Here are some exploring substances: acidic, basic, and neutral class 7 question answer for practice which help students to revise and strengthen their understanding of the concepts. Solving these will also prepare them for exams and improve their problem-solving skills.
NCERT Solutions for Class 7 Science Chapter 2 Exploring Substances: Acidic, Basic, and Neutral
Do you know why lemon tastes sour, what makes soap feel slippery, and why baking soda tastes bitter while turmeric tastes tangy? The answers to all these questions can be found in Exploring Substances: Acidic, Basic, and Neutral NCERT Solutions. In this chapter, you will learn how to test things to see whether they are acidic, basic, or neutral. You will be using magical tools like litmus paper, turmeric, and even red cabbage juice, which change color when they come into contact with acids or bases. Chemistry will come to life in the most vivid and startling ways, from your kitchen shelf to your school lab.
The important topics like acids, bases, indicators and neutralisation are all discussed in this chapter. These NCERT Solutions for Class 7 Science will offer a systematic way to learn the concepts through a series of solved questions. These Solutions will help you explore, experiment, and find out how the world around you is full of hidden hints. The activities are also included in this article to help you understand the concepts better. We have also added the points of approach that will help you answer the questions effectively. These NCERT Solutions are aligned with the latest CBSE curriculum and will serve as a valuable resource for students to enhance their exam performance.
This Story also Contains
- NCERT Solutions for Class 7 Science Chapter 2: Download PDF
- NCERT Solutions for Class 7 Science Chapter 2 (Exercise Questions with Answers)
- NCERT Solutions for Class 7 Science Chapter 2 (Activities)
- Practice Questions for Class 7 Science Chapter 2
- Approach to Solve Questions of Class 7 Science Chapter 2 Exploring Substances: Acidic, Basic, and Neutral
- Topics in NCERT Solutions for Chapter 2: Exploring Substances: Acidic, Basic, and Neutral
- Acids, Bases, and Neutral Substances Key Highlights
- What You Will Learn from Class 7 Science Chapter 2 – Exploring Substances: Acidic, Basic, and Neutral
- NCERT Solutions for Class 7 Science Chapter-Wise
- NCERT Books and NCERT Syllabus

NCERT Solutions for Class 7 Science Chapter 2: Download PDF
Students can easily download the NCERT Solutions for Class 7 Science Chapter 2 PDF. These solutions are prepared as per the latest syllabus and include NCERT solutions to help you understand the topic.
NCERT Solutions for Class 7 Science Chapter 2 (Exercise Questions with Answers)
Given below are the Exploring Substances: Acidic, Basic, and Neutral NCERT Solutions that help you master the concepts of acids, bases, and salts. This section includes all the questions and answers of the NCERT to help you revise effectively and score better in exams.
Question 1: A solution turns the red litmus paper blue. Excess addition of which of the following solutions would reverse the change?
(i) Lime water
(ii) Baking soda
(iii) Vinegar
(iv) Common salt solution
Answer:
A red litmus paper turns blue in a basic solution. To reverse the change, an acidic solution will be required. Lime water and baking soda are basic solutions and will turn red litmus blue. Common salt is a salt; It wouldn't affect the color change of the litmus paper. Vinegar contains acetic acid; hence, it will reverse the change and turn blue litmus red.
Question 2: You are provided with three unknown solutions labelled A, B, and C, but you do not know which of these is acidic, basic, or neutral. Upon adding a few drops of red litmus solution to solution A, it turns blue. When a few drops of turmeric solution are added to solution B, it turns red. Finally, after adding a few drops of red rose extract to solution C, it turns green.
Based on the observations, which of the following is the correct sequence for the nature of solutions A, B, and C?
(i) Acidic, acidic, and acidic
(ii) Neutral, basic, and basic
(iii) Basic, basic, and acidic
(iv) Basic, basic, and basic
Answer:
Solution A: The red litmus solution is turned to blue, that means solution A is acidic.
Solution B: Turmeric solutions turn red in basic solutions, so solution B is basic.
Solution C: Rose water extract is a natural pH indicator. It turns green in acidic solution. So, solution C is acidic.
So the correct sequence is acidic, basic, acidic.
Question 3: Observe and analyse Figs. 2.13, 2.14, and 2.15, in which red rose extract paper strips are used. Label the nature of solutions present in each of the containers.
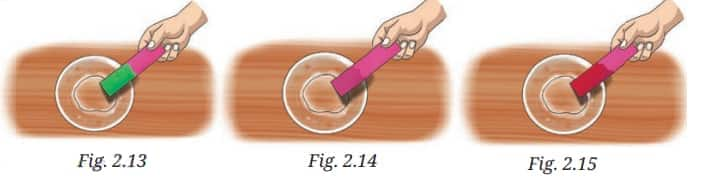
Answer:
Rose water extract gives different colors in acidic and basic solutions.
|
pH condition |
Color of Rose strip |
|
Acidic |
Bright red to pink |
|
Neutral |
Pink or no change in color |
|
Basic |
Green or blue-green |
So, the solution in Figure 2.13 is basic, as it turns the rose water color strip green. Solution in Figure 2.12 is neutral, as we see no change in the strip color. The solution in Figure 2.15 is acidic, as it turns the strip to bright red.
Question 4:
A liquid sample from the laboratory was tested using various indicators:
|
Indicator |
Red litmus |
Blue litmus |
Turmeric |
|
Change |
No change |
Turned red |
No change in colour |
Based on the tests, identify the acidic or basic nature of the liquid and justify your answer.
Answer:
We know that in the presence of an acidic solution, blue litmus turns red, and in the presence of a basic solution, red litmus turns blue. Also, turmeric solution turns red in the presence of a basic solution, and no color change is observed in acidic and neutral solution. So from this we can deduce that, since the liquid sample gives no change in red litmus and turmeric, it is acidic. This can be confirmed by the blue litmus turning red. So the liquid sample is acidic in nature.
Question 5: Manya is blindfolded. She is given two unknown solutions to test and determine whether they are acidic or basic. Which indicator should Manya use to test the solutions and why?
Answer:
Manya could use an indicator like litmus paper, but since she is blindfolded, she’ll use an olfactory indicator. One such indicator is an onion. She can do the following steps to test the solutions:
-
Take some finely chopped onions in a container, along with some strips of clean cotton cloth or filter paper.
-
Tightly close the container and leave it overnight.
-
Take two of the cotton cloth or filter paper strips from the container and check their odor. Keep them on a clean surface and put a few drops of the solution on the filter paper strips.
-
Allow the drops to spread on the strips.
-
Check the odor again.
So, in an acidic solution, the Onion smell remains, and in a basic solution, the Onion smell disappears. And therefore she can determine whether the solution is acidic or basic.
Question 6: Could you suggest various materials that can be used for writing the message on the white sheet of paper (given at the beginning of the chapter) and what could be in the spray bottle? Make a table of various possible combinations and the color of the writing obtained.
Answer:
|
Writing Material (on Paper) |
Spray Bottle Contents |
Type of Reaction |
Color of the Writing |
|
Turmeric paste |
Soap water (basic) |
Turmeric turns red in base |
Red / Reddish brown |
|
Phenolphthalein solution |
Soap water (basic) |
Colorless to pink in base |
Pink |
|
Lemon juice (acidic) |
Red cabbage extract |
Acid turns extract red |
Red / Pink |
|
Soap solution (basic) |
Red cabbage extract |
Base turns extract green |
Green / Blue-green |
|
Baking soda solution (basic) |
China rose (hibiscus) extract |
Base turns extract green |
Green |
|
Vinegar (acidic) |
China rose extract |
Acid turns extract pink |
Pink / Magenta |
|
Sodium carbonate solution (basic) |
Turmeric spray |
Turmeric turns red |
Red |
|
Lemon juice (acidic) |
Blue litmus solution |
Acid turns blue litmus red |
Red |
Question 7: Grape juice was mixed with red rose extract; the mixture got a tint of red color. What will happen if baking soda is added to this mixture? Justify your answer.
Answer: When grape juice is mixed with red rose extract (a natural pH indicator), the resulting mixture shows a red tint, indicating that grape juice is acidic. Now, if you add baking soda (a basic substance) to this mixture, the solution will neutralize the acid in the mixture, and the solution will become basic. Now, the color of rose water extract will change to green, indicating the basicity of the solution.
Question 8: Keerthi wrote a secret message to her grandmother on her birthday using orange juice. Can you assist her grandmother in revealing the message? Which indicator would you use to make it visible?
Answer:
Keerthi used orange juice to write a secret message, which means she used mild acid as invisible ink. This kind of writing dries clear and becomes visible only under certain conditions. To make it visible, Keerthi’s grandmother can do any of the following steps:
1. Gently heat the paper using a candle or bulb. The sugars in the orange juice will caramelize, turning brown, revealing the message.
Or
2. She can use red cabbage juice. Since orange juice is acidic and red cabbage juice is an indicator, it may turn the writing pink/red.
Question 9: How can natural indicators be prepared? Explain by giving an example.
Answer:
Natural indicators are substances obtained from plants that can show a color change when added to acidic or basic solutions. These indicators help us identify whether a substance is an acid or a base using simple color reactions. Let’s try making your indicators using red flowers.
-
Collect some fallen petals of red roses available in your surroundings. It is advised not to pluck flowers. You may pick petals or flowers fallen on the ground.
-
Take a fistful of the collected petals of red roses and wash them with water.
-
Crush the petals using a mortar and pestle.
-
Place them in a glass tumbler.
-
Pour some hot water into the glass tumbler to ensure that the crushed flower petals are completely immersed.
-
Caution— Perform this step under the supervision of an adult.
-
Cover the glass tumbler with a lid. Wait for 5 – 10 minutes till the water becomes colored, and filter it.
-
The filtrate (liquid after filtration) is the required flower extract to be used as an acid-base indicator.
Question 10: Three liquids are given to you. One is vinegar, another is a baking soda solution, and the third is a sugar solution. Can you identify them only using turmeric paper? Explain.
Answer:
|
Liquid |
Turmeric Paper Reaction |
Conclusion |
|
Vinegar (acidic) |
Stays yellow |
It is an acid |
|
Baking soda solution (basic) |
Turns reddish-brown |
It is a base |
|
Sugar solution (neutral) |
Stays yellow |
It is neutral |
Question 11: The extract of a red rose turns the liquid X green. What will the nature of liquid X be? What will happen when an excess of amla juice is added to liquid X?
Answer:
The extract of red rose turns liquid X green, which indicates that liquid X is basic. The red rose extract acts as a natural indicator that shows red or pink in acidic solutions and green in basic ones. Since the color turned green, it confirms that the solution is a base. When an excess amount of amla juice is added to liquid X, the acidic amla juice neutralizes the base. As more acid is added, the solution becomes less basic and eventually turns acidic. Due to this change in pH, the color of the mixture will gradually change from green back to red or pink, showing that the solution has now become acidic.
Question 12: Observe and analyze the information given in the following flowchart. Complete the missing information.
.png)
Answer:
A garden with plants showing signs of poor health. There can be two possibilities for the poor health of plants. They can be:
-
The soil can be acidic.
-
The soil can be basic.
We can use an indicator to know the nature of the soil. Litmus paper (or red cabbage juice as an alternative natural indicator) can be used as an indicator. After using the indicator, we can fix the soil by adding acidic or basic substances as required. The acidic soil can be treated with slaked lime (calcium hydroxide). The basic soil can be treated with organic matter (like compost or manure).
NCERT Solutions for Class 7 Science Chapter 2 (Activities)
The following are the activities that are mentioned in this chapter. Follow the class 7 science chapter 2 exploring substances: acidic, basic, and neutral solutions to learn more.
Activity 2.1 Page no. 8-9
Question 1. Table 2.1. Testing the nature of samples with blue and red litmus papers
|
Name of the Sample |
Colour of blue litmus paper after putting a drop of the sample |
Colour of red litmus paper after putting a drop of sample |
|
Lemon juice | ||
|
Soap solution | ||
|
Amla juice | ||
|
Tamarind water | ||
|
Vinegar | ||
|
Baking soda solution | ||
|
Lime water | ||
|
Tap water | ||
|
Washing powder solution | ||
|
Sugar solution | ||
|
Salt solution | ||
|
Orange juice |
Answer:
Based on the acidic and basic nature of the samples, observation are given below
|
Name of the Sample |
Colour of blue litmus paper after putting a drop of sample |
Colour of red litmus paper after putting a drop of sample |
|
Lemon juice |
Red |
Red |
|
Soap solution |
Blue |
Blue |
|
Amla juice |
Red |
Red |
|
Tamarind water |
Red |
Red |
|
Vinegar |
Red |
Red |
|
Baking soda solution |
Blue |
Blue |
|
Lime water |
Blue |
Blue |
|
Tap water |
Blue |
Red |
|
Washing powder solution |
Blue |
Blue |
|
Sugar solution |
Blue |
Red |
|
Salt solution |
Blue |
Red |
|
Orange juice |
Red |
Red |
Substances that turn blue litmus paper red are acidic in nature, while those that turn red litmus paper blue are basic in nature. Since litmus shows different colours in acidic and basic solutions, it is called an acid-base indicator. The substances that show no change are neutral.
Question 2. Now, let us analyse Table 2.1 and sort the samples into three groups as follows —
- Group A with samples that turn the blue litmus paper to red.
- Group B with samples that turn the red litmus paper to blue.
- Group C with samples that do not affect either of the two litmus papers.
|
Group A |
Group B |
Group C |
|
|
Answer:
- Substances in Group A, such as lemon juice and vinegar, are acids because they turn blue litmus paper red.
- Group B includes items like soap and baking soda, which are bases and turn red litmus paper blue.
- Group C, like tap water and sugar solution, is neutral since they do not change the colour of litmus paper.
|
Group A |
Group B |
Group C |
|
Lemon juice, Amla juice, Tamarind water, Vinegar, Orange juice |
Soap solution, Baking soda solution, Lime water, Washing powder solution |
Tap water, Sugar solution, Salt solution |
Activity 2.2 (Page no. 11)
Question 1. Find out and write the names of the most common acids present in the following substances — Lemon________, Curd________, Tamarind________, Vinegar________.
Answer:
Lemon juice, amla juice, tamarind water, vinegar and orange juice are edible and are acidic in nature. The common acids found in these substances are
- Lemon- Citric acid
- Curd- Lactic acid
- Tamarind- Tartaric acid
- Vinegar- Acetic acid
Activity 2.4 (page no. 12-13)
Question 1. Testing the nature of samples with red rose extract
|
Name of the Sample |
The colour of the red rose extract after adding the sample |
Nature of substance |
|
Lemon juice | ||
|
Soap solution | ||
|
Amla juice | ||
| Tamarind water | ||
|
Lime water | ||
| Washing powder solution | ||
|
Vinegar | ||
|
Orange juice | ||
|
Baking soda solution | ||
| Sugar solution | ||
|
Salt solution | ||
|
Tap water |
Answer:
The rose extract gives a red colour in an acidic solution, while a green colour in a basic solution. So, on the basis of the nature of the samples, the observations are given below.
|
Name of the Sample |
The colour of the red rose extract after adding the sample |
Nature of substance |
|
Lemon juice |
Red |
Acidic |
|
Soap solution |
Green |
Basic |
|
Amla juice |
Red |
Acidic |
| Tamarind water | Red | Acidic |
| Lime water | Green | Basic |
| Washing powder solution |
Green |
Basic |
|
Vinegar |
Red |
Acidic |
|
Orange juice |
Red |
Acidic |
|
Baking soda solution | Green | Basic |
| Sugar solution | No color change | Neutral |
|
Salt solution | No color change | Neutral |
| Tap water | No color change | Neutral |
So, here we can conclude that the red rose extract can also be used to test the nature of the substances; hence, it is another example of an acid-base indicator.
Activity 2.5 (Page no. 14-15)
Question 1. Testing the nature of samples with turmeric paper.
|
Name of the Sample |
The colour of turmeric paper after putting a drop of the sample |
|
Lemon juice | |
|
Soap solution | |
|
Amla juice | |
| Tamarind water | |
|
Lime water | |
| Washing powder solution | |
|
Vinegar | |
|
Orange juice | |
|
Baking soda solution | |
| Sugar solution | |
|
Salt solution | |
|
Tap water |
Answer:
|
Name of the Sample |
The colour of turmeric paper after putting a drop of the sample |
|
Lemon juice | Yellow |
|
Soap solution | Red |
|
Amla juice | Yellow |
| Tamarind water | Yellow |
|
Lime water | Red |
| Washing powder solution | Red |
|
Vinegar | Yellow |
|
Orange juice | Yellow |
|
Baking soda solution | Red |
| Sugar solution | Yellow |
|
Salt solution | Yellow |
|
Tap water | Yellow |
Turmeric paper changes colour from yellow to red in a basic solution but shows no change in acidic or neutral solutions. Therefore, we can conclude that turmeric paper can be used to test basic substances. However, it cannot differentiate between acidic and neutral substances. So, it cannot be used as an acid-base indicator.
Note: Activity 2.3 has already been discussed in the exercise question no. 9.
Practice Questions for Class 7 Science Chapter 2
Question 1. A solution turns blue litmus red but does not react with zinc. What is the most appropriate conclusion?
(1) The solution is basic
(2) The solution is neutral
(3) The solution is acidic but weak
(4) The solution is strongly acidic
Answer:
Turning blue litmus red shows the solution is acidic. Lack of reaction with zinc indicates it is not strongly acidic, hence a weak acid.
Hence, the correct answer is option (3).
Question 2. Which of the following pairs will give a neutral solution when mixed in equal amounts?
(1) Hydrochloric acid + Vinegar
(2) Sodium hydroxide + Lime water
(3) Hydrochloric acid + Sodium hydroxide
(4) Lemon juice + Water
Answer:
An acid + base in equal amounts undergo neutralization, forming salt and water, resulting in a neutral solution.
Hence, the correct answer is option (3).
Question 3. A student tests four colourless solutions using indicators. Which observation confirms that the solution is basic?
(1) Turns red litmus blue
(2) Turns blue litmus red
(3) No change in litmus paper
(4) Turns turmeric yellow
Answer:
Bases turn red litmus blue. Turmeric turns reddish-brown in bases, not yellow.
Hence, the correct answer is option (1).
Question 4. Why is factory waste containing acidic substances harmful when released into rivers?
(1) It increases water temperature
(2) It makes water salty
(3) It lowers the pH, harming aquatic life
(4) It increases oxygen content
Answer:
Acidic waste lowers the pH of water, which can kill fish and other aquatic organisms sensitive to pH changes.
Hence, the correct answer is option (3).
Question 5: What are Indicators? Give the names of some natural indicators.
Answer:
Indicators are substances that show change in colour when added an acidic or basic medium. They are used to detect whether the medium is acidic or basic.
Some examples of natural indicators are Litmus, tuemeric and china rose.
Question 6: What is neutralisation?
Answer:
It is a chemical reaction in which acids and bases react with each other to form salt and water.
Acid + Base $\rightarrow$ Salt + Water
Question 7: Why do we apply baking soda to the stung area when an ant bites?
Answer:
Ant sting contains formic acid which causes pain. Baking soda is a base. It neutralises the acid and gives relief from pain.
Question 8: How does turmeric affect acidic and basic substances?
Answer:
Turmeric remains yellow in acid but turns reddish- brown in bases. That's why it is used as a natural indicator.
Question 9: Why should we not taste substances to check if they are acidic or basic?
Answer:
We should not taste unknown substances because they might be harmful or poisonous. Instead, we use indicators like litmus paper or natural extracts to test if they are acids or bases safely.
Approach to Solve Questions of Class 7 Science Chapter 2 Exploring Substances: Acidic, Basic, and Neutral
To understand and solve the class 7 science chapter 2 exploring substances: acidic, basic, and neutral question answer, focus on the approaches given below to build strong conceptual clarity, apply examples from daily life, and practise solving different types of questions for better revision.
1. Before solving questions, it is very important to understand the key terms like acids, bases, neutral solutions, indicators and neutralisation.
2. It is very important to know what colour indicator shows in acidic, basic and neutral media because usually direct questions are asked about this topic. Learn more from the class 7 science chapter 2 exploring substances: acidic, basic, and neutral solution.
3. Neutralisation equations are usually asked in exams. So it is very important to understand the reactions between acids and bases and learn how to correctly write these equations
Acid + Base $\rightarrow$ Salt + Water
4. A lot of topics we usually experience around us like using baking soda on ant bite, tarting ingestion with antacids, and adding lime to acidic soil, are discussed in this chapter. Relating these concepts to real-life situations helps in better understanding and remembering the chapter.
5. To understand concepts of Exploring Substances: Acidic, Basic, and Neutral NCERT Solutions in a better way it is very important to practice questions regularly. Refer questions provided in the NCERT textbook.
Topics in NCERT Solutions for Chapter 2: Exploring Substances: Acidic, Basic, and Neutral
NCERT Solution for Class 7 Science Chapter 2 Exploring Substances: Acidic, Basic, and Neutral introduces students to the nature of substances and how they react with each other. These concepts are explained clearly in the NCERT book that will help the students grasp the fundamental concepts. Given below is the list of topics covered in this Chapter.
2.1 Nature - Our Science Laboratory
2.1.1 Litmus as an indicator
2.1.2 Red rose as an indicator
2.1.3 Turmeric as an indicator
2.2 What Happens When Acidic Substances Mix with Basic Substances?
2.3 Neutralisation in Daily Life
Acids, Bases, and Neutral Substances Key Highlights
These solutions help students to understand the key concepts of acids, bases and neutral substances. They are prepared by subject experts in a very comprehensive and systematic manner. Some of the main topics that students learn are given below:
- These NCERT Solution for Class 7 Science Chapter 2 Exploring Substances: Acidic, Basic, and Neutral explains the characterstics and properties of acids, bases and neutral substances.
- Uses of indicators both natural and synthetic are explain in detals.
- With the help of class 7 science chapter 2 exploring substances: acidic, basic, and neutral question answer students are going to learn how to identify a substances as acidic, basic and neutral.
- Real life applications of acids and bases are found in food, medicines, and daily activities. They play a vital role in cooking, healthcare, cleaning, and many household uses.
- They explains the concept of neutralisation and its importance in everyday situation.
- Chapter 2 exploring substances: acidic, basic, and neutral class 7 question answer helps students to developing curiosity and critical thinking to explore new ideas.
What You Will Learn from Class 7 Science Chapter 2 – Exploring Substances: Acidic, Basic, and Neutral
In this chapter, students will learn about the nature and behaviour of different substances based on their taste, reaction, and chemical properties. Some things that students will learn by using the class 7 science exploring substances: acidic, basic, and neutral question answer are given below:
1. This chapter tells about Acids, Bases, and Neutrals and what makes a substance acidic, basic, or neutral, and how they differ in taste and characteristics.
2. Here, students will learn about Natural Indicators and how substances like litmus, turmeric, and China rose can be used to identify acids and bases. Also, students will understand the use of laboratory indicators such as phenolphthalein and methyl orange
3. Using these class 7 science exploring substances: acidic, basic, and neutral question answer they understand how acids and bases react to form salt and water, and the importance of neutralisation in daily life.
4. Students will also understand how to handle acids and bases safely to prevent harm or accidents.
NCERT Solutions for Class 7 Science Chapter-Wise
Along with class 7 science chapter 2 exploring substances: acidic, basic, and neutral solutions, students can also refer to other NCERT chapters. The NCERT Solutions for Class 7 chapter-wise links are provided below.
NCERT Books and NCERT Syllabus
The links for the NCERT syllabus and NCERT books for class 7 are given below:
Frequently Asked Questions (FAQs)
NCERT Solutions for Class 7 Science Curiosity Chapter 2 are detailed answers to all the questions given in the textbook. They help students understand the concepts clearly, practise important questions, and prepare effectively for exams.
The NCERT textbook for Class 7 Science Chapter 2 provides questions and exercises for practice. However, the detailed answers and explanations are available in the NCERT Solutions, which guide students in understanding and solving those questions correctly.
NCERT Solutions for Class 7 Science are step by step answers to all the questions given in the NCERT textbook. They help students understand concepts easily, practise important topics, and prepare effectively for exams.
Indicators are substances that change color in response to the acidity or basicity of a solution. They are important because they provide a visual way to determine whether a substance is acidic, basic, or neutral.
Turmeric is a natural indicator. It stays yellow in acids and turns red when mixed with a base like a soap solution.
Neutralisation is important because it helps balance the effects of acids and bases in our daily life. When an acid and a base react, they form salt and water, which are neutral substances.
We can test for acids and bases at home by using readily available indicators like red cabbage juice, which changes color based on pH.
pH is a scale that measures how acidic or basic a substance is, ranging from 0 to 14. A pH less than 7 indicates acidity, while a pH greater than 7 indicates basicity. It is important because pH affects chemical reactions, biological functions, and overall environmental balance.
Acids and bases react to neutralize each other, producing water and a salt. This reaction is called a neutralization reaction. For example, when hydrochloric acid reacts with sodium hydroxide, it produces water and sodium chloride, which is table salt.
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
