NCERT Solutions for Class 7 Science Chapter 7 Heat Transfer in Nature
Have you ever questioned yourself why summers are so hot or how we can keep ourselves warm in cold winters? That is precisely what the NCERT Solutions for Class 7 Science Chapter 7 - Heat Transfer in Nature does by discussing the interesting phenomenon of heat transfer in nature. The chapter discusses the movement of heat between hot and cold objects, the use of conductors and insulators, as well as the ways of measuring temperature in real-life scenarios. It gives students a clear knowledge of heat conduction, convection, and radiation with easy explanations and examples that can be relatable.
This Story also Contains
- Download Class 7 Science Chapter 7 - Heat Transfer in Nature Question Answers PDF
- NCERT Solutions Heat Transfer in Nature Class 7: Exercise Questions
- Class 7 Science Chapter 7 - Heat Transfer in Nature: Additional Questions
- NCERT Solutions for Class 7 Science Chapter 7: Topics
- Approach to Solve Questions of Class 7 Science Chapter 7 – Heat: Transfer in Nature
- Benefits of NCERT Solutions for Class 7 Science Curiosity Chapter 7
- What Students Learn from NCERT Solutions for Class 7 Science Chapter 7: Heat Transfer in Nature?
- NCERT Solutions for Class 7 Science: Chapter-Wise
- NCERT Solutions for Class 7- Subject Wise
- NCERT Books and NCERT Syllabus

The NCERT Solutions for Class 7 Science Chapter 7 - Heat Transfer in Nature are well prepared by subject experts following the latest CBSE syllabus 2025-26. The NCERT solutions are step-by-step answers to the textbook questions, which makes the learning process easy and systematic. Students can also review difficult subjects very fast, enhance their ability to solve problems and approach exams without fear. Simple language and practical examples explain complex concepts in simple language so that they are better remembered. These NCERT Solutions for Class 7 Science Chapter 7 - Heat Transfer in Nature PDFs serve as an ideal source of study to revise in exams, to do homework and assignments. Their conceptual clarity and exam-oriented approach enable the students to gain better marks in Class 7 Science exams.
Download Class 7 Science Chapter 7 - Heat Transfer in Nature Question Answers PDF
Heat Transfer in Nature NCERT Solutions are provided in an easy-to-download format to enable the students to better prepare for exams. These NCERT solutions give step-by-step solutions to every question in the textbook, including the most important concepts of heat transfer, conductors and insulators, temperature measurement, and convection and radiation. Through this free PDF, students can make revisions anytime they want, on their mobile, laptop or tablet, even when not connected to the internet. The Heat Transfer in Nature class 7 question answers are prepared by subject experts according to the newest NCERT syllabus (2025-26) and assist learners in developing a coherent vision of the concepts, save time on revision, and improve the results in the exam. These class 7 science chapter 7 Heat Transfer in Nature question answers simplify the process of learning and make the students very comfortable when answering the textbook-based questions, as well as the questions in the exams.
NCERT Solutions Heat Transfer in Nature Class 7: Exercise Questions
These Class 7 Science Chapter 7 - Heat Transfer in Nature question answers offer solutions to all the exercise questions of the textbook in detail. These class 7 science chapter 7 Heat Transfer in Nature question answers are made to make students clear of important concepts such as conduction, convection and radiation. They keep up with the new CBSE instructions and make learning practical based on simple step-by-step explanations.
Q1.(i) Choose the correct option in each case. (i) Your father bought a saucepan made of two different materials, A and B, as shown in Fig. 7.14. The materials A and B have the following properties —
(a) Both A and B are good conductors of heat
(b) Both A and B are poor conductors of heat
(c) A is a good conductor and B is a poor conductor of heat
(d) A is a poor conductor and B is a good conductor of heat
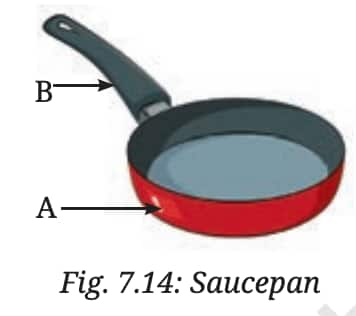
Answer: (c) A is a good conductor and B is a poor conductor of heat
Q1.(ii) Pins are stuck to a metal strip with wax, and a burning candle is kept below the rod, as shown in Fig. 7.15. Which of the following will happen?
(a) All the pins will fall almost at the same time
(b) Pins I and II will fall earlier than pins III and IV
(c) Pins I and II will fall later than pins III and IV
(d) Pins II and III will fall almost at the same time

Answer: (b) Pins I and II will fall earlier than pins III and IV
Explanation: Heat travels from the heated end to the other end via conduction. The pins near the flame fall first as the wax melts.
Q1. (iii) A smoke detector is a device that detects smoke and sounds an alarm. Suppose you are fitting a smoke detector in your room. The most suitable place for this device will be:
(a) Near the floor
(b) In the middle of a wall
(c) On the ceiling
(d) Anywhere in the room
Answer:(c) On the ceiling
Explanation: Smoke rises up due to convection, so detectors must be placed at the highest point.
Q2. A shopkeeper serves you cold lassi in a tumbler. By chance, the tumbler had a small leak. You were given another tumbler by the shopkeeper to put the leaky tumbler in it. Will this arrangement help to keep the lassi cold for a longer time? Explain.
Answer: Yes, it will help. The air between the two tumblers acts as an insulator (a poor conductor of heat) and slows down the heat transfer from outside.
Q3. State with reason(s) whether the following statements are True [T] or False [F].
(i) Heat transfer takes place in solids through convection. [ ]
(ii) Heat transfer through convection takes place by the actual movement of particles. [ ]
(iii) Areas with clay materials allow more seepage of water than those with sandy materials. [ ]
(iv) The movement of cooler air from land to sea is called a land breeze. [ ]
Answer:(i) False
Reason: In solids, heat transfer happens through conduction, not convection.
(ii) True
Reason: In convection, particles of liquids and gases move to transfer heat.
(iii) False
Reason: Clay has smaller pores than sand, so it allows less seepage.
(iv) True
Reason: At night, land cools faster, and cooler air moves towards the sea.
Q4. Some ice cubes placed in a dish melt into water after some time. Where do the ice cubes get heat for this transformation?
Answer: When ice cubes are placed in a dish, they start to melt and turn into water. For this transformation to happen, the ice needs to absorb heat energy.
- The ice cubes get heat from the surrounding environment.
- This includes heat from the air around them, the surface of the dish, and even the table they are placed on.
- The heat causes the ice to melt and change into water.
- This heat makes the ice melt and turn into water.
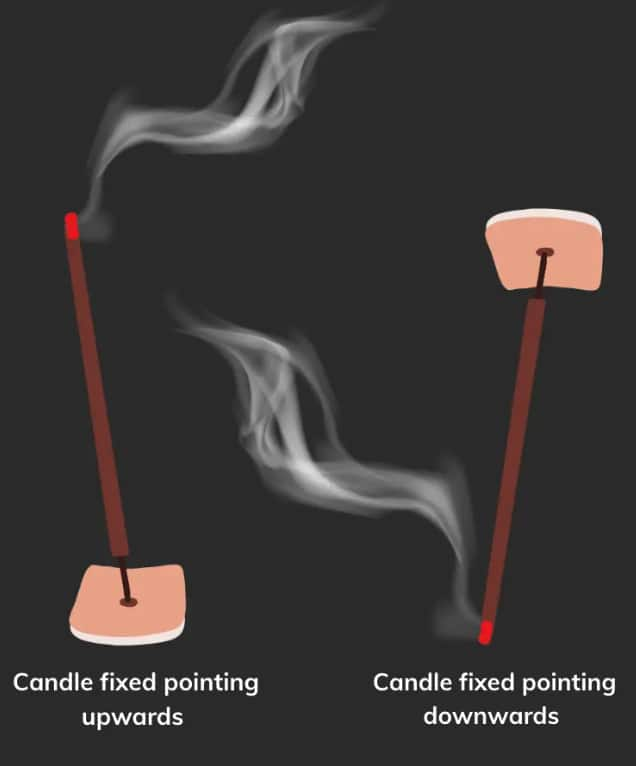
Q5. A burning incense stick is fixed, pointing downwards. In which direction would the smoke from the incense stick move? Show the movement of smoke with a diagram.
Answer: When an incense stick is burning and fixed pointing downwards, the smoke will move upwards. This happens because smoke rises due to convection.
As the incense stick burns, it heats the air around it, making the air less dense. The cooler, denser air pushes the hot air (and smoke) upward. So, even if the incense stick is pointing downwards, the smoke will still rise.
Q6. Two test tubes with water are heated by a candle flame as shown in Fig. 7.16. Which thermometers (Fig. 7.16a or Fig. 7.16b) will record a higher temperature? Explain.
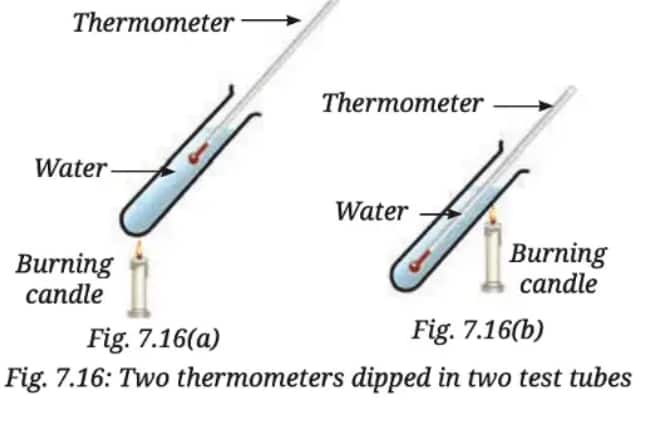
Answer:
In the given experiment, the test tube in Fig. 7.16(b), where the thermometer is closer to the flame, will show a higher temperature.
When water is heated by the candle, it becomes hot at the bottom of the test tube. The hot water rises because it is less dense than the cooler water. This movement of water from the bottom to the top is called convection.
Now, let's look at both test tubes:
- In Fig. 7.16(a): The thermometer is placed higher up, away from the hot water. It does not measure the rising hot water directly. So, it will show a lower temperature.
- In Fig. 7.16(b): The thermometer is placed in the area where the hot water is rising. Since the hot water is directly in contact with the thermometer, it will measure the higher temperature.
Therefore, the thermometer in Fig. 7.16(b) will show a higher temperature.
Q7. Why are hollow bricks used to construct the outer walls of houses in hot regions?
Answer: Hollow bricks are used because they trap air inside the spaces between the bricks. Air is a poor conductor of heat, meaning it does not allow heat to pass through easily. This helps to reduce heat transfer from the outside to the inside of the house. As a result, the house stays cooler in hot regions, as less heat enters through the walls.
Q8. Explain how large water bodies prevent extreme temperatures in areas around them.
Answer: Large water bodies, such as oceans and seas, help prevent extreme temperatures in nearby areas through the sea and land breeze effect.
Q9. Explain how water seeps through the surface of the Earth and gets stored as groundwater.
Answer:
Water seeps through the surface of the Earth:
- When it rains, water falls to the ground and moves through the soil and rocks. This process is called infiltration.
- The water travels down through the layers of soil and rocks, filling the gaps and spaces between them. Storage in aquifers.
- Eventually, the water reaches underground layers called aquifers, which are large areas of rock or soil that can hold water. These aquifers store the water as groundwater.
Thus, water seeps through the soil and rocks into the ground, where it gets stored as groundwater.
Q10. The water cycle helps in the redistribution and replenishment of water on the Earth. Justify the statement.
Answer: The water cycle helps move water around the Earth through evaporation, condensation, and precipitation. It brings rain, fills rivers and lakes, and refills underground water. This way, it redistributes and replenishes water everywhere.
Class 7 Science Chapter 7 - Heat Transfer in Nature: Additional Questions
The purpose of the additional questions in Class 7 Science Chapter 7: Heat Transfer in Nature is to help students better understand how heat moves in the environment. By covering conduction, convection, and radiation in everyday phenomena, these questions assist students in making the connection between theory and practical applications. Students who practice these questions improve their ability to solve problems and get ready for tests.
Q1: Look at the figure below:
.jpeg)
The length of wire PQ in case of A is equal to the diameter of the semicircle formed by the wire CDE, in case B. One pin is attached to each wire with the help of wax, as shown in Figure 4.9. Which pin will fall first? Explain.
Answer:
PQ's pin will fall first. The transfer of heat from objects with higher temperatures to those with lower temperatures is referred to here as conduction. The pin on the wire in Figure "A" will therefore fall first under the conditions described, since heat will reach it before the pin in Figure "B."
Q2: For setting curd, a small amount of curd is added to warm milk. The microbes present in the curd help in setting if the temperature of the mixture remains approximately between 35°C and 40 °C. At places where the room temperature remains much below the range, the setting of the curd becomes difficult. Suggest a way to set the curd in such a situation.
Answer:
The container may be covered with wool or any other poor heat conductor to keep the mixture at the appropriate temperature. As an alternative, the combination can be stored in a container that can withstand heat. While cooking, the container can be placed next to the gas stove or in the sun.
Q3: A circular metal loop is heated at point O as shown in the Figure below:
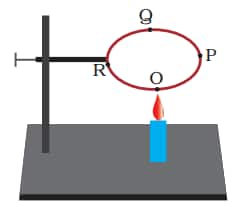
(i) In which direction would heat flow in the loop?
(ii) In which order do the pins at points P, Q and R fixed with the help of wax fall if points O, P, Q and R are equidistant from each other?
Answer:
(i). The heat will flow in both directions, i.e. from O to P and from O to R.
(ii). At first, the pins at R and P will fall simultaneously, followed by the pin at Q.
Q4: To keep her soup warm, Paheli wrapped the container in which it was kept with a woollen cloth. Can she apply the same method to keep a glass of cold drink cool? Give a reason for your answer.
Answer:
Yes, Paheli can apply the same to keep a glass of cold drink cool. A woollen cloth is not a good conductor of heat, which is why it cannot allow heat to go through it easily. A cold drink kept in a woollen cloth does not allow the heat of the surroundings to penetrate the glass. This makes the cold beverage remain cold longer. Similarly, hot soup when covered with a woollen cloth will not lose heat, hence the soup will remain warm. So in both situations, we can use the assistance of woollen cloth in order to reduce the speed of heat transfer, either when we want to retain something hot or cold.
Q5: In a mercury thermometer, the level of mercury rises when its bulb comes in contact with a hot object. What is the reason for this rise in the level of mercury?
Answer:
When a mercury thermometer is exposed to a hot body, the amount of mercury on the bulb increases due to the expansion of mercury on heating. The heat is passed onto the mercury in the bulb when the bulb of the thermometer comes in contact with a hot object. Mercury is a liquid, which expands on heating. As it grows, it requires additional space, and thus it ascends through the fine glass tube of the thermometer. This increase in the mercury level assists in determining the temperature of the hot object with some degree of precision.
NCERT Solutions for Class 7 Science Chapter 7: Topics
Chapter 7 of Class 7 Science is called Heat Transfer in Nature, which explains the flow of heat as an aspect of energy flow in the surrounding world. It describes the three primary modes of heat transfer, conduction (in solids), convection (in liquids and gases), and radiation (in empty space) and how they act individually upon nature and our lives with simple activities and real-life descriptions that most people can understand. This information enables learners to be familiar with the mechanisms, such as warming of the earth by the Sun, the land and sea breeze, as well as real-life scenarios, such as the energy-efficient houses, etc.
7.1 Conduction of Heat
Conduction is the means of heat transfer through solids, such as a metal spoon. One particle transfers the heat energy to the other without the actual motion of the particles out of the location.
7.2 Convection
Convection is the transfer of heat in fluids and gases. Lighter, warmer fluid thus rises and cooler, denser fluid sinks to the pan, and this circular movement is known as a convection current, which transports heat.
7.2.1 Land and Sea Breeze
Sea Breeze: Land absorbs heat rapidly in comparison to the sea during the day. The land air is heated up, which expands and rises, leaving a region of low pressure behind. The cooler, higher-pressure air that is found above the sea draws towards land to fill this space. This cool wind from the sea is called a sea breeze.
Land Breeze: During the night, the process is reversed. The land will cool more than the sea. The hot air, now becoming lighter than the colder on-shore air, rises, and the latter moves towards the sea to take its place. This is what is referred to as a land breeze.
7.3 Radiation
Radiation is the transfer of heat, which does not require a medium; it is instead propagated in the form of waves through empty space. It is by this that the heat of the Sun is transmitted to the Earth so that you can feel the heat of a fire across the table.
7.4 Water Cycle
The water cycle is the process of water movement on the Earth; it is stimulated by the Sun. It encompasses evaporation of water bodies and the formation of clouds and rain/snow, which fall back to the earth through condensation.
7.4.1 Seepage of water beneath the Earth
Also known as infiltration, it involves the soaking of water that comes with rain following the soil and cracks on the ground. This water recharges underground catchments, turning into groundwater.
Approach to Solve Questions of Class 7 Science Chapter 7 – Heat: Transfer in Nature
Chapter 7 of Class 7 Science explains the interesting phenomenon of heat transfer in nature, including conduction, convection, radiation, and the way temperature is measured in the real world. In order to be successful in this chapter, students are to concentrate on the acquisition of the concepts through examples rather than memorisation. To master this topic, the following step-by-step strategy is given below:
- Start by understanding heat as an energy which flows from a body at a higher temperature to a body at a lower temperature. For example, a hot cup of tea left open on the table cools down after some time as heat flows from the tea to the surroundings.
- Rather than memorising, compare different ways of heat transfer: conduction (spoon in hot tea), convection (boiling water) and radiation (sunlight on earth).
- Do easy experiments, such as putting a spoon in hot soup or watching sea and land breezes, because you remember better when you do something yourself.
- Start learning important scientific definitions, such as conduction, convection currents, radiation and insulators, and attempt to answer them in your own words
- To make your answers clearer, draw diagrams of such things as convection currents, heat flow, and thermometers.
- Use concepts in real-life contexts, e.g. why did we ever have to use woolly clothes to keep us warm, or why do you use wooden pan handles?
- Lastly, keep on writing and revising definitions, examples and important points, and use short notes or mind maps to simplify the revising process.
Benefits of NCERT Solutions for Class 7 Science Curiosity Chapter 7
These NCERT Solutions of Class 7 Science Curiosity Chapter 7: Heat Transfer in nature aims at demonstrating to the student how heat moves and how it affects various materials. These Heat Transfer in Nature class 7 question answers use the current syllabus and explain the concepts in an accessible manner. They are good for building concrete foundations and instil confidence in students during examinations.
1. Easy-to-Follow Explanations
The solutions introduce the three most common types of heat transfer (conduction, convection and radiation) and explain in simple diagrams and everyday examples what each involves, so that students can identify easily the way heat moves through solids, liquids and empty space.
2. Real-World Connections
There are natural examples throughout the book, and the solutions provide the reasoning behind why everyday things such as land and sea breezes (convection), the role of the sun in the water cycle (radiation and convection), and dressing smart (radiation) occur.
3. Logical and Analytical Thinking
The solutions are more in-depth than mere definitions, educating students to respond to the questions of why and how in line with the Higher Order Thinking Skills (HOTS). These are the essential skills during exam preparation.
4. Exam Readiness
The answers are up-to-date, according to recent CBSE and NCERT guidelines, addressing both essential topics and commonly asked questions, thus providing students with a solid base to prepare for exams.
5. Supports Practical and Hands-On Learning
Activities to measure the materials tested as conductors or insulators, and the different colours on heat absorption are given. All activities have a distinct procedure, results, and conclusions, but they are made to support practical knowledge.
6. Builds a Strong Foundation for Future Studies
A good understanding of the subject of heat transfer forms the foundation of most of the physics and geography subjects that students will encounter at higher levels of study, and therefore leads to more advanced science.
What Students Learn from NCERT Solutions for Class 7 Science Chapter 7: Heat Transfer in Nature?
Heat Transfer in Nature NCERT Solutions help students understand how heat moves from one place to another in a clear and easy way. These Class 7 Science Chapter Heat Transfer in Nature solutions describe natural heating processes and assist learners develop solid foundation of knowledge of thermal concepts in real life and at advanced levels.
- The Class 7 Science Heat Transfer in Nature question answers provide a clear explanation of the principle of heat transfer by explaining the nature of heat and its transfer between a hotter substance and a colder one.
- The three primary means of heat transfer, conduction, convection and radiation, are explained in a simple manner with examples given to students.
- The solved questions will help the students in knowing how heat is transferred in nature, like a sea breeze, a land breeze and the warming of the Earth by the Sun.
- Question and answer format enables the students to connect the concepts of heat transfer to their daily experiences,s such as heating food, rooms, and getting warmed by the sun.
NCERT Solutions for Class 7 Science: Chapter-Wise
NCERT Solutions Class 7 Science are the surest method to identify the right, question-by-question solutions that correspond to the most recent syllabus of the CBSE. This arrangement of the answers allows students to grasp the key concepts and to prepare to pass exams at school. The links of each chapter help to review them faster and easier to follow.
NCERT Solutions for Class 7- Subject Wise
NCERT Solution of Class 7 gives step-by-step answers to all subjects according to the new CBSE syllabus. These subject-wise links enable students to get the solutions to each chapter quickly, making learning easier and enabling students to prepare adequately to pass their examinations.
NCERT Books and NCERT Syllabus
NCERT Books and NCERT Syllabus of Class 7 provide access to the official textbook and the most recent CBSE curriculum in a single location, so that the students can find it easily. These resources support planned study, clear topic coverage, and effective exam preparation throughout the academic year.
Frequently Asked Questions (FAQs)
NCERT Science Book Class 7 Solutions for Chapter 7, Heat Transfer in Nature, teach about conduction, convection, and radiation, helping students understand how heat moves around us.
With simple explanations, clear diagrams and real-life examples, NCERT Solutions explain difficult heat transfer concepts in a smooth way and simplifying the learning experience by making it much easier and enjoyable.
The entire Solutions have been revised to reflect the latest CBSE, Class 7 Science syllabus (2025-26) and align with the new NCERT textbook, titled Curiosity.
Start by reading the textbook yourself. Next, read the NCERT Solutions to revise important concepts, attempt the exercise questions, and know of how to write in the answer-format answers that will give you scores in exams.
Sea breeze is the cool air that blows from the sea to the land during the day due to unequal heating.
In order to follow the right process as outlined in the CBSE 2025–26 syllabus, students should use NCERT Solutions to understand concepts, get clarification on their questions, and practice the step-by-step solutions.
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
