NCERT Solutions for Class 7 Science Chapter 3 Electricity: Circuits and their Components
Have you ever flicked a switch and turned on a bulb, or flicked a switch and turned on a fan with a single button? All these daily activities are possible due to electricity, which is a strong source of energy that is transmitted via wires and circuits to ensure that our lives are made comfortable. With the NCERT Solutions for Class 7 Science Chapter 3 - Electricity: Circuits and their Components, students are introduced to the fundamentals of the operation of electric circuits, the components of the electric circuit and how electricity is used in our day-to-day lives.
This Story also Contains
- Download Class 7 Science Chapter 3 - Electricity: Circuits and their Components Question Answers PDF
- Electricity: Circuits and their Components NCERT Solutions: Exercise Questions
- Electricity: Circuits and their Components NCERT Solutions: Exploratory Projects
- Class 7 Science Chapter 3 - Electricity: Circuits and their Components: Additional Questions
- Electricity: Circuits and their Components Class 7 Science Chapter 3: Topics
- Approach to Solve Questions of Class 7 Science Chapter 3 – Electricity: Circuits and Their Components
- Benefits of NCERT Solutions for Class 7 Science Curiosity Chapter 3
- What Students Learn from NCERT Solutions for Class 7 Science Chapter 3?
- NCERT Solutions for Class 7 Science Chapter Wise
- NCERT Solutions for Class 7 Subject Wise
- NCERT Books and NCERT Syllabus
.jpg)
The NCERT Solutions for Class 7 Science Chapter 3 - Electricity: Circuits and their Components cover clear step-by-step answers to all questions asked in the in-text and exercises, which makes concepts easy to understand. These NCERT Solutions elaborate on how to sketch circuit diagrams with standard symbols, which is an important skill in science and engineering. By paying attention to practical and real-life uses of electricity, they also make the chapter interesting and practical. Revising the important concepts, definitions and principles of electricity and circuits that students need to learn in their examinations is easy by studying the Class 7 Science Chapter 3 - Electricity: Circuits and their Components question answers. These NCERT Solutions for Class 7 Science Chapter 3 - Electricity: Circuits and their Components can be used as a good study guideline to reinforce knowledge, gain confidence and achieve improved performance in exams. Whenever students want to revise something in a hurry, they can download some free PDF solutions and do so.
Download Class 7 Science Chapter 3 - Electricity: Circuits and their Components Question Answers PDF
Electricity: Circuits and their Components Class 7 question answers are available to download so that you can prepare your exam easily and effectively. These Electricity: Circuits and their Components NCERT Solutions give step-by-step solutions to all the questions in the textbook, which include essential concepts such as electric circuits, symbols, conductors, insulators, switches and practical applications of electricity. Through this free PDF, students are able to make revisions at any time, whether using a mobile, a laptop, or a tablet, without needing the internet. The class 7 science chapter 3 Electricity: Circuits and their Components question answers are made by subject professionals according to the latest NCERT syllabus, which will assist students in developing a clear understanding, save time in revision and perform better in examinations.
Electricity: Circuits and their Components NCERT Solutions: Exercise Questions
Class 7 Science Chapter 3 - Electricity: Circuits and their Components question answers assist Class 7 students to learn the fundamentals of electric circuits, circuit symbols, conductors, and insulators in simple terms. These step-by-step class 7 science chapter 3 Electricity: Circuits and their Components question answers are also made ready according to the new NCERT syllabus, so that learning becomes easy and effective. With such solutions, students will be able to revise fast, clear doubts, and perform well on exams.
Q1. Choose the incorrect statement.
(i) A switch is the source of electric current in a circuit.
(ii) A switch helps to complete or break the circuit.
(iii) A switch helps us to use electricity as per our requirement.
(iv) When the switch is in the ‘OFF’ position, there is an air gap between its terminals.
Answer:
(i): This statement is wrong; a switch does not provide electric current in a circuit. The electric current supplied to a circuit is generally provided by a battery or power source. A switch simply opens and closes the circuit and therefore controls the flow of current.
(ii): This statement is right. The job of a switch is to close (or complete) and open (or break) an electrical circuit, which then allows current to flow or stops the flow of current.
(iii): This statement is right. By using switches, we have control over when and where electricity flows, and we only have to use electric devices when we actually need to do something.
(iv): This statement is right. When the switch is in the 'OFF' position, there is a break in the electrical pathway (that break is typically filled with air, which is an insulator), and therefore, current cannot flow.
Q2. Observe Fig. 3.16. With which material connected between the ends A and B, the lamp will not glow?
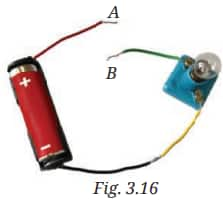
Answer:
The lamp will not glow if the material between the ends A and B is an insulator (like rubber, plastic, or wood). These materials do not allow electricity to flow through them.
Q3. In Fig. 3.17, if the filament of one of the lamps is broken, will the other glow? Justify your answer.

Answer:
If one lamp has a broken filament, then the other lamp will not light up, either.
- When one lamp's filament breaks, it opens a gap in the circuit.
- This gap indicates that the path for the electric current is discontinuous. When the circuit is open, the current can no longer flow through the circuit.
- Although the other lamp may be perfectly fine and operating, it will not glow because no electricity is reaching it due to the gap/disconnection.

Q4. A student forgot to remove the insulator covering from the connecting wires while making a circuit. If the lamp and the cell are working properly, will the lamp glow?
Answer:
The insulator stops the electric current from flowing through the wire, which is required for the lamp to glow; therefore, no, it won't.
You must remove the insulation from the sections of the wire that link to the cell and the light in order for the circuit to function and the lamp to glow. The lamp will light up as a result of the power flowing through this!
Q5. Draw a circuit diagram for a simple torch using symbols for electric components.
Answer:
A simple torch circuit can be represented as:
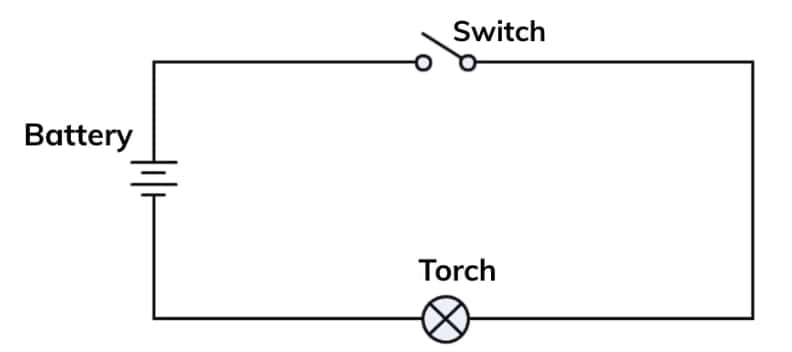
This is a simple series circuit where the battery provides power, the switch controls the current, and the lamp glows when the current flows.
Q6. In Fig. 3.18:
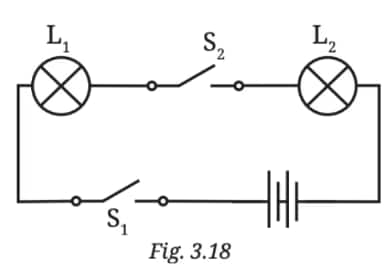
(i) If S2 is in ‘ON’ position, S1 is in ‘OFF’ position, which lamp(s) will glow?
(ii) If S2 is in ‘OFF’ position, S1 is in ‘ON’ position, which lamp(s) will glow?
(iii) If S1 and S2 both are in ‘ON’ position, which lamp(s) will glow?
(iv) If both S1 and S2 are in ‘OFF’ position, which lamp(s) will glow?
Answer:
(i) Ans: Neither lamp will glow because both switch 1 is open.
(ii) Ans: Neither lamp will glow because both switches are open.
(iii) Ans: Both Lamp 1 and Lamp 2 will glow, as both switches are closed, allowing current to flow to both lamps.
(iv) Ans: Neither lamp will glow because both switches are open, preventing current flow.
Q7. Vidyut has made the circuit as shown in Fig. 3.19. Even after closing the circuit, the lamp does not glow. What can be the possible reasons? List as many possible reasons as you can for this faulty operation. What will you do to find out why the lamp did not glow?

Answer:
Some possible reasons are:
- The lamp has a broken filament (if it’s an incandescent lamp).
- Loose connections or poor contact in its electrical terms.
- Battery is dead(done) or battery terminals are not placed correctly.
- The wires are not properly connected to the lamp or battery.
- The circuit has an open circuit due to a faulty switch or a broken connection in the circuit.
In order to find out why the lamp did not glow, you should:
- Inspect the lamp for a broken filament.
- Make sure everything has secure connections.
- Use a simple tester to test the battery and make sure it is working.
- The switch is in the ‘ON’ position.
- Check the wiring in order to make sure there is electrical contact.
Q8. In Fig. 3.20, in which case(s) will the lamp not glow when the switch is closed?
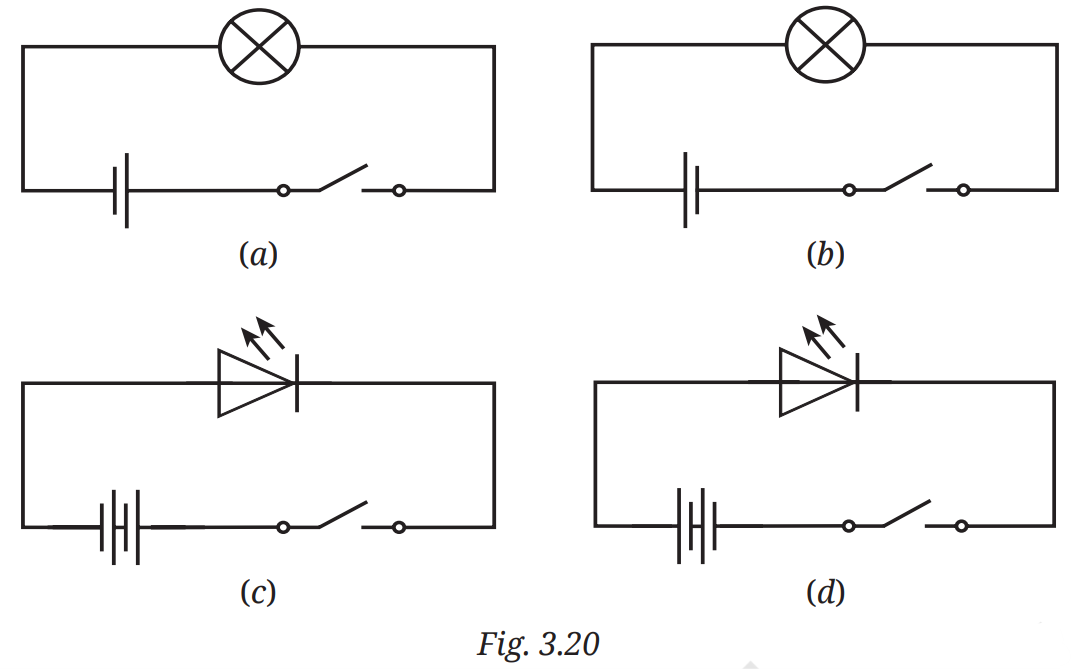
Answer:
Cases (a), (b), and (d) will light up when the switch is closed, but case (c) will not light up because the battery's negative terminal is connected to the LED's positive terminal, which means that it is reverse-connected. In this case, current will not flow through the LED, and it will not light up.
Q9. Suppose the ‘+’ and ‘–’ symbols cannot be read on a battery. Suggest a method to identify the two terminals of this battery.
Answer:
We can test the ' + ' and ' - ' of a battery by connecting it to an LED. We know that the longer wire of an LED is the positive terminal of the LED, and the shorter wire of the LED is the negative terminal. After connecting the LED to the cell, if the LED glows, then the terminal connected to the longer side of the LED is the positive terminal of the Cell, and the other side is the negative terminal of the cell.
If the LED does not glow after connecting with the cell, then exchange the connection of the terminal and test again.
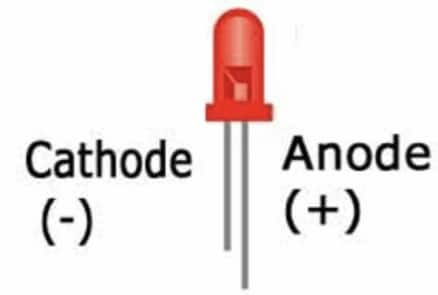
Q10. You are given six cells marked A, B, C, D, E, and F. Some of these are working and some are not. Design an activity to identify which of them is working. (i) List the items that you require. (ii) Write the procedure that you will follow. (iii) With the items, carry out the activity to identify the cells that are working.
Answer:
i.) To test which of the batteries is working, we will require a working bulb and connecting wires.
ii.) We will connect each of the batteries with the bulb using the connecting wires and check if the bulb glows or not.
iii.) First, we will connect the connecting wires to the terminals of the bulb. Then we will connect the cells one by one to the wires. The bulb will glow in some cases, and in some cases it will not. The cells that are able to glow the bulb are identified as working.
Q11. An LED requires two cells in series to glow. Tanya made the circuit as shown in Fig. 3.21. Will the lamp glow? If not, draw the wires for correct connections.
Answer:
No, the LED will not glow if the cells are not connected in the correct polarity. To make the LED glow, the positive terminal of the battery should be connected to the positive terminal of the LED (longer wire), and the negative terminal of the battery should be connected to the negative terminal of the LED (shorter wire).
Corrected connection:

Electricity: Circuits and their Components NCERT Solutions: Exploratory Projects
Exploratory projects in the chapter Electricity: Circuits and Their Components help students understand how electricity works through fun, hands-on activities. These projects encourage curiosity by letting students build simple circuits, test conductors and insulators, and observe how switches control current. Such practical learning strengthens understanding of electric concepts beyond the textbook and builds problem-solving skills.
Q1. Suppose that due to some problem, the power supply is disrupted in your area for two days. List out which actions from your daily life you would not be able to do.
Answer:
If there is no electricity in my area for two days, many of my daily activities will be affected. Some things I won’t be able to do are:
Switch on the lights – I will not be able to study or see things properly at night.
Use fans or AC – It will be very hot and uncomfortable, especially during summer.
Keep food fresh – The fridge will not work, so milk, fruits, and vegetables may spoil.
Charge my mobile phone or tablet – I won’t be able to call anyone or attend online classes.
Watch TV or use a computer – I will miss my favourite shows and won’t be able to use the internet.
Cooking using electric appliances – I cannot use the microwave, toaster, or induction stove.
Pump water – If the water pump doesn't work, we may run out of water.
Iron clothes – My school uniform may remain wrinkled.
Q2. Using a solar panel (Fig. 3.22a) as a source of electrical energy, make a circuit to run a toy fan (Fig. 3.22b) as shown in Fig. 3.22c.
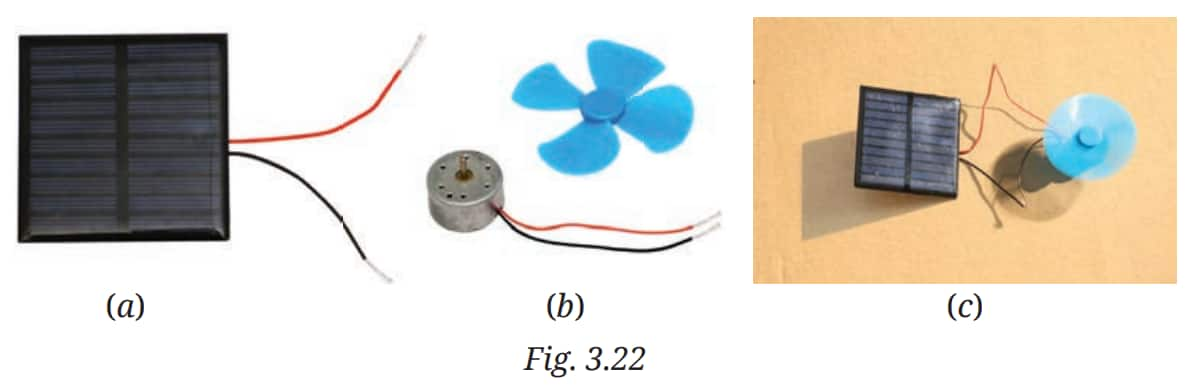
Answer:
Q3. Visit an electrical items shop. With the help of the shopkeeper, identify the various types of cells available. For each cell, also find out which device(s) it is used for. Prepare a report.
Answer:
Visit Date: [Write a date]
Place: [Name of shop]
Accompanied by: Shopkeeper [Mr./Ms. Name]
Introduction:
I visited a local electrical supplies store to learn about the types of cells (batteries) that are used in everyday things. The shopkeeper assisted in my questioning of how I could tell the difference between the types of cells currently available and insisted on noting the types of cells and the appliances they are used for.
Conclusion:
This visit made me aware of and understand the different types of cells and their importance in the operation of a range of electrical appliances. Some are single-use (disposable), while some can be recharged and reused - which is better for our environment!
Q4. Prepare a list of objects in your home under three categories:
(i) Objects which are electrical insulators only
(ii) Objects which are electrical conductors only
(iii) Objects which are made of both, whose some parts are insulators and some are electrical conductors
Answer:
(i) Objects that only act as Electrical Insulators:
These objects do not conduct electricity through them:
- Plastic bucket
- Rubber gloves
- Wooden chair
- Glass cup
- Woolen blanket
- Plastic comb
- Thermocol plate
(ii) Objects that only act as Electrical Conductors:
These objects conduct electricity through them easily:
- Copper wires
- Iron nails
- Steel spoon
- Aluminium foil
- Coins
- Metal taps
- Keys
(iii) Objects that contain both Conductors and Insulators:
These objects are a combination of both ( conductor and insulator parts)
- Electric iron (metal base - conductor, plastic handle - insulator)
- Mixer grinder (metal blades - conductor, plastic body - insulator)
- Electric kettle (metal coil - conductor, plastic handle - insulator)
- Extension board (internal wires - conductor, outer casing - insulator)
- Mobile charger (metal pins - conductor, plastic body - insulator)
Class 7 Science Chapter 3 - Electricity: Circuits and their Components: Additional Questions
The additional questions from Electricity: Circuits and Their Components help students revise and test their understanding of how electric current flows through circuits. These questions cover important ideas like conductors, insulators, switches, and types of circuits. Practising them strengthens conceptual clarity and prepares students well for exams and real-life applications of electricity.
Q1: A torch is not functioning, though the contact points in the torch are in working condition. What can be the possible reasons for this? Mention any three.
Answer:
The possible reasons could be:
(i) The switch may be faulty.
(ii) The cells may not be placed in the correct order.
(iii) The bulb may be fused..
(iv) The filament may be fused in the bulb.
Q2: Boojho has a cell and a single piece of connecting wire. Without cutting the wire in two, will he be able to make the bulb glow? Explain with the help of a circuit diagram.
Answer:
Boojho will not be able to light up the bulb using only a piece of wire and a cell. To turn on a bulb, a complete circuit is required, and this implies an unbroken line through which the electric current can pass through the positive terminal of the cell, through the bulb, and on to the negative terminal of the cell.
And this is an easy description of what a complete circuit would be:
Cell: Is the source of electrical energy.
Bulb: This is what is lit up when current is passed through it.
Wires: Use the wire to connect the cell to the bulb and back to the cell.
Two pieces of wire would be required in a complete circuit:
One wire, which then links the positive terminal of the cell to one of the terminals of the bulb.
The other wire will be used to link the other terminal of the bulb back to the negative terminal of the cell.
Boojho does not have enough wire and can not complete the circuit since he has only one piece of wire, and the bulb does not glow.
Q3: Paheli wanted to glow a torch bulb using a cell. She could not get connecting wires; instead, she got two strips of aluminium foil. Will she succeed? Explain how?
Answer:
To complete a circuit, we need a cell, connecting wires, and a lightbulb. When these parts are connected correctly, the lightbulb will illuminate. Electricity is carried by connecting wires. If connecting wires are not available, we can utilise another conductor between the two terminals. We can replace the connecting wires with aluminium since it is an excellent conductor.
Electricity: Circuits and their Components Class 7 Science Chapter 3: Topics
Electricity: Circuits and Their Components in Class 7 Science teaches students the concepts of electric circuits, such as the flow of electricity through a circuit and the parts that it requires to complete a circuit. Subsequently, this chapter develops background knowledge about electricity and its practical applications in day-to-day life.
3.1 A Torchlight
One of the common tools that are used to emit light with the help of electricity is a torchlight. It involves a bulb, a switch, and electric cell, and connecting wires. When the switch is switched on, the current of the cell passes through the bulb and the bulb glows. When the switch is shut, the current is no longer flowing and the bulb is switched off. Torchlight only works when all the parts are well connected. It enables us to know the way electricity is applied in our day-to-day lives.
3.2 A Simple Electrical Circuit
A complete path of flow of electric current is a simple electrical circuit. It consists of an electric cell, wires and a bulb and a switch. Electricity is only conducted when the circuit is complete. When the circuit is broken, then the current ceases to flow. Simple circuits are used to ensure we get to know how electrical devices operate. They are the foundation of any electrical system.
3.2.1 Electric cell
Electric cell is a source of electricity. It contains two terminals which are the positive terminal, which is abbreviated as + and the negative terminal, which is abbreviated as -. The chemicals contained within the cell generate electrical energy. Electricity is conducted through a circuit when the terminals are connected in a circuit. Devices such as remote controls, toys and clocks utilize electric cells. A cell is able to supply electricity temporarily.
3.2.2 Battery
A battery is a combination of two or more electric cells. It delivers a higher electrical energy when compared to one cell. Devices that require more power such as torches, radios and even mobile phones use batteries. There is an appropriate order of the cells in a battery. Devices last longer with the assistance of batteries. They play a significant role in providing electricity.
3.2.3 Electric lamp
An electric lamp is a gadget that lightens upon the flow of electricity through the gadget. Within it, there is a fine wire known as a filament. When current is passed through the filament it becomes hot and emits light. Lighting is done in homes, schools and streets using lamps. They are only working when they are linked to a complete circuit. The electrical energy is transformed into light energy with the help of an electric lamp.
3.2.4 Making an electric lamp glow using an electric cell or battery
The electric cell or a battery needs to be wired to the lamp in order to make the lamp glow. The positive terminal of the cell is connected to the lamp by one wire, and the other connected the lamp to the negative terminal. This completes the circuit. Electricity passes and the lamp lights when an electric circuit is complete. The lamp will not glow in case any connection is loose. This exercise demonstrates the significance of a closed circuit.
3.2.5 An electrical circuit
An electrical circuit refers to a closed circuit in which electricity flows. It consists of some source of power, wires, a switch, and one electrical object such as a bulb. Electricity will only flow when the circuit is closed. When the circuit is not closed, the flow of electricity is not possible. All electrical appliances make use of circuits. Circuit theory aids us in the safe use of electricity.
3.2.6 Electric switch
An electric switch is a device that is used to turn on or off the flow of electricity in a circuit. When the switch is in the ON position, the circuit is complete, and the current is flowing. When the switch is turned off, the circuit is broken, and the current is no longer flowing. Electrical devices are easily controlled using switches. They are utilised in machines, schools, and homes. Switches are more convenient and better as far as safety is concerned.
3.3 Circuit Diagrams
Circuit diagrams are diagrams that are not difficult to draw, and they depict how electrical components are joined together in a circuit. They have unique symbols to represent parts such as cells, bulbs, switches, and wires. A complex circuit is easily understood using circuit diagrams. Scientists and electricians utilise them. Circuit drawing assists students in gaining knowledge of circuits in a clean manner. They save time and minimise confusion.
3.4 Electrical Conductors and Insulators
Conductors are materials through which one can pass electricity, such as copper and iron. Substances that are not conductive to electricity are referred to as insulators, and they include plastic, rubber and wood. Wires are made by means of conductors. Wires are covered with insulators to avoid electric shocks. This division assists in the safe operation of electricity. This is quite essential in everyday life to know conductors and insulators.
Approach to Solve Questions of Class 7 Science Chapter 3 – Electricity: Circuits and Their Components
Class 7 Science Chapter 3 presents the students with the intriguing world of electric current and circuits, containing the explanation of how the electric current flows, why some conductors and insulators are needed, and how to compose the circuit diagram with the help of the standard symbols. Students are advised to learn the concepts instead of memorising them to effectively answer the questions in this chapter. To learn to master this chapter, the following method will assist:
- Start by learning what electricity is and how it assists in our daily lives, such as when we turn on a bulb or a mobile device.
- Learn about the important parts of a circuit and what each of them does. For example, a cell gives power, wires carry electricity, and switches on/off tell whether the circuit is open/closed.
- Then practice how a circuit diagram is drawn by connecting different circuit elements.
- Try out simple experiments by connecting a bulb, switch, resistor and wire and observe when the bulb glows. When it does, we understand the current is flowing.
- Try to remember important terms such as conductor (things through which electricity may pass, such as copper) and insulator (things through which it cannot pass, such as rubber and plastic).
- Try to connect the concepts with real-life examples happening around us, for example, take an example of a torch--it has got cells, a switch and a bulb. It is a mere demonstration of an electric circuit
- Solve the NCERT exercise and additional questions to get command over the chapter and learn how to write answers to get higher marks in the examination
Benefits of NCERT Solutions for Class 7 Science Curiosity Chapter 3
Electricity: Circuits and their Components Class 7 question answers give students a clear and systematic approach to learn about the fundamentals of electricity. These Electricity: Circuits and their Components class 7 question answers present concepts such as open and closed circuits, conductors, insulators, switches, and circuit symbols in a simplistic way. They are very useful in the preparation of exams, homework and using the concept of science in real-life activities so that the learners have a solid background in the study of electricity.
1. Detailed Step-by-Step Solutions
All the exercise questions are answered with a clear understanding, so that the students can trace the right reasoning to draw the circuit diagrams and solve the problems on electric current, switches, bulbs, and cells.
2. Concept Clarity
Using simple language and familiar examples, solutions explain scientific terminologies- such as conductors, insulators, open/closed circuits, series and parallel, etc.- and concepts are easier to grasp.
3. Boosts Exam Preparation
The class 7 science Electricity: Circuits and their Components question answers are according to the current NCERT examination pattern and thus the student is accustomed to the format and style required to clear the tests and exams.
4. Improves Problem-Solving Abilities
The higher-order thinking questions and reasoning are answered adequately with explanations, and the reasoning improves analysis competency, powers of observation, as well as drawing ability in the students.
5. Saves Time on Revision
Access to all solutions would be quick, enabling students to self-check answers immediately, a larger number of chapters to be covered as well as by a smaller number of times in a given period and also it helps build self-confidence.
6. Supports Practical Activities
Solutions to activity-based questions (e.g. making a simple circuit or testing materials to determine whether they are conductors or not) provide step-by-step procedures, expected observations, and desired answers.
7. Creates a solid base of upper classes
Learning and grasping the elementary basics of electricity using NCERT solutions equips the students to advance to more complicated physics in higher classes.
What Students Learn from NCERT Solutions for Class 7 Science Chapter 3?
The Electricity: Circuits and their Components NCERT Solutions provide the students with the elementary ideas of electricity in a simple and comprehensible way. Through these Electricity: Circuits and their Components Class 6 Question Answers, students are able to understand how electricity is transported and how various electrical components interact with each other, which will enable them to develop a solid background for the future topics of science.
- The Class 7 Science Chapter 3 Electricity: Circuits and their Components solutions describe what electricity is and the flow of electric current through a circuit, but only when the circuit is complete.
- Students are taught about significant components of an electrical circuit (electric cells, batteries, bulbs, wires, and switches) and understand how they work.
- Simple activities and solved questions explain how an electric lamp glows and how a switch controls the flow of electricity in a circuit.
- The question-answer nature suggests that the students can associate the concept of electricity with the real life objects, such as torchlights, switches at home and battery-powered objects.
NCERT Solutions for Class 7 Science Chapter Wise
The NCERT Solutions of Class 7 Science give step-by-step solutions to all the chapters of the new NCERT textbook. These solutions assist students in developing a good foundation, planning well before exams, and comprehending concepts easily. It is easy to revise or access any topic with the chapter-wise links, making it easier to learn and more organised.
NCERT Solutions for Class 7 Subject Wise
NCERT Solution of Class 7 gives step-by-step answers to all subjects according to the new CBSE syllabus. These subject-wise links enable students to get the solutions to each chapter quickly, making learning easier and enabling students to prepare adequately to pass their examinations.
NCERT Books and NCERT Syllabus
NCERT Books and NCERT Syllabus of Class 7 provide access to the official textbook and the most recent CBSE curriculum in a single location, so that the students can find it easily. These resources support planned study, clear topic coverage, and effective exam preparation throughout the academic year.
Frequently Asked Questions (FAQs)
Lights, fans, TVs, refrigerators and lots of appliances are powered by electricity, thus making our life more comfortable.
The filament of a bulb heats and emits light when the electric current is applied through the bulb in a closed circuit through which the electric current flows.
A switch is used to open or close an electric circuit. It controls the flow of electricity. When the switch is closed (ON), the circuit is complete, and current flows; when it's open (OFF), the current stops.
Plastic is insulating material, which barricades electric shocks by not allowing supply of current out of the wire.
The reason is due to the fact that electricity may lead to electric shocks, burns or even fires if electrical devices are handled carelessly.
Chapter 10 of Class 7 Science solution describes how an electromagnet drives an electric bell. The application of the magnetic effects of electric current is demonstrated when electricity runs and an iron strip is drawn to the electromagnet, causing the bell to ring. At the same time, the circuit is disconnected to cease the ringing.
Correct functioning, safety, and the avoidance of short circuits are all made possible by proper wiring and an awareness of circuit diagrams. NCERT Solutions help students develop the fundamental abilities needed to identify and resolve real-world electrical problems in both higher education and everyday life.
To get good marks, especially in CBSE Class 7 Science exams, you just need to:
- Respond to questions step by step according to the textbook.
- Provide diagrams and definitions as requested.
- Do intext question and back exercise questions.
- Revise significant questions and significant definitions.
The most common exam questions from Class 7 Science Chapter 3 include:
- Label and draw a basic circuit diagram of electricity.
- Define: conductor, insulator, switch, open, and closed circuit.
- Distinguish between the series and parallel circuits.
- Discuss the working of a bulb in a circuit.
- Determine faults in a specific circuit diagram.
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
