Aakash Repeater Courses
ApplyTake Aakash iACST and get instant scholarship on coaching programs.
Hydrocarbons, as their name suggests, are those compounds that consist of Carbon and Hydrogen only. Hydrocarbons play a vital role in our day-to-day lives as they are the primary energy source. For example, Petrol and Diesel are hydrocarbons that are used as fuel for vehicles, LPG is also a hydrocarbon that is used for cooking, and Natural gas used for heat is a hydrocarbon. Some of the important topics covered are types of hydrocarbons, their properties, formation, and reactions.
JEE Main Scholarship Test Kit (Class 11): Narayana | Physics Wallah | Aakash | Unacademy
NEET Scholarship Test Kit (Class 11): Narayana | Physics Wallah | Aakash | ALLEN

NCERT solutions are prepared by Subject experts in a very comprehensive and systematic way, which helps students develop a clear understanding of the topics used to solve particular problems. To get a clear understanding of Hydrocarbons, students need to study the chapter sincerely and solve the questions provided in the textbook. By referring to the NCERT solutions for class 11 Chemistry, students can understand all the important concepts and practice questions well enough before their examination.
Also Read:
Students can download the PDF of Chapter 9 from here:
Question 9.1 How do you account for the formation of ethane during the chlorination of methane?
Answer:
Chlorination of methane is by free radical mechanism and it takes place in three steps-
(i) Initiation-
First, homolytic cleavage of the

(ii) Propagation-
Chlorine-free radicals attack methane molecules and generate methyl-free radicals as

This methyl radical reacts with other molecules of chlorine (

Then chlorine free radical reacts with methyl chloride and this way propagation occurs.

(iii) Termination-
Ethane will be produced as a final product in this step. When two methyl free radicals react with each other ethane will be formed.

Question 9.2(a) Write IUPAC names of the following compounds
Answer:
The IUPAC name of the given compound is 2-methyl but-2-ene
Question 9.2(b) Write IUPAC names of the following compounds
Answer:
The IUPAC name of the compound is Pent-1-ene-3-yne
Question 9.2(c) Write IUPAC names of the following compounds
Answer:
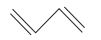
The IUPAC name of the compound is 1, 3-Butadiene or Buta-1, 3-diene
Question 9.2(d) Write IUPAC names of the following compounds :
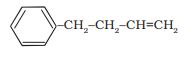
Answer:
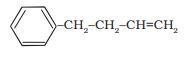
The IUPAC name of the compound is 4-phenylbut-1-ene
Question 9.2(e) Write IUPAC names of the following compounds :

Answer:

The IUPAC name of the compound is 2-methylphenol
Question 9.2 (f) Write IUPAC names of the following compounds :
Answer:
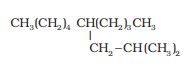
The IUPAC name of the compound is- 5-(2-methyl propyl)-decane
Question 9.2(g) Write IUPAC names of the following compounds :

Answer:

The IUPAC name of the compound is- 4-ethylDeca-1, 4, 8-triene
Answer:
 | But-1-ene |
 | But-2-ene |
 | 2-Methylprop-1-ene |
Question 9.4(i) Write IUPAC names of the products obtained by the ozonolysis of the following compounds :
Pent-2-ene
Answer:
Ozonolysis of Pent-2-ene gives two product both are aldehyde compounds. In this process, the ozone molecules attach at double bond to the molecule and break it into two products

The IUPAC name of the compounds-(i) ethanal (ii) propanal
Question 9.4 (ii) Write IUPAC names of the products obtained by the ozonolysis of the following compounds :
3,4-Dimethylhept-3-ene
Answer:
Ozonolysis of 3,4-Dimethylhept-3-ene gives two products of keto-compounds. The ozone molecules attach at the double bond.

The IUPAC name of the compound is -(i) Butan-2-one (ii) Pentan-2-one
Question 9.4(iii) Write IUPAC names of the products obtained by the ozonolysis of the following compounds:
2-Ethylbut-1-ene
Answer:
Ozonolysisof 2-Ethylbut-1-ene gives two products one is keto compound and another is an aldehyde.
2-Ethylbut-1-ene + O 3
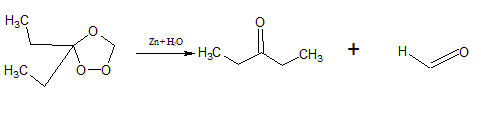
aldehyde The IUPAC name of the compound (i) is Pentan-3-one and the name of the (ii) compound is methanal.
Question 9.4 (iv) Write IUPAC names of the products obtained by the ozonolysis of the following compounds :
1-Phenylbut-1-ene
Answer:
On ozonolysis of 1-Phenylbut-1-ene gives two products of aldehyde compound. one is aromatic in nature and the other is an aliphatic aldehyde.

The IUPAC name of the compound is-(i) Benzaldehyde (ii)propanal
Question 9.5 An alkene ‘A’ on ozonolysis gives a mixture of ethanal and pentan-3- one. Write structure and IUPAC name of ‘A’.
Answer:
In the process of ozonolysis, an ozonide, cyclic ring structure intermediate is formed, which undergoes cleavage to give the product. The compound A produces pentan-3-one and ethanal. So, the possible structure of A should be

Thus, by removing the ozone from ozonide we can get the parent alkene structure.

Answer:
As per the given data, compound A on ozonolysis give two moles of aldehyde, having molar mass 44u. It indicates that the compound has an identical structure around (both sides) a double bond. So, the possible general structure of
A =
There are eight
Now, by combining all the observations, the structure of the A would be-

The ozonolysis reaction is shown below-

The atomic mass of ethanal is 44 u.
Question 9.7 Propanal and pentane-3-one are the ozonolysis products of an alkene? What is the structural formula of the alkene?
Answer:
In the process of ozonolysis, an ozonide, cyclic ring structure intermediate is formed, which undergoes cleavage to give the product. The parent compound produces pentan-3-one and propanal. So, the possible structure should be-

Here in this above structure, the right side Pent-3-one and the left-hand side propanal structure.
Thus, by removing the ozone from ozonide we can get the parent alkene structure.
Therefore the structure of the parent alkene is

(3-ethyl-3-hexene)
Question 9.8(i) Write chemical equations for the combustion reaction of the following hydrocarbons:
Butane
Answer:
Combustion means the reaction of a compound with the dioxygen(
Question 9.8(ii) Write chemical equations for combustion reaction of the following hydrocarbons:
Pentene
Answer:
Combustion means the reaction of a compound with the dioxygen(
Question 9.8 (iii) Write chemical equations for combustion reaction of the following hydrocarbons:
Hexyne
Answer:
Combustion means the reaction of the given compound with the dioxygen(
Question 9.8(iv) Write chemical equations for combustion reaction of the following hydrocarbons:
Toluene
Answer:
Combustion means the reaction of the given compound with the dioxygen(
Question 9.9 Draw the cis and trans structures of hex-2-ene. Which isomer will have higher b.p. and why?
Answer:
The structure of the hex-2-ene is shown here-

Now, the Geometrical, cis and trans isomers -

Cis-isomer has a higher boiling point than trans-isomers due to more dipole-dipole interaction between the molecules. The cis-form is more polar than the trans-form because it has a higher dipole moment than the trans-form. Trans-form is almost non-polar in nature.
Question 9.10 Why is benzene extraordinarily stable though it contains three double bonds?
Answer:
Benzene is a hybrid of various resonating structures.

Each carbon of benzene is in
There are 6-
Question 9.11 What are the necessary conditions for any system to be aromatic?
Answer:
The necessary conditions for any system to be aromatic are -
Question 9.12 (i) Explain why the following systems are not aromatic?
Answer:
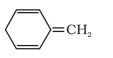
Not an aromatic compound because the
Question 9.12(ii) Explain why the following systems are not aromatic?

Answer:
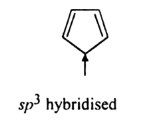
There is no complete conjugation of a
Question 9.12(iii) Explain why the following systems are not aromatic?
Answer:
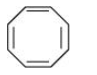
It disobeys the Huckle rule of (4n+2)
Question 9.13(i) How will you convert benzene into
p-nitrobromobenzene
Answer:
Bromination of a benzene ring in the presence of anhydrous
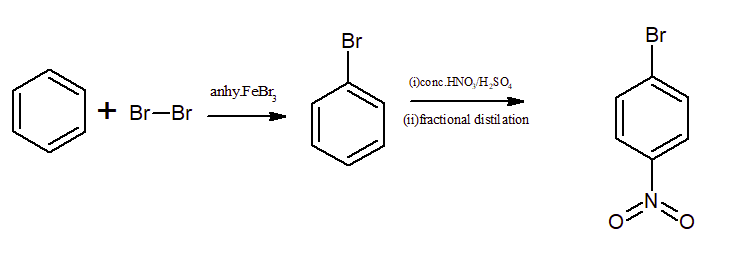
Question 9.13 (ii) How will you convert benzene into
m- nitrochlorobenzene
Answer:
Benzene on treatment with conc. nitric acid and sulphuric acid gives nitrobenzene which on further treatment with chlorine in the presence of anhydrous aluminium chloride (
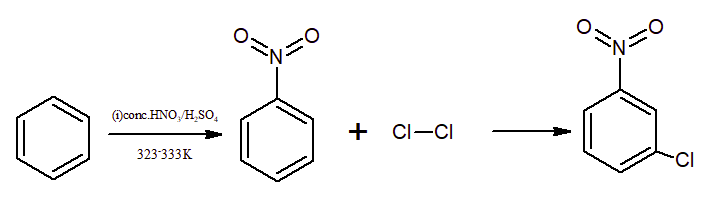
Question 9.13(iii) How will you convert benzene into
p - nitrotoluene
Answer:
Alkylation of benzene gives in the presence of anhydrous aluminium chloride give methylbenzene and

Question 9.13 (iv) How will you convert benzene into
acetophenone
Answer:
Benzene on reacting with an acyl chloride in the presence of anhydrous aluminium chloride gives acetophenone and hydrochloric acid as a by-product.

Answer:

Question 9.15 What effect does branching of an alkane chain has on its boiling point?
Answer:
On an increase in the branching of the alkane, the boiling point of the alkane is decreased. Alkane experience inter-molecular van der Waals forces. The strong is the force, strong will be the boiling point. When we increase the branching, the surface area of the molecule decreases, as a result, of the van der Waals force also decreases.
Answer:
Addition of
In this addition, an electrophile

a secondary carbocation is more stable than the primary carbocation. Thus bromide ion attacks the carbocation to form 2-bromopropane as a major product. (This mechanism is followed by Markovnikov's rule)

Answer:
Ortho-xylene has two resonant structure so,

Since all three products, methylglyoxal, 1, 2-methylglyoxal and glyoxal are cannot be obtained from any one of the two structure (i and ii). Hence we can say that o-xylene is a resonant hybrid of two Kekule structure (I and II)
Question 9.18 Arrange benzene,
Answer:
Acidic character of a species is defined on the basis of the ease with which it can lose its H– atoms.
The acidic character decreases in the order: Ethyne > Benzene > Hexane.
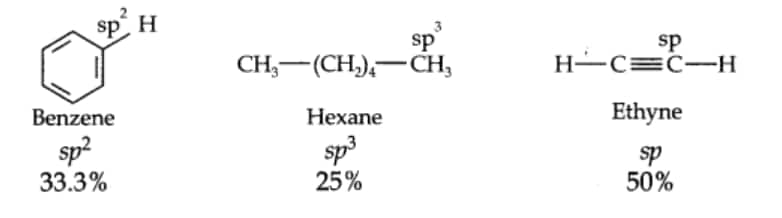
As the s – character decreases, carbon electronegativity decreases and C – H bond pair electrons lie away from the carbon atom. As a result, H– atom partially positive charge increases, and H+ ions are set free.
Question 9.19 Why does benzene undergo electrophilic substitution reactions easily and nucleophilic substitutions with difficulty?
Answer:
In benzene, the
Question 9.20 (i) How would you convert the following compounds into benzene?
Ethyne
Answer:
By cyclic polymerisation of ethyne, Ethyne on passing by red hot tube(made of iron) at 873K. Three molecules of ethyne polymerises to form benzene.

Question 9.20(ii) How would you convert the following compounds into benzene?
Ethene
Answer:
By converting ethene to ethyene by reacting with bromine in the presence of carbon tetrachloride. And then heating in presence of alc. KOH followed by

Question 9.20(iii) How would you convert the following compounds into benzene?
Hexane
Answer:
The cyclisation of hexane in the presence of

Question 9.21 Write structures of all the alkenes which on hydrogenation give 2-methylbutane.
Answer:
Structures of all the alkenes which on hydrogenation give 2-methylbutane.
the general structure of the 2-methylbutane is shown here;

As per the above structure, the following alkene compounds produces 2-methylbutane by hydrogenation.



Question 9.22 (a) Arrange the following set of compounds in order of their decreasing relative reactivity with an electrophile, E +
Chlorobenzene, 2,4-dinitrochlorobenzene, p-nitrochlorobenzene
Answer:
Electrophiles are electron deficient species, so they want a nucleophile which donates electrons to them. The higher the electron density on a benzene ring, the higher is the reactivity towards electrophile.
Decreasing order of their reactivity with an electrophile(
Chlorobenzene > p-nitrochlorobenzene > 2, 4-dinitrochlorobenzene
Question 9.22 (b) Arrange the following set of compounds in order of their decreasing relative reactivity with an electrophile, E +
Toluene,
Answer:
Electrophiles are electron deficient species, so they want a nucleophile which donates electrons to them. The higher the electron density on a benzene ring, the higher is the reactivity towards electrophile.
since the methyl group is electron donating group it increases the electron density on the benzene ring. And more the number of EWG lesser reactive towards electrophile.
therefore, the decreasing order of reactivity towards electrophile -
toluene
Question 9.23 Out of benzene, m–dinitrobenzene and toluene which will undergo nitration most easily and why?
Answer:
Nitration is occurred by an electrophilic substitution reaction, in which an electron rich species is attacked by an electron deficient molecule known as an electrophile. In nitration
Question 9.24 Suggest the name of a Lewis acid other than anhydrous aluminium chloride which can be used during ethylation of benzene.
Answer:
Lewis acid like anhydrous ferric chloride
Answer:
Wurtz reaction-

Wurtz reaction not preferred for the preparation of alkanes containing an odd number of carbon atoms because if we take two dissimilar alkyl halide as a reactant, the product will be a mixture of alkane but the reaction is by a free radical mechanism it will produce an alkene also. example- bromomethane and iodoethane.

all the products in the mixture have nearly close boiling point. So, the separation will be difficult.
Question: Given below are two statements :
Statement (I) : On nitration of
Statement (II) :
In the light of the above statements, choose the correct answer from the options given below:
1) Both Statement I and Statement II are false
2) Statement I is false but Statement II is true
3) Both Statement I and Statement II are true
4) Statement I is true but Statement II is false
Answer:
Statement-I

Statement-II
Hence, the correct answer is option (3).
Question: Given below are two statements :
Statement I : Ozonolysis followed by treatment with
Statement II : The production obtained by ozonolysis followed by treatment with
In the light of the above statements, choose the correct answer from the options given below :
(1) Both Statement I and Statement II are true
(2) Statement I is false but Statement II are true
(3) Statement I is true but Statement II is false
(4) Both Statement I and Statement II are false
Answer:
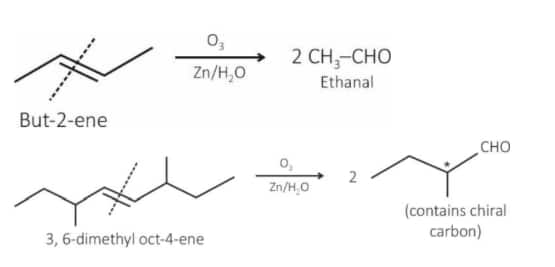
Statement I: Correct statement
Statement II: Incorrect statement because the product has a chiral center.
Hence, the correct answer is option (3).
A structured approach that works well for both theory-based and numerical problems is given below
Students can start by identifying the key topics covered: Types of hydrocarbons- alkanes, alkenes, alkynes, aromatic hydrocarbons, nomenclature rules, Isomerism, preparation and reactions of hydrocarbons, and their physical and chemical properties.
Categorise questions into conceptual/theoretical questions, structural drawing, reaction-based questions, IUPAC nomenclature, numerical questions
While solving questions, it is very important to read the question carefully and identify exactly what is being asked.
While solving questions of organic chemistry, it is very important to note down the information given an then relate it to the concepts of the chapter and also use bullet points for clarity when writing answers.
5) Students can refer to the solved examples of the textbook and then solve the intext questions. Students can also solve the NCERT exemplar of this chapter for effective learning.
Given below are topics that are covered in latest NCERT textbook:
9.1 Classification
9.2 Alkanes
9.2.1 Nomenclature and Isomerism
9.2.2 Preparation
9.2.3 Properties
9.2.4 Conformations
9.3 Alkenes
9.3.1 Structure of Double Bond
9.3.2 Nomenclature
9.3.3 Isomerism
9.3.4 Preparation
9.3.5 Properties
9.4 Alkynes
9.4.1 Nomenclature and Isomerism
9.4.2 Structure of Triple Bond
9.4.3 Preparation
9.4.4 Properties
9.5 Aromatic Hydrocarbon
9.5.1 Nomenclature and Isomerism
9.5.2 Structure of Benzene
9.5.3 Aromaticity
9.5.4 Preparation of Benzene
9.5.5 Properties
9.5.6 Directive influence of a functional group in monosubstituted benzene
9.6 Carcinogenicity and Toxicity
Given below a comparison table highlighting what to study beyond NCERT for JEE:
Below are the chapter-wise solutions-
The hyperlinks of the NCERT solution of class 11 are given below:
Students can refer to the links given below for the NCERT books and Syllabus:
Hydrocarbons are organic compounds consisting entirely of hydrogen and carbon atoms. They can be found in various forms, including gases, liquids, and solids. Hydrocarbons are primarily categorized into two types: aliphatic hydrocarbons which include alkanes, alkenes, and alkynes and aromatic hydrocarbons.
Markovnikov’s Rule states that in the addition of HX to an unsymmetrical alkene, the hydrogen attaches to the carbon with more hydrogen atoms.
The study of hydrocarbons is crucial because they form the fundamental building blocks for a vast range of organic molecules. Because hydrocarbons helps in fields like pharmacology, petrochemicals, and environmental science, as they serve as fuels and raw materials for numerous industrial applications.
Isomerism in hydrocarbons refers to compounds that have the same molecular formula but different structural arrangements. It is significant because it leads to variations in physical and chemical properties, influencing reactivity and usability in different applications.

Take Aakash iACST and get instant scholarship on coaching programs.

This ebook serves as a valuable study guide for NEET 2025 exam.

This e-book offers NEET PYQ and serves as an indispensable NEET study material.

As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters

As per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
As per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE