NCERT Solutions for Class 7 Science Chapter 4 The World of Metals and Non-metals
Items we see in our daily life, such as pans, buckets, tongs, and farming tools like spades, axes, and trowels are made of various materials. Do you know what these materials are? The answer to this question lies in class 7 science chapter 4 the world of metals and non-metals solutions. From kitchen utensils to skyscrapers, they are all around us. This chapter covers topics like metals and non-metals, their physical and chemical properties, characteristics, and reactivity. This chapter helps students to build a strong foundation for understanding science and connecting that to the real world.
This Story also Contains
- NCERT Solutions for Class 7 Science Chapter 4: Download PDF
- NCERT Solutions for Class 7 Science Chapter 4 (Exercise Questions with Answers)
- Practice Questions for Class 7 Science Chapter 4
- Approach to Solve Questions of Class 7 Science Chapter 4
- Topics and Subtopics Covered in the NCERT Textbook
- Understanding Class 7 Science Chapter 4 Metals and Non-Metals
- What You Will Learn from Class 7 Science Chapter 4: The World of Metals and Non-metals
- NCERT Solutions for Class 7 Science Chapter-wise
- NCERT Books and NCERT Syllabus

NCERT Solutions for Class 7 Science help you to explore, experiment, and find out how the world around you is full of hidden hints that you may find out by reading The World of Metals and Non metals.The practice questions are also included in the article to improve your critical thinking. These NCERT Solutions serve as an important resource for mastering the world of metals and non-metals class 7 question answer.
NCERT Solutions for Class 7 Science Chapter 4: Download PDF
The NCERT Solutions for Class 7 Science Chapter 4 PDF can be downloaded from the button given below and is designed to help students understand important concepts, practice textbook questions, and prepare effectively for exams. NCERT Solutions for Class 7 are given here in a well-structured and student-friendly format.
NCERT Solutions for Class 7 Science Chapter 4 (Exercise Questions with Answers)
The World of Metals and Non-metals NCERT Solutions provide clear and accurate answers to all the exercise questions. They help students understand the concepts better and practise effectively for exams. These solutions of NCERT are explained in simple words to help you understand the concepts better and score good marks in your exams.
Question 1: Which metal is commonly used to make food packaging materials, as it is cheaper, and its thin sheets can be folded easily into any shape?
(i) Aluminium
(ii) Copper
(iii) Iron
(iv) Gold
Answer:
Aluminium foil is commonly used to make food packaging materials. Due to its malleability, it can be beaten into thin sheets.
Hence, the correct answer is option (i).
Question 2: Which of the following metals catches fire when it comes in contact with water?
(i) Copper
(ii) Aluminium
(iii) Zinc
(iv) Sodium
Answer:
The reactivity of Sodium with water is very high; therefore, it generates heat when it comes into contact with water. To prevent this, it is stored in kerosene.
Hence, the correct answer is option (iv).
Question 3: State with reason(s) whether the following statements are True [T] or False [F].
(i) Aluminium and copper are examples of non-metals used for making utensils and statues. [ ]
(ii) Metals form oxides when combined with oxygen, the solution of which turns blue litmus paper
red. [ ]
(iii) Oxygen is a non-metal essential for respiration. [ ]
(iv) Copper vessels are used for boiling water because they are good conductors of electricity. [ ]
Answer:
(i) [F] False, Aluminium and copper are known as metals, for their properties like Malleability,
lustre, and hardness.
(ii) [F] False, Metal oxides are generally basic in nature, as we know basic solutions turn red litmus paper into blue.
(iii) [T] True, Oxygen is a non-metal essential for respiration. When we breathe, we inhale oxygen and exhale carbon dioxide.
(iv) [F] False, Copper vessels are used for boiling water because they are good conductors of heat.
Question 4: Why are only a few metals suitable for making jewellery?
Answer:
Metals are Suitable for jewellery making due to these specific properties:
-
Malleability & Ductility: Easily shaped and drawn into wires
-
Lustre: Has a shiny appearance
-
Resistance to Corrosion: doesn’t tarnish easily
-
High Value or Rarity: Metals like silver and gold possess these qualities well.
Question 5: Match the uses of metals and non-metals given in Column I with the jumbled names of metals and non-metals given in Column II.
|
Column I |
Column II |
|
(i) Used in electrical wiring |
(a) E N X Y G O |
|
(ii) Most malleable and ductile |
(b) N E C O H I R L |
|
(iii) Living organisms cannot survive without it. |
(c) P E P O R C |
|
(iv) Plants grow healthy when fertilisers containing it are added to the soil. |
(d) T E N G O I N R |
|
(v) Used in water purification |
(e) O G D L |
Answer:
|
Column I |
Column II |
|
(i) Used in electrical wiring |
(c) P E P O R C (COPPER) |
|
(ii) Most malleable and ductile |
(e) O G D L (GOLD) |
|
(iii) Living organisms cannot survive without it. |
(a) E N X Y G O (OXYGEN) |
|
(iv) Plants grow healthy when fertilisers containing it are added to the soil. |
(d) T E N G O I N R (NITROGEN) |
|
(v) Used in water purification |
(b) N E C O H I R L (CHLORINE) |
Question 6: What happens when oxygen reacts with magnesium and sulfur? What are the main differences in the nature of the products formed?
Answer:
When oxygen reacts with magnesium (a metal), it forms magnesium oxide. When oxygen reacts with sulfur (a non-metal), it forms sulfur dioxide gas. Main Difference: Magnesium oxide forms a basic solution in water. Sulfur dioxide forms an acidic solution in water. As we know, generally, metal oxides are basic, and non-metal oxides are acidic.
Question 7: Complete the following flow chart:
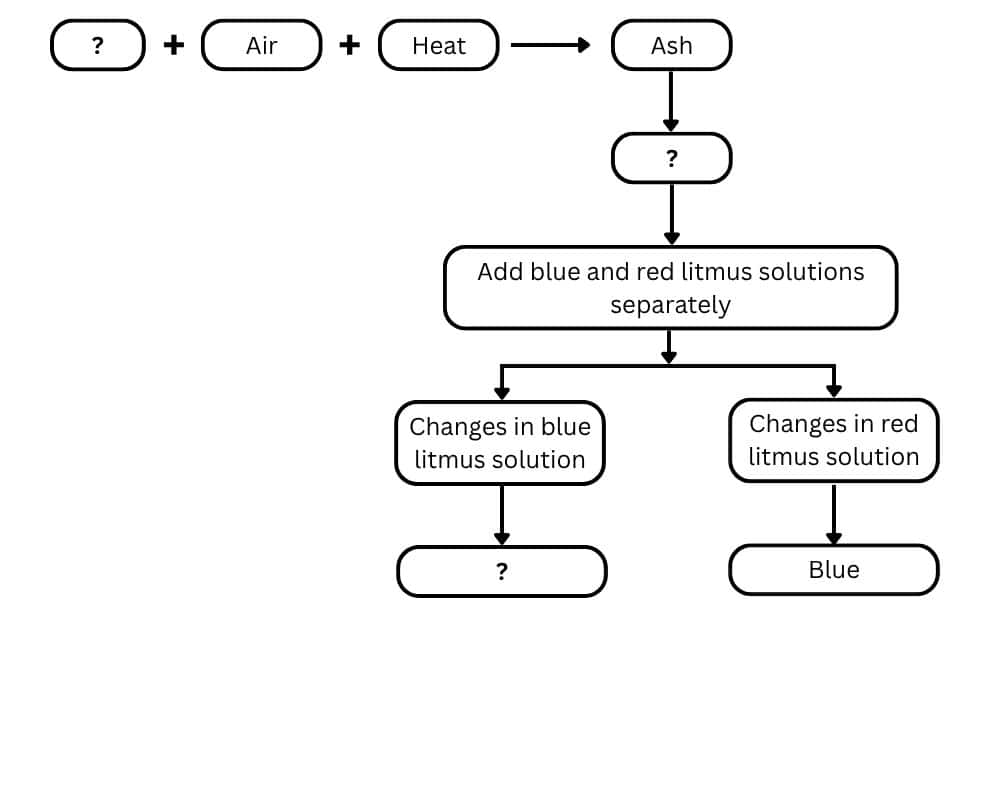
Answer:

Question 8: You are provided with the following materials. Discuss which material would be your choice to make a pan that is most suitable for boiling water and why?

Answer:
To make pans for boiling water, we need to transfer heat efficiently. As we know, metals (Iron, Copper) are good conductors of heat. Materials like sulfur, coal, plastic, wood, and cardboard are poor conductors of heat, so these are unsuitable for cooking vessels. Therefore, the best choice would be copper or iron.
Question 9: You are provided with three iron nails, each dipped in oil, water, and vinegar. Which iron nail will not rust, and why?
Answer:
As we know, rusting requires both air (oxygen) and water. Oil prevents the iron surface from contacting air and moisture, which inhibits rust. Therefore, the iron nail dipped in oil will not rust or rust much slower than the others.
Question 10: How do the different properties of metals and non-metals determine their uses in everyday life?
Answer:
The different properties of metals and non-metals determine their uses in everyday:
Metals: Good Conductor of electricity ( used in electric wires), Good Conductor of heat ( used in making cookware), Malleability/Ductility (used in making sheets, wires, and jewellery), Highly Lustrous (decoration), etc.
Non-metals: Poor conductors of heat (insulation, handles), Chemical Properties (Chlorine for purification, Iodine as antiseptic, etc.), Gaseous State (Nitrogen for fertilizers and Oxygen for breathing), etc.
Question 11: One of the methods of protecting iron from rusting is to put a thin coating of zinc metal over it. Since sulfur does not react with water, can it be used for this purpose? Justify your answer.
Answer:
Sulfur is brittle in nature and does not form a durable protective layer like zinc does in galvanization. That’s why sulfur coating can be easily cracked. Therefore, we can not use sulfur for this purpose.
Question 12: An ironsmith heats iron before making tools. Why is heating necessary in this process?
Answer:
Heating the iron makes it more malleable and softer. This allows the ironsmith to beat the hot iron and shape it into desirable forms relatively easily.
Practice Questions for Class 7 Science Chapter 4
Here are some the world of metals and non-metals class 7 question answer designed to strengthen your understanding of concepts. Solving them will not only improve your knowledge but also boost your confidence for exams. These question answer are great for revision and exam preparation.
Question 1: Which of the following catches fire when it comes in contact with water?
-
Sodium
-
Copper
-
Gold
-
Aluminium
Answer:
The reactivity of Sodium with water is very high, therefore, it generates heat when it comes into contact with water. To prevent this, it is stored in kerosene.
Hence, the correct answer is option (A)
Question 2: Why are only a few metals suitable for making jewellery?
Answer:
Metals are Suitable for jewellery making due to these specific properties:
-
Malleability & Ductility: Easily shaped and drawn into wires
-
Lustre: Has a shiny appearance
-
Resistance to Corrosion: doesn’t tarnish easily
-
High Value or Rarity: Metals like silver and gold possess these qualities well.
Question 3: What happens when oxygen reacts with magnesium and sulfur? What are the main differences in the nature of the products formed?
Answer:
When oxygen reacts with magnesium (a metal), it forms magnesium oxide. When oxygen reacts with sulfur (a non-metal), it forms sulfur dioxide gas. Main Difference: Magnesium oxide forms a basic solution in water. Sulfur dioxide forms an acidic solution in water. As we know, generally, metal oxides are basic, and non-metal oxides are acidic.
Question 4. What is the effect of air and water on iron metal?
Answer:
We know that Iron is a reactive metal, and when it comes into contact with air (oxygen) and water (moisture), it follows a redox reaction that leads to corrosion, commonly known as rusting.
Question 5. Explain why metals are good conductors of electricity.
Answer:
Question 6. Metals have free electrons that allow electricity to flow through them easily, making them good conductors of electricity.
Which property best explains why metals are used to make electrical wires?
(1) Malleability
(2) Ductility
(3) Sonority
(4) Hardness
Answer:
Ductility is the ability to be drawn into thin wires. This is why metals like copper and aluminium are used for wiring.
Hence, the correct answer is option (2)
Question 7. Which of the following is a non-metal but good conductor of electricity?
(1) Sulphur
(2) Phosphorus
(3) Graphite
(4) Iodine
Answer:
Graphite (a form of carbon) conducts electricity due to free-moving electrons, even though it is a non-metal.
Hence, the correct answer is option (3)
Question 8. Which pair is incorrectly matched?
(1) Iron – Magnetic
(2) Copper – Conductor
(3) Sulphur – Malleable
(4) Aluminium – Lightweight
Answer:
Sulphur is a non-metal and is brittle, not malleable.
Hence, the correct answer is option (3)
Approach to Solve Questions of Class 7 Science Chapter 4
To understand and solve class 7 science chapter 4 the world of metals and non-metals question answer focus on the approaches given below. Go through the concepts thoroughly to strengthen your understanding and improve application skills.
1. Before solving questions, it is very important to understand the key concepts like physical and chemical properties, characteristics, and reactivity of metals and nonmetals.
2. Physical properties are one of the most important topics discussed, and questions are asked frequently about this topic. Some of the important physical properties are:
- Lustre
- Malleability
- Ductility
- Conductivity
- Hardness
3. Reactions of metals and non-metals with oxygen, water, and acids are important. Learn how to write simple word equations like:
Metal + Acid $\rightarrow$ Salt + Hydrogen gas
4. A lot of topics we usually experience around us are discussed in class 7 science chapter 4 the world of metals and non-metals solutions, for example, the use of copper in electrical wires due to its high conductivity, aluminium in kitchen utensils because of its light weight and resistance to corrosion, or how iron rusts when left in moisture. So connecting concepts with daily life is helpful for students to understand the concepts of this chapter.
5. To understand concepts in a better way, it is very important to practice questions regularly. Refer questions provided in the NCERT textbook, and for better understanding, you can also refer
The World of Metals and Non-metals NCERT Solutions.
Topics and Subtopics Covered in the NCERT Textbook
The topics discussed in the world of metals and non-metals class 7 question answer help students understand the basic differences, properties, and uses of metals and non-metals through simple explanations and examples. Given below the topics discussed in NCERT Book.
4.1 Properties of materials:
4.1.1 Malleability
4.1.2 Ductility
4.1.3 Sonority
4.1.4 Conduction of heat
4.1.5 Conduction of electricity
4.2 Effect of air and water on metal: Iron
4.2 Effect of air and water on other metals
4.4 Substances that behave differently from metals in air and water
4.6 Are non-metals essential in everyday life?
Understanding Class 7 Science Chapter 4 Metals and Non-Metals
These class 7 science chapter 4 the world of metals and non-metals solutions help students to understand the key concepts simply and easily. They provide clear explanations and examples from daily life. Some of the main topics that students learn using these solutions are given below:
- They explain the characteristics and properties of metals and non-metals.
- The difference between metals and non-metals based on conductivity, appearance, hardness and malleability is explained with the help of solved questions.
- The World of Metals and Non-metals NCERT Solutions explain everyday uses of metals and non-metals in tools, utensils, wires, medicines, and agriculture.
- With the help of these solutions, the concept of alloys and their importance is explained.
- The reactions of metals and non-metals with air, water, and acids are explained in these solutions.
What You Will Learn from Class 7 Science Chapter 4: The World of Metals and Non-metals
In this chapter, students will learn about the different types of elements that make up our world. Some things that students will learn by using the class 7 science the world of metals and non-metals question answer given below:
1. In this chapter, students will understand what elements are and how they are classified into metals and non-metals.
2. Here they will learn about Physical Properties of Metals and Non-metals and why metals are generally hard, shiny, malleable, ductile, and good conductors of heat and electricity. At the same time, non-metals are often dull, brittle, and poor conductors.
3. Using this class 7 science chapter 4 the world of metals and non-metals question answer, students will understand Chemical Properties and Uses of Metals and Non-metals.
4. This chapter also explains where metals are found in nature, how they are extracted from ores, and the importance of mining and refining.
NCERT Solutions for Class 7 Science Chapter-wise
Along with class 7 science chapter 4 the world of metals and non-metals solutions students can also explore other chapters. The complete chapter-wise solutions are provided below.
NCERT Books and NCERT Syllabus
The NCERT books and syllabus links for class 7 are given below:
Frequently Asked Questions (FAQs)
Metals are generally hard, shiny, malleable, ductile, and good conductors of heat and electricity. Non-metals are typically dull, brittle, and poor conductors of heat and electricity.
NCERT Solution for Class 7 Science Chapter 4 The World of Metals and Non-metals provide detailed answers to all the questions in this chapter. They help students understand the properties, uses, and reactions of metals and non-metals, and guide them in solving textbook exercises accurately.
According to NCERT Curiosity Chapter 4 solutions metals have various uses. For example, iron is used in construction for making steel, copper is used in electrical wiring due to its excellent conductivity, and aluminum is often used in packaging, such as foil and cans because it is lightweight and resistant to corrosion.
Studying The World of Metals and Non-Metals helps students understand the fundamental properties, differences, and uses of metals and non-metals. It also connects scientific concepts to real-life applications, enabling students to identify materials around them and apply their knowledge in daily life and experiments.
Only a few metals are suitable for making jewellery because they are malleable, durable, and attractive. Metals like gold and silver are easy to shape into designs, do not rust or tarnish, and have a shiny appearance that makes them perfect for jewelry. Also, they are non-reactive.
Metals are typically shiny, dense, and solid at room temperature. They can conduct heat and electricity effectively. Non-metals, on the other hand, are often dull, less dense, and can exist in solid, liquid, or gas forms. They are generally poor conductors of heat and electricity.
Metals react with oxygen to form metal oxides, which are often solid compounds. For example, when iron reacts with oxygen, it forms iron oxide.
Metals are generally shiny, hard, malleable, ductile, and good conductors of heat and electricity, forming basic oxides and reacting with acids. Non-metals are dull, brittle, poor conductors, and form acidic or neutral oxides, playing key roles in life processes and industries.
Metals are good conductors of electricity because they have free electrons that can move easily throughout the metal lattice. This movement of electrons allows electric current to flow, which is why metals like copper and aluminum are commonly used in electrical wiring.
Metals are more reactive when they lose electrons during chemical reactions. For instance, alkali metals like sodium react vigorously with water. Non-metals, on the other hand, typically gain electrons during reactions and can be less reactive overall, although some non-metals like chlorine are highly reactive.
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
