NCERT Solutions for Class 7 Science Chapter 5 Changes Around Us: Physical and Chemical
Have you ever wondered why an ice cube melts into water when left at room temperature and why milk turns into curd? The answer to these questions lies in changes around us: physical and chemical ncert solutions. There are various changes that we observe around us every day, like the ripening of fruits, rusting of iron, freezing of water, burning of candles, etc. Out of these, some changes are Physical changes, while others are chemical changes, some are irreversible changes, while others are reversible changes, some changes are fast, while others are slow.
This Story also Contains
- NCERT Solutions for Class 7 Science Chapter 5: Download PDF
- NCERT Solutions for Class 7 Science Chapter 5 (Exercise Questions with Answers)
- Practice Questions for Class 7 Science Chapter 5
- Approach to Solve Questions of Class 7 Science Chapter 5
- Topics and Subtopics Covered in the NCERT Textbook
- Understanding Class 7 Science Chapter 5 Changes Around Us: Physical and Chemical
- What You Will Learn from Chapter 5 Changes Around Us: Physical and Chemical
- NCERT Solutions for Class 7 Science Chapter-wise
- NCERT Books and NCERT Syllabus

NCERT solutions offer a systematic and structured approach for changes around us: physical and chemical class 7 question answer. These Solutions will help you explore, experiment, and find out how the world around us constantly changes whether through physical or chemical processes. These NCERT Solutions for Class 7 are designed to help students in exams by offering step-by-step solutions to all the exercise questions, approaches to solve questions, and some additional questions for practice. We have also added the points of approach that will help you answer the questions effectively.
NCERT Solutions for Class 7 Science Chapter 5: Download PDF
Click the button given below to download NCERT solutions for Class 7 Science Chapter 5 Changes Around Us: Physical and Chemical to study anytime, anywhere. These NCERT Solutions for Class 7 Science are designed to help you understand concepts clearly, solve questions effectively, and revise the chapter quickly before exams.
NCERT Solutions for Class 7 Science Chapter 5 (Exercise Questions with Answers)
Find all the exercise questions with clear and accurate answers from class 7 science changes around us: physical and chemical question answer. These solutions of NCERT cover important topics related to Physical and chemical changes, helping students grasp concepts easily and practise effectively for exams.
Question 1: Which of the following statements are the characteristics of a physical change?
(i) The state of the substance may or may not change.
(ii) A substance with different properties is formed.
(iii) No new substance is formed.
(iv) The substance undergoes a chemical reaction.
(a) (i) and (ii)
(c) (i) and (iii)
(b) (ii) and (iii)
(d) (iii) and (iv)
Answer:
(c) (i) and (iii)
(i) The state of the substance may or may not change - Physical changes can involve changes in state (like melting or freezing), but no new substance is formed.
(iii) No new substance is formed - In a physical change, the substance remains the same, only its form or state changes.
Question 2: Predict which of the following changes can be reversed and which cannot be reversed. If you are not sure, you may write that down. Why are you not sure about these?
(i) Stitching cloth to a shirt
Answer (i): Stitching cloth to a shirt - Cannot be reversed: Once stitched, it is hard to undo without damage.
(ii) Twisting of straight string
Answer(ii): Twisting of straight string - Can be reversed: The string can be untwisted.
(iii) Making idlis from a batter
Answer(iii):
Making idlis from a batter - Cannot be reversed: The batter cannot be returned to its original form after steaming.
(iv) Dissolving sugar in water
Answer(iv): Dissolving sugar in water - Can be reversed: Sugar can be recovered by evaporating water.
(v) Drawing water from a well
Answer(v): Drawing water from a well - Can be reversed: Water can be returned to the well.
(vi) Ripening of fruits
Answer(vi): Ripening of fruits - Cannot be reversed: Once ripe, fruits cannot go back to unripe.
(vii) Boiling water in an open pan
Answer(vii): Boiling water in an open pan - Can be reversed: Evaporated water can be obtained back by condensation.
(viii) Rolling up a mat
Answer(viii): Rolling up a mat - Can be reversed: The mat can be unrolled.
(ix) Grinding wheat grains to flour
Answer(ix): Grinding wheat grains to flour - Cannot be reversed: Flour cannot be turned back into grains.
(x) Forming of soil from rocks
Answer(x): Forming of soil from rocks - Cannot be reversed: Soil formation is a slow process and cannot form rock back.
Question 3: State whether the following statements are True or False. In case a statement is False, write the correct statement.
1. Melting of wax is necessary for burning a candle. (True/False)
Answer 1: True
2. Collecting water vapour by condensing involves a chemical change. (True/False)
Answer 2: False
Correct statement: Collecting water vapour by condensing involves a physical change.
3. The process of converting leaves into compost is a chemical change. (True/False)
Answer 3: True
4. Mixing baking soda with lemon juice is a chemical change. (True/False)
Answer 4: True
Question 4. Fill in the blanks in the following statements:
(i) Nalini observed that the handle of her cycle has got brown deposits. The brown deposits are due to—-----------and this is a —----- change.
Answer: rusting, chemical
(ii) Folding a handkerchief is a —----------change and can be —---------.
Answer: physical, reversed
(iii) A chemical process in which a substance reacts with oxygen with evolution of heat is called —-------- and this is a —------- change.
Answer: combustion, chemical
(iv) Magnesium, when burnt in air, produces a substance called —-------. The substance formed is —--------- in nature. Burning of magnesium is a —----------change.
Answer: magnesium oxide, basic, chemical
Question 5: Are the changes of water to ice and water to steam, physical or chemical? Explain.
Answer: Both, the change of water to ice and water to steam are physical changes. In both processes, the chemical composition of water remains the same; only its state changes from liquid to solid or from liquid to gas. No new substance is formed which is a characteristic of physical changes.
Question 6: Is curdling of milk a physical or chemical change? Justify your statement.
Answer: The curdling of milk is a chemical change because, during this process, milk reacts with acid or bacteria, forming new substances like curd. This change cannot be reversed, which makes it a chemical change.
Question 7: Natural factors, such as wind, rain, etc., help in the formation of soil from rocks. Is this change physical or chemical and why?
Answer: The formation of soil from rocks involves both physical and chemical changes. Natural factors like wind, rain and temperature break down rocks into smaller pieces (physical change), while chemical processes, like weathering, also change the minerals in the rocks (chemical change). Both types of changes work together to form soil.
Question 8: Read the following story titled 'Eco-friendly Prithin', and tick the most appropriate option(s) given in the brackets. Provide a suitable title of your choice for the story.
Prithvi is preparing a meal in the kitchen. He chops vegetables, peels potatoes, and cuts fruits (physical changes/chemical changes). He collects the seeds, fruits, and vegetable peels into a clay pot (physical change/ chemical change). The fruits, vegetable peels, and other materials begin to decompose due to the action of bacteria and fungi, forming compost (physical change/chemical change). He decides to plant seeds in the compost and water them regularly. After a few days, he notices that the seeds begin to germinate and small plants start to grow, eventually blooming into colourful flowers (physical change/chemical change). His efforts are appreciated by all his family members.
Answer: Prithvi's Green Kitchen
1. Prithvi chops vegetables, peels potatoes, and cuts fruits are physical changes
2. He collects the seeds, fruits, and vegetable peels into a clay pot are physical change
3. The fruits, vegetable peels, and other materials decompose into compost are chemical change
5. Seeds germinate and grow into plants are chemical change
Question 9: Some changes are given here. Write physical changes in the area marked 'A' and chemical changes in the area marked 'B'. Enter the changes which are both physical and chemical in the area marked 'C
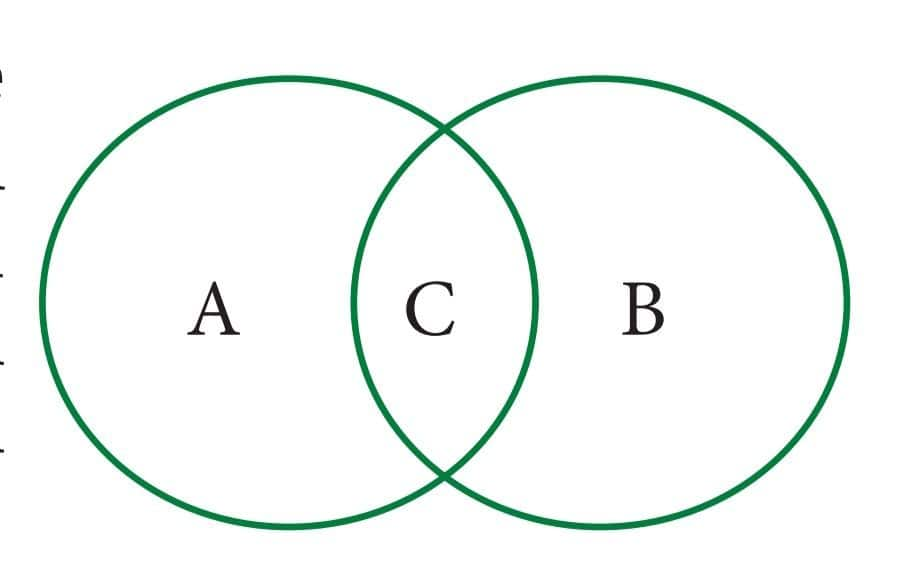
Process of burning a candle; Tearing of paper; Rusting; Curdling of milk; Ripening of fruits; Melting of ice; Folding of clothes; Burning of magnesium and Mixing baking soda with vinegar.
Answer 9: A (Physical changes)
-
Tearing of paper
-
Melting of iron
-
Folding of clothes
B (Chemical Changes)
-
Rusting
-
Curdling of milk
-
Ripening of fruits
-
Burning of magnesium
-
Mixing baking soda with vinegar
C ( Both physical and chemical changes)
-
Process of burning of candle
Question 10: The experiments shown in Fig. 5.11a, b, c, and d were performed. Find out in which case(s) did lime water turn milky and why?

Answer: In Figure (a), when vinegar reacts with baking soda, carbon dioxide gas is released. This carbon dioxide gas travels through the straw into the test tube with lime water, where it reacts to form calcium carbonate, a white solid substance that makes the lime water appear milky.
Practice Questions for Class 7 Science Chapter 5
Students can strengthen their understanding of the chapter with changes around us: physical and chemical class 7 question answer. These questions are designed to test your knowledge of key concepts related to this chapter, and help you prepare confidently for exams:
Question 1. Which of the following is a chemical change?
1) Melting of wax
2) Tearing of paper
3) Burning of wood
4) Dissolving sugar in water
Answer. Burning produces new substances like ash and gases, making it a chemical change.
Hence, the correct answer is option (3).
Question 2. Which of the following changes is reversible?
1) Rusting of iron
2) Cooking of rice
3) Freezing of water
4) Burning of a candle
Answer: Water can freeze into ice and melt back, making it a reversible physical change.
Hence, the correct answer is option (3).
Question 3. Is a chemical change reversible?
Answer: Typically, chemical changes are not easily reversible. While some chemical reactions can be reversed under specific conditions, it usually requires additional energy or other chemical reactions. Therefore, It is difficult.
Question 4. What is the difference between physical and chemical changes?
Answer: Physical change are those changes in which no new substance is formed and usually reversible while Chemical change are those changes in which new substance is formed and are usually irreversible; may produce heat, light, gas, or colour change.
Question 5. Is melting of butter a physical or chemical change?
Answer: Melting of butter is a physical change because it only changes from solid to liquid; no new substance is formed.
Question 6. Which of the following changes is both irreversible and involves formation of a new substance?
(1) Melting of ice
(2) Tearing of paper
(3) Rusting of iron
(4) Dissolving sugar in water
Answer:
Rusting forms a new substance called iron oxide. It is irreversible and a chemical change.
Hence, the correct answer is option (3).
Question 7. A student burns magnesium ribbon and observes a white powder. Which statement is correct?
(1) The change is physical and reversible
(2) No new substance is formed
(3) The mass of magnesium decreases
(4) Magnesium oxide is formed
Answer:
Burning magnesium produces magnesium oxide, a new substance. Hence, it is a chemical change.
Hence, the correct answer is option (4).
Question 8. Which of the following is a physical change but irreversible?
(1) Freezing of water
(2) Boiling of water
(3) Breaking of glass
(4) Dissolving salt in water
Answer:
Breaking glass does not form a new substance, so it’s a physical change, but it cannot be reversed.
Hence, the correct answer is option (3).
Approach to Solve Questions of Class 7 Science Chapter 5
To solve the class 7 science chapter 5 changes around us: physical and chemical question answer, it is very important to follow the approaches given below. These approaches, make learning easier:
1) Before solving questions from this chapter it is very important to understand the basic concepts like Physical changes, Chemical changes, Rusting, Combustion and Erosion.
2) Try to identify what is given by identifying key terms given in the question, like physical changes, chemical changes, combustion, then determine what we need to find out.
3) Relate changes around us: physical and chemical ncert solutions to real life example like change in the length of a rubber band by stretching, which is a physical change, burning of a candle, which is both a physical as well as chemical change and Ripening of fruits, which is a chemical change.
4) Simple diagrams like that of burning, melting, and dissolving can help solving question effectively
5) To understand concepts in better way it is very important to practice questions regularly. Refer questions provided in NCERT textbook and for better understanding you can also refer class 7 science chapter 5 changes around us: physical and chemical solutions.
Topics and Subtopics Covered in the NCERT Textbook
The topics covered in NCERT textbook are given below. With the help of the class 7 science chapter 5 changes around us: physical and chemical question answer, students can easily understand all the related topics:
5.1 A substance may change in appearance but remain the same
5.2 A substance may change in appearance and not remain the same
5.3 Some other processes involving chemical changes
5.3.1 Rusting
5.3.2 Combustion
5.4 Can physical and chemical changes occur in the same process
5.5 Are changes permanent
5.6 Are all changes desirable
5.7 Some slow natural changes
5.7.1 Weathering of rocks
5.7.2 Erosion
Understanding Class 7 Science Chapter 5 Changes Around Us: Physical and Chemical
The changes around us: physical and chemical class 7 question answers make it easier for students to understand the different concepts. These solutions explain concepts with clear examples, helping students connect the chapter with real life situations.
- They help students to understand the difference between physical and chemical changes.
- Through ncert solutions students can learn to identify the reversible and irreversible changes.
- Using class 7 science chapter 5 changes around us: physical and chemical solutions, students learn the concepts with the help of real life examples which makes it easier to understand the concept of physical and chemical changes.
- With the help of NCERT Solutions, students understand why and how different changes occurs.
What You Will Learn from Chapter 5 Changes Around Us: Physical and Chemical
In this chapter students will learn about many changes happening around us. Some changes are temporary and reversible, while others are permanent and form new substances. Some things that students will learn by using the class 7 science chapter 5 changes around us: physical and chemical question answer are given below:
1. This chapter is about the types of changes in our surroundings.
2. Here students mainly understand about Physical Changes i.e, changes in shape, size, or state of a substance without forming a new substance and Chemical Changes i.e, changes that produce new substances with different properties
3. These class 7 science changes around us: physical and chemical question answers also explain the differences between Physical and Chemical Changes and how to identify and distinguish them.
4. In this chapter students will understand which changes can be reversed and which cannot. Along with that they will learn about indicators of chemical changes.
NCERT Solutions for Class 7 Science Chapter-wise
In addition to changes around us: physical and chemical ncert solutions, students may also refer to other chapters from the NCERT syllabus. To make learning easier, we have provided chapter-wise links.
NCERT Books and NCERT Syllabus
The NCERT books and syllabus links for class 7 are given below:
Frequently Asked Questions (FAQs)
A physical change is a change in the form of a substance; it doesn't change the chemical composition of the substance. The substance is still the same material, even if it looks different. Chemical changes result in the formation of new substances with different chemical properties. These changes involve a reaction between substances. For example, the rusting of iron, burning wood, and cooking an egg are all examples of chemical changes.
Science NCERT Solutions for Class 7 Chapter 5 provide step by step answers to all textbook questions from the chapter Changes Around Us. These solutions help students understand concepts like physical and chemical changes, reversible and irreversible changes.
Class 7 Science Chapter 5 is Changes Around Us that deals with the different types of changes that occur in the environment and daily life. It explains physical changes like melting and dissolving and chemical changes like rusting and burning.
Rusting of iron is the process where iron objects develop a reddish-brown flaky substance called rust on their surface. This happens when iron reacts with oxygen (from the air) and water (or water vapour). It is a chemical change because a new substance, iron oxide (rust), is formed, which has different properties from iron.
No, boiling water is not a chemical change; it is a physical change. When water boils, it changes from liquid to gas, but its chemical composition remains unchanged. This process can be reversed by cooling down the steam back to water.
CBSE NCERT solutions help students by providing clear answers to all textbook questions. They make it easier to understand concepts, prepare for exams, practice effectively, and score better by offering accurate explanations and examples.
Signs include colour change, gas formation, precipitate formation, temperature change, or light emission.
Yes, many physical changes are reversible. For example, melting ice can be refrozen, and sugar can be crystallized back from a solution. However, chemical changes are generally irreversible. Once a chemical reaction occurs, the original substances cannot simply be restored to their initial state.
Temperature and pressure can significantly affect physical and chemical changes. For instance, heating can speed up chemical reactions, and higher pressure might change states of matter like water boiling at higher temperatures under pressure.
Catalysts are substances that speed up chemical reactions without being consumed in the process. They lower the activation energy needed for a reaction to occur, allowing it to happen more efficiently.
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
