valence electrons in order to have an octet in their valence shells. This is known as the octet rule.
Significance: It is quite useful for understanding the structures of most of the organic compounds and it applies mainly to the second-period elements of the periodic table
Limitations: There are three types of exceptions to the octet rule.
The incomplete octet of the central atom - the number of electrons surrounding the central atom is less than eight. Examples are LiCl,BeH2,BCl3.
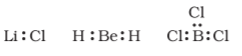
Odd-electron molecules - the octet rule is not satisfied for all the atoms in NO and NO2.
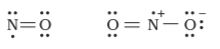
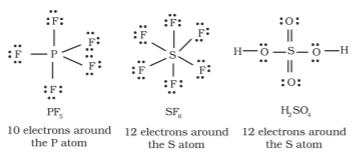
The expanded octet - there are more than eight valence electrons around the central atom. This is termed as the expanded octet. Some of the examples of such compounds are PF5, SF6, H2SO4 and a number of coordination compounds.
Question 4.6 Write the favourable factors for the formation of ionic bond.
Answer :
The formation of an ionic bond takes place by the transfer of one or more electrons from one atom to another. So, ionic bond formation mainly depends upon the ease with which neutral atoms can lose or gain electrons.
The bond formation also depends upon the lattice energy of the compound formed.
Ionic bonds will be formed more easily between elements with comparatively low ionization enthalpies and elements with a comparatively high negative value of electron gain enthalpy.
Question 4.7(a) Discuss the shape of the following molecules using the VSEPR model:
BeCl2 ,
Answer :
Using the VSEPR model, we have BeCl2
The central atom has no lone pair, and there are two bond pairs.
BeCl2 is of the type AB2
Hence, it has a linear shape.
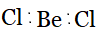
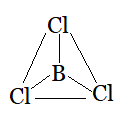
Question 4.7(b) Discuss the shape of the following molecules using the VSEPR model:
(b) BCl3
Answer :
Using the VSEPR model, we have BCl3
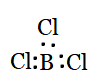
The central atom has no lone pair and there are three bond pairs.
BCl3 is of the type AB3
Hence, it has a trigonal planar shape.
Question 4.7(c) Discuss the shape of the following molecules using the VSEPR model:
(c) SiCl4
Answer :
Using the VSEPR model, we have SiCl4
The central atom has no lone pair, and there are four bond pairs.
SiCl4 is of the type AB4.
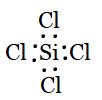
Hence, it has a tetrahedral shape.
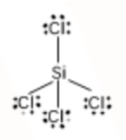
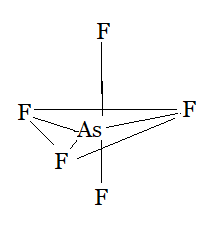
Question 4.7(d) Discuss the shape of the following molecules using the VSEPR model:
(d) AsF5
Answer:
Using the VSEPR model, we have AsF5
The central atom has no lone pair and there are five bond pairs.
AsF5 is of the type AB5
Hence, it has a trigonal bipyramidal shape.
Question 4.7 (e) Discuss the shape of the following molecules using the VSEPR model:
Answer:
Using the VSEPR model we have, H2S
The central atom has no lone pair and there are two bond pairs.
H2S is of the type AB2E
Hence, it has a bent shape.
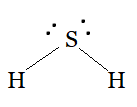
Question 4.7(f) Discuss the shape of the following molecules using the VSEPR model:
(f) PH3
Answer :
Using the VSEPR model, we have PH3
The central atom has no lone pair and there are three bond pairs.
PH3 is of the type AB3E
Hence, it has a trigonal bipyramidal shape.
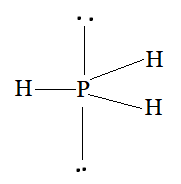
Question 4.9 How do you express the bond strength in terms of bond order?
Answer :
Bond strength refers to the amount of energy required to break the bond between two atoms in a molecule.
So, with an increase in bond order, bond enthalpy increases as a result, bond strength increases.
Question 4.10 Define the bond length.
Answer :
Bond length is defined as the equilibrium distance between the nuclei of two bonded atoms in a molecule.
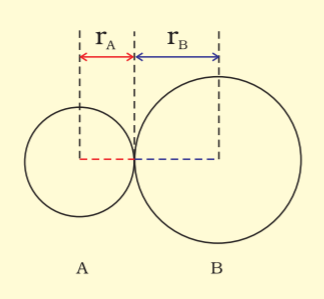
The bond length in a covalent molecule AB.
R=rA+rB where (R is the bond length and rA and rB are covalent radii of atoms A and B respectively.
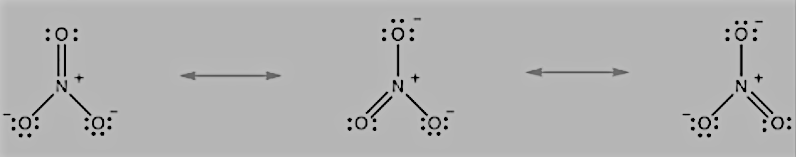
Question 4.11 Explain the important aspects of resonance with reference to the CO32− ion
Answer :
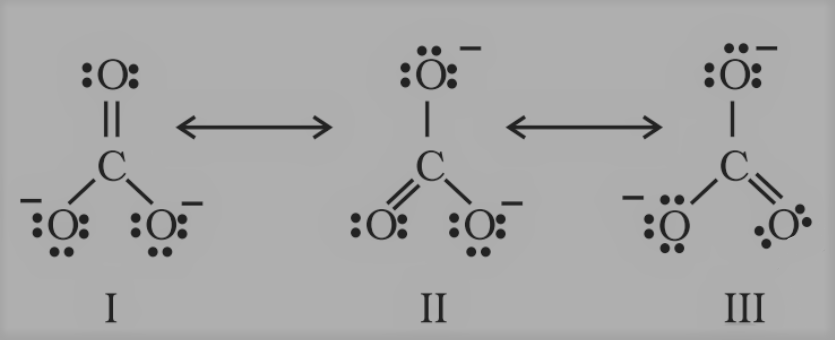
The single Lewis structure based on the presence of two single bonds and one double bond between carbon and oxygen atoms is inadequate to represent the molecule accurately, as it represents unequal bonds. According to the experimental findings, all carbon-to-oxygen bonds in
CO32- are equivalent. Therefore, the carbonate ion is best described as a resonance hybrid of the canonical forms I, II, and III shown below.
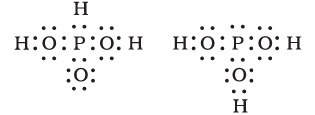
Answer :
The structures have different types of bonding. In the left one, P is doubly bonded to one oxygen, and there are 2 OH groups, while in the other, P is bonded to 3 OH groups.
Hence, the given structures cannot be taken as the canonical forms of the resonance hybrid.
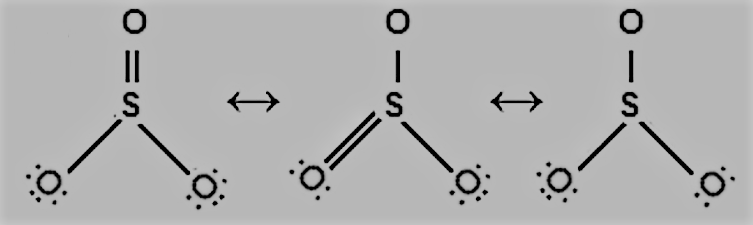
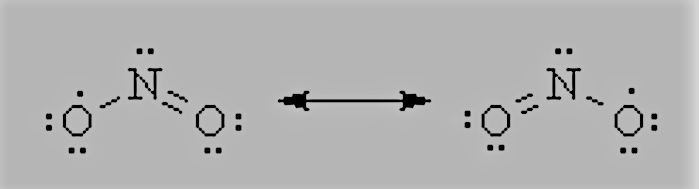
Question 4.13 Write the resonance structures for SO3, NO2, and NO3−.
Answer :
The resonance structures SO3
The resonance structures NO2
The resonance structures NO3−
Question 4.14(a) Use Lewis symbols to show electron transfer between the following atoms to form cations and anions :
Answer :
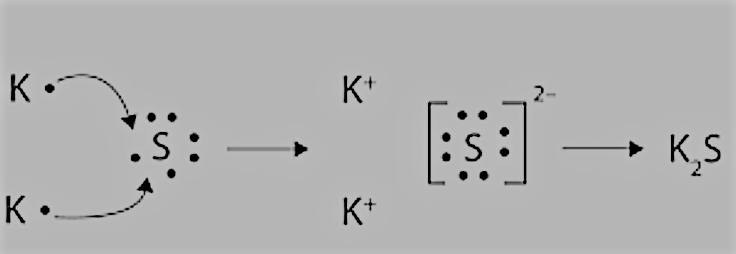
K and S:
We have the electronic configurations of both:
K=2,8,8,1 having 1 electron in the valence shell, and it can donate 1 electron to get to the nearest noble gas configuration.
S=2,8,6 having 6 electrons in the valence shell, so it can complete its octet by accepting 2 more electrons.
So, there will be an electron transfer between them as follows:
Question 4.14(b) Use Lewis symbols to show electron transfer between the following atoms to form cations and anions :
Answer :
CaandO :
We have the electronic configurations of both:
Ca=2,8,8,2 has 2 electrons in the valence shell, and it can donate 2 electrons to get to the nearest noble gas configuration.
O=2,6 has 6 electrons in the valence shell, so it can complete its octet by accepting 2 more electrons.
So, there will be an electron transfer between them as follows:
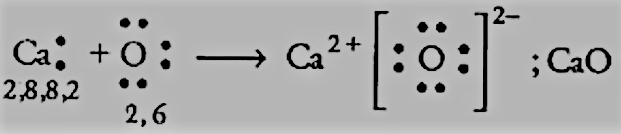
Question 4.14(c) Use Lewis symbols to show electron transfer between the following atoms to form cations and anions :
Answer :
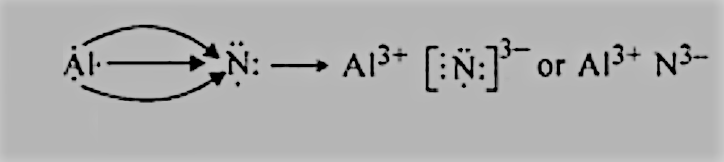
AlandN. :
We have the electronic configurations of both:
Al=2,8,3 has 3 electrons in the valence shell, and it can donate 3 electrons to get to the nearest noble gas configuration.
N=2,5 has 3 electrons in the valence shell, so it can complete its octet by accepting 2 more electrons.
So, there will be electron transfer between them as follows:
Answer :
H2O molecule, which has a bent structure, the two O–H bonds are oriented at an angle of 104.50. The net dipole moment of 6.17 × 10-30 C m is the resultant of the dipole moments of two O–H bonds.
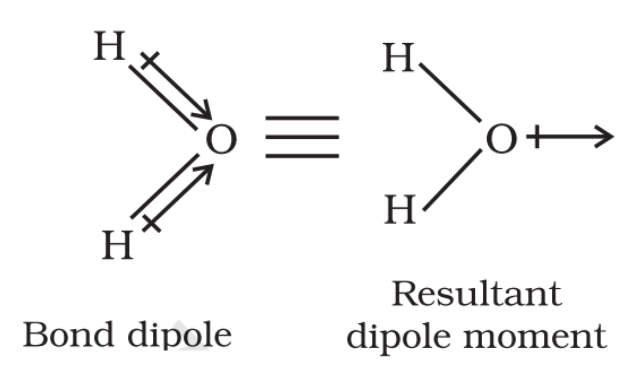
On the other hand, the dipole moment of carbon dioxide is zero. This may be because of linear shape of the molecule as it has two C-O bonds, which have opposite dipole moments cancelling each other.
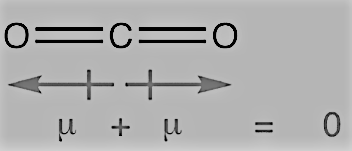
Question 4.16 Write the significance/applications of dipole moment.
Answer :
Some of the important significance of the dipole moment is as follows:
1. We can determine the shape of the molecule. Symmetrical molecules like linear, etc., do have zero dipole moment, whereas if not symmetrical, then they take different shapes, such as a bent shape or some angular shapes.
2. For determining the polarity of the molecules, the greater the dipole moment value, the greater will be the polarity and vice versa.
3. We can say that if a molecule has a zero dipole moment, then it must be non-polar, and if it is non-zero, then it must have some polar character.
Question 4.17 Define electronegativity. How does it differ from electron gain enthalpy?
Answer :
Electronegativity is the ability of an atom in a compound to attract a bond pair of electrons towards itself. It cannot be measured and it is a relative number.
The electron gain enthalpy, △egH, is the enthalpy change when a gas-phase atom in its ground state gains an electron. The electron gain process may be exothermic or endothermic.
An element has a constant value of the electron gain enthalpy that can be measured experimentally.
Question 4.18 Explain with the help of suitable example polar covalent bond.
Answer :
A polar covalent bond forms when two different atoms share electrons unequally in a covalent bond due to a difference in their electronegativities that causes the shared electron pair to shift toward the more electronegative atom.
For examples, in H2O ,
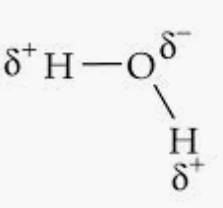
Here, slightly positive charges are developed in hydrogen atoms and slightly negative charges are developed in oxygen atoms as oxygen is more electronegative than hydrogen. Thus, opposite poles are developed in the molecule.
Hence, the bond pair lies towards the oxygen atom.
Question 4.19 Arrange the bonds in order of increasing ionic character in the molecules:
Answer :
The ionic character in a molecule depends on the electronegativity difference between the constituting atoms. The greater the difference more ionic the character of the molecule.
So, on this basis, we have the order of increasing ionic character in the given molecules.
N2<SO2<ClF3<K2O<LiF .
Question 4.20 The skeletal structure of CH3COOH as shown below is correct, but some of the bonds are shown incorrectly
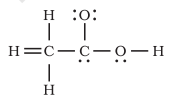
Answer :
Here hydrogen atom is bonded to carbon with a double bond, which is not possible because hydrogen has only one electron to share with carbon.
Also, the second carbon does not have its valency satisfied means it has formed five bonds instead of four.
Therefore, the correct skeletal structure of CH3COOH as shown below:
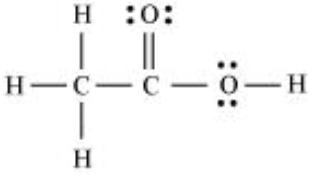
Answer :
The electronic configuration of a carbon atom is C:1s22s22p2.
Where it has s-orbital, p-orbital only, and there is no d-orbital present.
Hence, the carbon atom undergoes sp3 hybridization in the methane molecule and takes a tetrahedral shape.
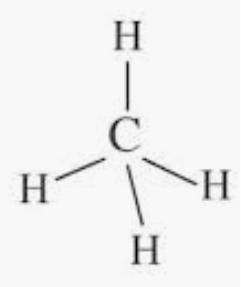
And for a molecule to have a square planar structure, it must have a d orbital present.
But here the absence of d-orbital, as a result, it does not undergo dsp2 hybridization, the structure of methane cannot be square planar.
Also, the reason that the bond angle in square planar 90∘ which makes the molecule more unstable because of repulsion between the bond pairs.
Hence, according to VSEPR theory CH4 molecule takes a tetrahedral structure.
Question 4.22 Explain why BeH2 molecule has a zero dipole moment, although the Be−H bonds are polar.
Answer :
BeH2 molecule has a zero dipole moment because the two equal bond dipoles point in opposite directions and cancel the effect of each other.
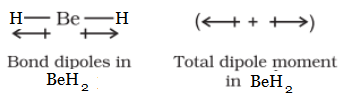
Question 4.23 Which out of NH3 and NF3 has a higher dipole moment and why?
Answer :
Here, both have nitrogen as a central atom, and it has a lone pair of electrons with three bond pairs.
Hence, both molecules have a pyramidal shape.
The electronegativity of fluorine is more as compared than that of hydrogen. Hence, it is expected that the net dipole moment of NF3 is greater than NH3.
However, NH3 has the net dipole moment of 1.46D and NF3 has the net dipole moment of 0.24D. which is greater than NF3 .
This is because of the direction of the dipole moments of each bond in NH3 and NF3.
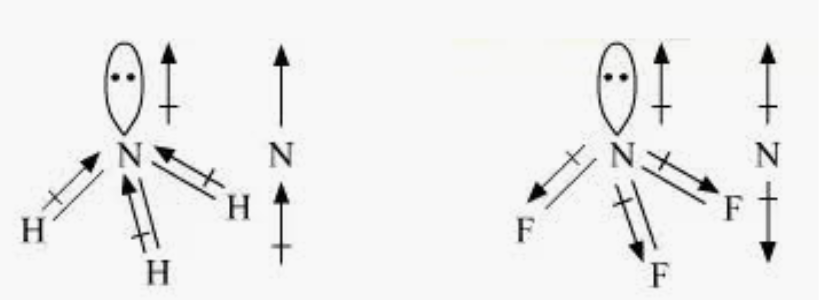
The moments of the lone pair in NF3 partly cancel out. But in NH3, the resultant moment adds up to the bond moment of the lone pair.
Question 4.24 What is meant by hybridisation of atomic orbitals? Describe the shapes of sp , sp2 , sp3 hybrid orbitals.
Answer :
Hybridisation can be defined as the process of intermixing the orbitals of slightly different energies so as to redistribute their energies, resulting in the formation of a new set of orbitals of equivalent energies and shape.
The shapes of sp , sp2 , sp3 hybrid orbitals are shown:
sp hybrid orbital: It is linear in shape and formed by the intermixing of s and p orbitals.
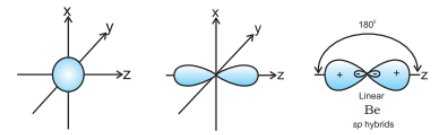
sp2 hybrid orbital: It is the trigonal planar shape and is formed by the intermixing of one s-orbital and two 2p-orbitals.
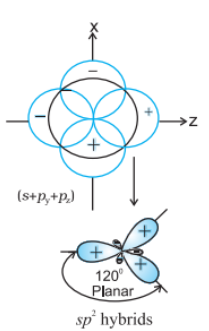
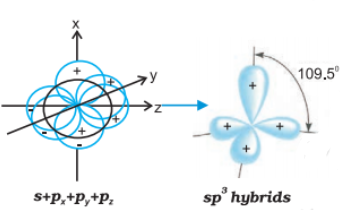
sp3 hybrid orbital: It is tetrahedral in shape and is formed by the intermixing of one s-orbital and three p-orbitals.
Question 4.25 Describe the change in hybridisation (if any) of the Al atom in the following reaction.
Answer :
Initially, the aluminium is in the ground state, and the valence orbital can be shown as:
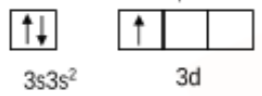
Then the electron gets excited so the valence orbital can be shown as:
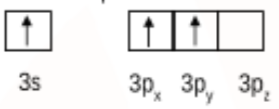
So, initially, aluminium (AlCl3) had sp2 hybridisation and hence had a trigonal planar shape.
Then it reacts with chloride ion to form AlCl4−. Where it has the empty 3pz orbital which gets involved and the hybridisation changes from sp2→sp3.
Hence, there is a shape change from trigonal planar to tetrahedral.
Question 4.26 Is there any change in the hybridisation of B and N atoms as a result of the following reaction?
Answer :
Initially boron atom BF3 was in sp2 hybridised. The valence orbital of boron in the excited state can be shown as:
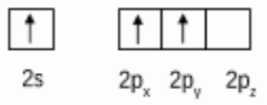
And nitrogen atom in NH3 is sp3 hybridised. The valence orbital of nitrogen in the excited state can be shown as:
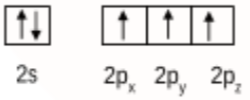
Then, after the reaction has occurred, the product F3B.NH3 is formed by the hybridisation of 'B' changes to sp3. However, the hybridisation of 'N' remains unchanged.
Question 4.27 Draw diagrams showing the formation of a double bond and a triple bond between carbon atoms in C2H4 and C2H2 molecules.
Answer :
We have the electronic configuration of the C-atom in the excited state:
C=1s22s12px12py12pz1
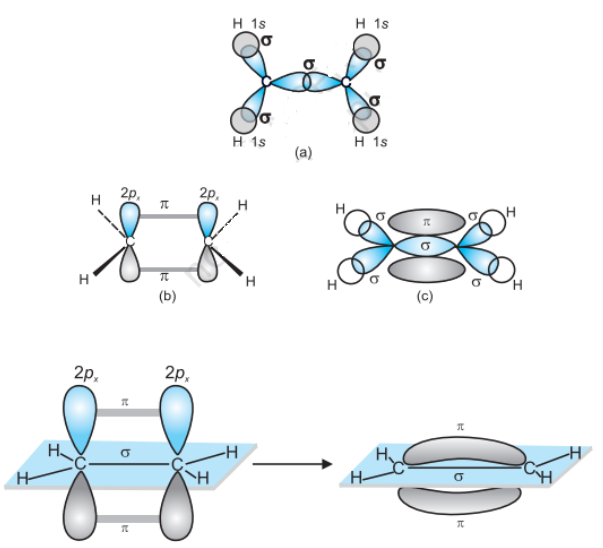
Formation of an ethane molecule (C2H4) by overlapping of a sp2 hybridized orbital of another carbon atom, thereby forming a C−C sigma bond.
The remaining two sp2 orbitals of each carbon atom form a sp2−s sigma bond with two hydrogen atoms. The unhybridized orbital of one carbon atom undergoes sidewise overlap with the orbital of a similar kind present on another carbon atom to form a weak n-bond.
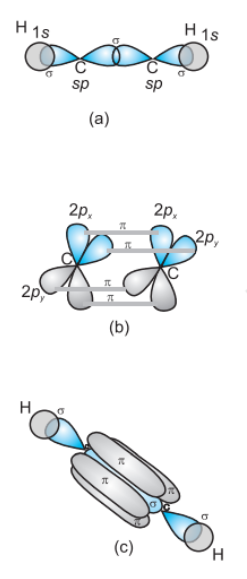
Formation of C2H2 molecule- each C-atom is sp-hybridized with two 2p-orbitals in an unhybridized state.
One sp hybrid orbital of one carbon atom overlaps axially with the sp hybrid orbital of the other carbon atom to form a C-C sigma bond, while the other hybridised orbital of each carbon atom overlaps axially with the half-filled s orbital of the hydrogen atoms forming σ bonds.
Each of the two unhybridised p orbitals of both the carbon atoms overlaps sideways to form two π bonds between the carbon atoms. So the triple bond between the two carbon atoms is made up of one sigma and two pi bonds as shown in Fig
Question 4.28(a) What is the total number of sigma and pi bonds in the following molecules?
Answer :
Given molecule C2H2 :
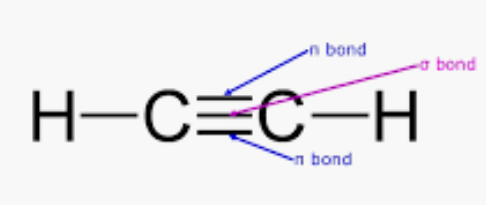
So, there are three sigma (2C-H bonds + 1 C-C bond) and two pi-bonds (2 C-C bonds) in C2H2.
Question 4.28(b) What is the total number of sigma and pi bonds in the following molecules?
Answer :
Given molecule C2H4 :
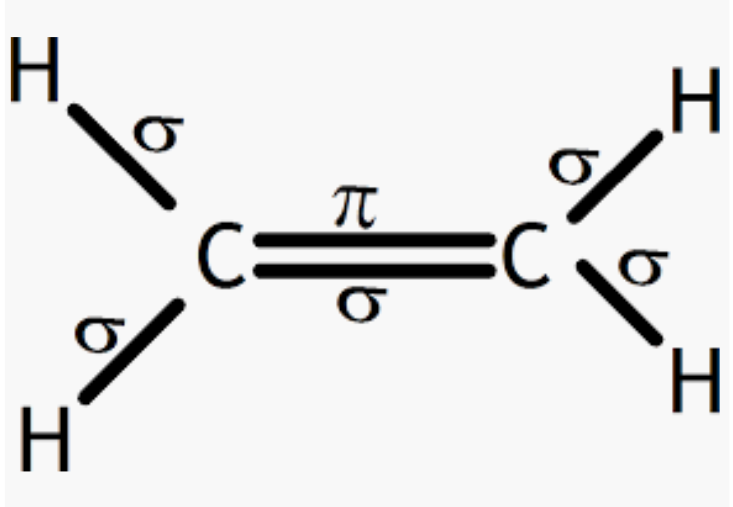
So, there are five sigma (4C-H bonds + 1 C-C bond) and one pi-bond (C-C bonds) in C2H4.
Question 4.29(a) Considering x-axis as the internuclear axis which out of the following will not form a sigma bond and why?
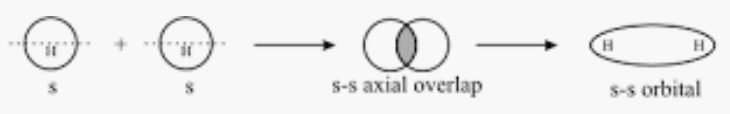
Answer :
Orbitals 1sand1s will form a sigma bond as both orbitals are spherical and can combine along the x-axis as the internuclear axis.
Question 4.29(b) Considering x-axis as the internuclear axis which out of the following will not form a sigma bond and why?
Answer :
Orbitals 1sand2px will form a sigma bond as 1s orbital and 2p x orbital are aligned such that they can combine along the x-axis as the internuclear axis.

Question 4.29(c) Considering x-axis as the internuclear axis which out of the following will not form a sigma bond and why?
Answer :
Orbitals 2pyand2py will not form a sigma bond as both 2py orbitals are aligned in y-direction but the internuclear axis is the x-axis.
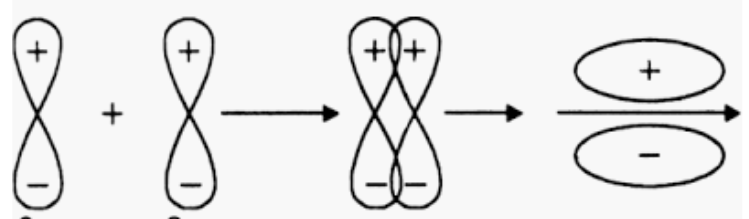
The formation of a pi bond takes place.
Question 4.29(d) Considering x-axis as the internuclear axis which out of the following will not form a sigma bond and why?
Answer :
Orbitals 1sand2s. will form a sigma bond as both 1s and 2s orbitals are spherical and can combine along the x-axis as the internuclear axis.
Question 4.30(a) Which hybrid orbitals are used by carbon atoms in the following molecules?
Answer :
CH3−CH3
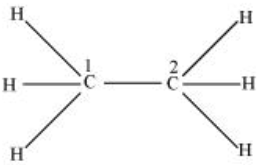
There are four sigma bonds (single bond), each with the help of one s hybrid orbital and three p hybrid orbitals. Hence, C1 and C2 are sp3 hybridized.
Question 4.30(b) Which hybrid orbitals are used by carbon atoms in the following molecules?
Answer :
CH3−CH=CH2;
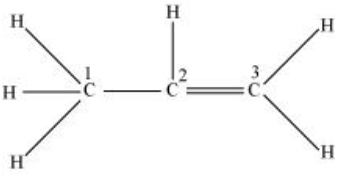
C1 is making 4 sigma bonds (single bond) therefore it is sp3 hybridised.
While C2 and C3 are making a double bond. (1sigma bond+1 pi bond)
Therefore, they both are sp2 hybridized.
Question 4.30(c) Which hybrid orbitals are used by carbon atoms in the following molecules?
Answer :
CH3−CH2−OH
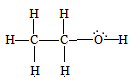
C1 is making 4 sigma bonds (single bonds), therefore it is sp3 hybridised.
and C2 is also making a 4 sigma bond. Therefore, it is also sp3 hybridised.
Therefore, they both are sp3 hybridized.
Question 4.30(d) Which hybrid orbitals are used by carbon atoms in the following molecules?
Answer :
CH3−CHO
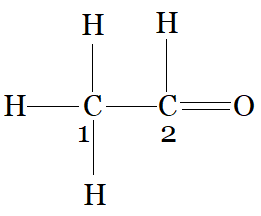
C1 is making 4 sigma bonds (single bonds), therefore it is sp3 hybridised.
and C2 is making a 3 sigma bonds with hydrogen, carbon and oxygen. and one pi bond with oxygen,n therefore it is sp2 hybridised.
Question 4.30(e) Which hybrid orbitals are used by carbon atoms in the following molecules?
Answer :
CH3COOH
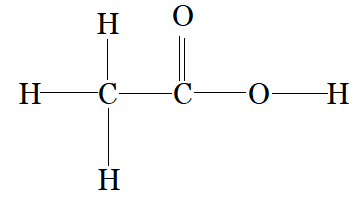
C1 is making 4 sigma bonds (single bond) therefore it is sp3 hybridised.
and C2 is making a 2 sigma bonds with carbon and 1 sigma bond with oxygen and one pi bond with oxygen therefore, it is sp2 hybridised.
Question 4.31 What do you understand by bond pairs and lone pairs of electrons? Illustrate by giving one exmaple of each type.
Answer :
The shared pairs of electrons present between the bonded atoms are called bond pairs.
And all valence electrons may not participate in bonding; those electron pairs that do not participate in bonding are called lone pairs of electrons.
For example,
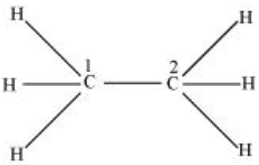
In C2H6 ethane, there are seven bond pairs but no lone pair is present.
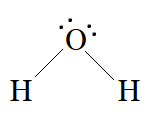
In H2O , there are two bond pairs and two lone pairs on the central atom (oxygen).
Question 4.32 Distinguish between a sigma and a pi bond.
Answer :
The difference between the sigma bond and the pi bond is shown in the table below:
Sigma (σ) Bond | Pi (π) Bond |
(a) Formed by end-to-end overlapping of orbitals. | Formed by the lateral overlapping of orbitals |
(b) Sigma bonds are stronger than the pi bond. | Weak bond. |
(c) The orbitals involved in the overlapping are s-s, s-p, and p-p. | Bonds are formed only with the overlapping of p-p orbitals. |
(d) The electron cloud is symmetrical about an internuclear axis. | The electron cloud is not symmetrical. |
(e) Free rotation is possible in the case of a sigma bond. | Rotation is restricted in the case of pi-bonds. |
Question 4.33 Explain the formation of H2 molecule on the basis of valence bond theory.
Answer :
Formation of H2 molecule:
Assume that two hydrogen atoms (A and B) with nuclei (NA and NB) and electrons (eA and eB) are taken to undergo a reaction to form a hydrogen molecule.
When the two atoms are at a large distance, there is no interaction between them. As they approach each other, the attractive and repulsive forces start operating.
An attractive force arises between:
(a) The nucleus of one atom and its electron, i.e., NA−eA and NB−eB .
(b) The nucleus of one atom and an electron of another atom i.e., NA−eB and NB−eA
Repulsive force arises between:
(a) Electrons of two atoms i.e., eA−eB .
(b) Nuclei of two atoms i.e., NA−NB .
The force of attraction brings the two atoms together, whereas the force of repulsion tends to push them apart.
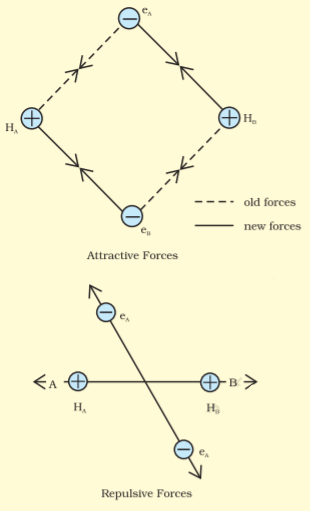
The attractive force overcomes the repulsive force. Hence, the two atoms approach each other. As a result, the potential energy decreases. Finally, a state is achieved when the attractive forces balance the repulsive forces and the system acquires minimum energy. This leads to the formation of a dihydrogen molecule.
Question 4.34 Write the important conditions required for the linear combination of atomic orbitals to form molecular orbitals.
Answer :
The important conditions required for the linear combination of atomic orbitals to form molecular orbitals are as follows:
1. The combining atomic orbitals must have the same or nearly the same energy.
2..The combining atomic orbitals must have the same symmetry about the molecular axis.
3. The combining atomic orbitals must overlap to the maximum extent.
Question 4.35 Use molecular orbital theory to explain why the Be2 molecule does not exist.
Answer :
The electronic configuration of Be is 1s22s2 .
From the molecular orbital electronic configuration, we have for Be2 molecule,
σ1s2σ1s∗2σ2s2σ2s∗2
We can calculate the bond order for Be2 is =12(Nb−Na) where,
Nb is the number of electrons in bonding orbitals and Na is the number of electrons in anti-bonding orbitals.
So, therefore we have,
Bond order of Be2=12(4−4)=0
that means that the molecule is unstable.
Hence, Be2 molecule does not exist.
Question 4.36 Compare the relative stability of the following species and indicate their magnetic properties;
O2,O2+,O2−−(superoxide), O22−(peroxide)
Answer :
The electronic configuration of O2 molecule can be written as:
(σ1s)2(σ∗1s)2(σ2s)2(σ∗2s)2(σ2pz)2
(π2px2≡π2py2)(π∗2px1≡π∗2py1)
Here, the number of bonding electrons is Nb=10 and the number of antibonding electrons is Na=6.
Therefore,
Bond order=12(Nb−Na)
=12(10−6)=2
The electronic configuration of O2+ molecule can be written as:
(σ1s)2(σ∗1s)2(σ2s)2(σ∗2s)2(σ2pz)2
(π2px2≡π2py2)(π∗2px1)
Here, the number of bonding electrons is Nb=10 and the number of antibonding electrons is Na=5.
Therefore,
Bond order=12(Nb−Na)
=12(10−5)=2.5
The electronic configuration of O2− molecule can be written as:
(σ1s)2(σ∗1s)2(σ2s)2(σ∗2s)2(σ2pz)2
(π2px2≡π2py2)(π∗2px2≡π∗2py1)
Here, the number of bonding electrons is Nb=10 and the number of antibonding electrons is Na=7.
Therefore,
Bond order=12(Nb−Na)
=12(10−7)=1.5
The electronic configuration of O22− molecule can be written as:
(σ1s)2(σ∗1s)2(σ2s)2(σ∗2s)2(σ2pz)2
(π2px2≡π2py2)(π∗2px2≡π∗2py2)
Here, the number of bonding electrons is Nb=10 and the number of antibonding electrons is Na=8.
Therefore,
Bond order=12(Nb−Na)
=12(10−8)=1
Therefore, the bond dissociation energy is directly proportional to the bond order.
Thus, the higher the bond order, the greater will be the stability.
We get this order of stability:
O2+>O2>O2−>O22−
Question 4.37 Write the significance of a plus and a minus sign shown in representing the orbitals.
Answer :
Wave functions can be used to represent molecular orbitals. The plus and minus represent the positive wave function and negative wave function, respectively.
Question 4.38 Describe the hybridisation in case of PCl5. Why are the axial bonds longer as compared to equatorial bonds?
Answer :
The initial ground state and final excited state electronic configuration of phosphorus (P) are:
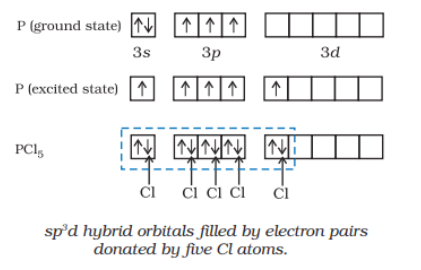
So, the phosphorus atom is sp3d hybridized in the excited state. The donated electron pairs by five Cl atoms are filled and make PCl5..
The resultant shape is trigonal bipyramidal and the five sp3d hybrid orbitals are directed towards the five corners.
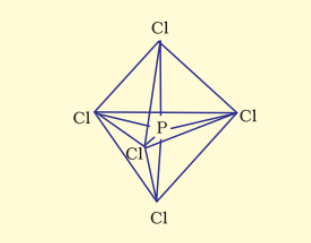
In the five P-Cl sigma bonds, three lie in one plane and make 120∘ with each other, are equatorial bonds and the two P-Cl bonds lie above and below the equatorial plane making an angle of 90∘ with the plane, are axial bonds.
So, because of more repulsion from the equatorial bond pairs, the axial bonds are slightly longer than the equatorial bonds.
Question 4.39 Define hydrogen bond. Is it weaker or stronger than the van der Waals forces?
Answer :
A hydrogen bond can be defined as the attractive force acting between the hydrogen atom of one molecule with the electronegative atom (F, O or N) of another molecule.
Because of the difference between electro-negativities, the bond pair between hydrogen and the electronegative atom drifts towards a more electronegative atom. As a result, the hydrogen atom becomes slightly positively charged.
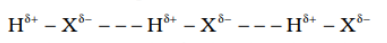
Hydrogen bonds are stronger than van der Waals forces because H-bonds are considered as an extreme form of dipole-dipole interaction.
Question 4.40 What is meant by the term bond order? Calculate the bond order of :
Answer :
Bond order (B.O.) is defined as one-half the difference between the number of electrons present in the bonding and the antibonding orbitals of a molecule.
Bond Order=12(Nb−Na)
Where Nb andNa are the number of electrons occupying bonding orbitals and the number occupying the antibonding orbitals, respectively.
So, the bond orders for different molecules are:
N2 : The electronic configuration is (σ1s)2(σ∗1s)2(σ2s)2(σ∗2s)2
(π2px)2(π2py)2(σ2pz)2
Where, the number of bonding electrons Nb=10 and number of antibonding electrons, Na=4
So, Bond order of nitrogen molecule =12(10−4)=3
O2 : The electronic configuration is (σ1s)2(σ∗1s)2(σ2s)2(σ∗2s)2(σ2pz)2
(π2px2≡π2py2)(π∗2px1≡π∗2py1)
Where, the number of bonding electrons Nb=10 and number of antibonding electrons, Na=6
So, Bond order of nitrogen molecule =12(10−6)=2
O2+ : The electronic configuration is (σ1s)2(σ∗1s)2(σ2s)2(σ∗2s)2(σ2pz)2
(π2px2≡π2py2)(π∗2px1)
Where, the number of bonding electrons Nb=8 and number of antibonding electrons, Na=3
So, Bond order of O2+ molecule =12(8−3)=2.5
O2− : The electronic configuration is (σ1s)2(σ∗1s)2(σ2s)2(σ∗2s)2(σ2pz)2
(π2px2≡π2py2)(π∗2px2≡π∗2py1)
Where, the number of bonding electrons Nb=8 and number of antibonding electrons, Na=5
So, Bond order of O2− molecule =12(8−5)=1.5
These Higher Order Thinking Skills questions are based on NCERT Solutions for Class 11 Chemistry Chapter 4 Chemical Bonding and Molecular Structure. Practise these questions to develop conceptual understanding and problem solving ability.
Question: Which of the following molecules(s) show/s paramagnetic behavior ?
(A) O2
(B) N2
(C) F2
(D) S2
(E) Cl2
Choose the correct answer from the options given below :
1) B only
2) A & C only
3) A & E only
4) A & D only
Answer:
| No. of umpaired e− | ||
| (A) | O2 | 2 |
| (B) | N2 | 0 |
| (C) | F2 | 0 |
| (D) | S2 | 2 |
| (E) | Cl2 | 0 |
If species contain unpaired electron than it is paramagnetic.
So A & D are paramagnetic.
Hence, the correct answer is option (4).
Question: Which of the following is not a postulate of VSEPR theory?
(1) The shape of a molecule depends upon the number of valence shell electron pairs (bonded or non-bonded) around the central atom.
(2) Pairs of electrons in the valence shell repel one another since their electron clouds are negatively charged.
(3) The positions of the electron pairs in space around the central atom are such that they maximise repulsion and thus minimise the distance between them.
(4) The magnitudes of the different types of electronic repulsions follow the order given below: Lone pair - Lone pair > Lone pair - Bonding pair > Bonding pair - Bonding pair
Answer:
The main postulates of VSEPR theory are:
The actual shape of a molecule depends upon the number of electron pairs (bonded or non–bonded) around the central atom.
The electron pairs tend to repel each other due to their negative charge.
Electron pairs arrange themselves in such a way that there exists a minimum repulsion between them.
The valence shell is considered as a sphere with the electron pairs placed at a distance.
A multiple bond is treated as if it is a single electron pair & the electron pairs that constitute the bond as a single pair.
The repulsive interaction of electron pairs decreases in the order as mentioned below:
Lone pair (lp) – Lone pair (lp) > Lone pair (lp) –
Bond pair (bp) > Bond pair (bp) – Bond pair (bp).
Double bonds cause more repulsion than single bonds, and triple bonds cause more repulsion than double bonds. This repulsion decreases sharply with increasing bond angle between the electron pairs.
The third statement is wrong as it says the elctrons arrangement is such that it maximize repulsion which is not true.
Hence, the correct answer is option (3).
Question: Which of the following compounds has the lowest melting point?
(1) NaCl
(2) MgCl2
(3) AlCl3
(4) BeCl2
Answer:
AlCl3 has the lowest melting point due to its covalent character, which is a result of the high charge density of Al3+. BeCl2 also has a lower melting point but higher than AlCl3.
Hence, the correct answer is option (3).
Students can refer to the effective approaches to solve Chemical Bonding and Molecular Structure questions and answers. The following points will help you build a good strategy for Class 11 Chemical Bonding and Molecular Structure NCERT Solutions.
1. Review the chapter
Before solving questions it is very important to understand the structure of the chapter and break the chapter into manageable sections.
2. Understand the concepts
Try to learn and memorize key concepts like Lewis structure, octet rule, bond parameters, hybridization, etc. Most of the Chemical Bonding and Molecular Structure questions and answers are often asked directly from these topics. You can revise these concepts from notes available on our website.
3. Learn bonding theories
The theories like valence bond theory, valence shell electron pair repulsion theory and molecular orbital theory are too crucial to understand. Make sure to understand their applications and limitations. Also, use NCERT solutions for class 11 chemistry chapter 4 PDF for effective learning.
4. Attempt questions wisely
First, read the questions thoroughly and note down the given information. Apply the concepts learned and solve in a step-wise manner.
5. Practice regularly
Solve the NCERT in-text and exercise questions. You can also refer to the solved examples to learn how to answer the question. The NCERT Solutions for Class 11 Chemistry Chapter 4 Chemical Bonding and Molecular Structure will make your learning feasible.
All the topics and subtopics listed below are well explained in the Class 11 Chemistry Chapter 4 Notes. Students can access these notes available on our website.
4.1 Kössel-Lewis Approach to Chemical Bonding
4.1.1 Octet Rule
4.1.2 Covalent Bond
4.1.3 Lewis Representation of Simple Molecules( the Lewis Structures)
4.1.4 Formal Charge
4.1.5 Limitations of the Octet Rule
4.2 Ionic or Electrovalent Bond
4.2.1 Lattice Enthalpy
4.3 Bond Parameters
4.3.1 Bond Length
4.3.2 Bond Angle
4.3.3 Bond Enthalpy
4.3.4 Bond Order
4.3.5 Resonance Structures
4.3.6 Polarity of Bonds
4.4 The Valence Shell Electron Pair Repulsion (VSEPR) Theory
4.5 Valence Bond Theory
4.5.1 Orbital Overlap Theory
4.5.2 Directional Properties of Bonds
4.5.3 Overlapping of Atomic Orbitals
4.5.4 Types of Overlapping and Nature of Covalent Bonds
4.5.5 Strength of Sigma and Pi Bonds
4.6 Hybridisation
4.6.1 Types of Hybridisation
4.6.2 Other Examples of sp3, sp2 and sp Hybridisation
4.6.3 Hybridisation of Elements Involving d Orbitals
4.7 Molecular Orbital Theory
4.7.1 Formation of Molecular Orbitals Linear Combination of Atomic Orbitals (LCAO)
4.7.2 Conditions for the Combination of Atomic Orbitals
4.7.3 Types of Molecular Orbitals
4.7.4 Energy Level Diagram for Molecular Orbitals
4.7.5 Electronic Configuration and Molecular Behaviour
4.8 Bonding in Some Homonuclear Diatomic Molecules
4.9 Hydrogen Bonding
4.9.1 Cause of Formation of Hydrogen Bond
4.9.2 Types of H-Bonds
Excel your exam preparations by solving NCERT chapter-wise solutions. Click on the link below
Follow the links below to get access to the NCERT solutions for other subjects as well.
| NCERT solutions for Class 11 Biology |
| NCERT solutions for Class 11 Chemistry |
| NCERT solutions for Class 11 Maths |
| NCERT solutions for Class 11 Physics |
Click on the links below to get the syllabus and recommended books.
| NCERT Books Class 11 Chemistry |
| NCERT Books Class 11 |
| NCERT Syllabus Class 11 Chemistry |
| NCERT Syllabus Class 11 |
Atoms combine to achieve a state of lower energy and greater stability. They tend to attain the stable electron configuration of the nearest noble gas. This can be achieved by losing, gaining, or sharing electrons.
Hybridization is the concept of intermixing atomic orbitals of slightly different energies belonging to the same atom to form a new set of equivalent orbitals called hybrid orbitals. These hybrid orbitals are equal in energy and shape and are more effective in forming stable bonds.
Valence Shell Electron Pair Repulsion Theory states that electron pairs, both lone pairs and bond pairs, in the valence shell of the central atom repel each other and arrange themselves in space to minimize this repulsion. The minimized repulsion leads to a specific geometric arrangement of electron pairs, which in turn determines the molecular geometry and bond angles. Lone pairs exert more repulsion than bond pairs (lp-lp > lp-bp > bp-bp). Topics like these are well explained in our class 11 chemical bonding notes.
It is a measure of the polarity of a bond or molecule. It is the product of the magnitude of the charge and the distance between the charges. Nonpolar molecules have a zero or very small net dipole moment, while polar molecules have a net dipole moment.
Bond strength and bond order are directly proportional to each other. As bond order increases, bond strength also increases which means a higher bond order corresponds to a stronger bond between two atoms.
Application Date:24 July,2025 - 23 August,2025
Application Date:24 July,2025 - 23 August,2025

Take Aakash iACST and get instant scholarship on coaching programs.

This ebook serves as a valuable study guide for NEET 2025 exam.

This e-book offers NEET PYQ and serves as an indispensable NEET study material.

As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
As per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
As per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE