NCERT Class 11 Chemistry Chapter 12 Notes Organic Chemistry- Some Basic Principles and Techniques
Ever wondered what makes carbon the backbone of chemistry, how single elements give rise to millions of compounds, and why life on Earth is carbon-based? The answer to all such questions lies in Organic compounds and its basic principles. Organic compounds are those compounds which are made up of living organisms or occur naturally, but apart from that, organic compounds can be prepared in labs as well. Approximately 10 million compounds are carbon-based. From the food we eat, the plastic we use to the important organic reactions in industries, Organic compounds are everywhere.
This Story also Contains
- NCERT Notes for Class 11 Chemistry Chapter 8 Some basic principles and techniques of organic chemistry: Download PDF
- NCERT Notes for Class 11 Chemistry Chapter 8
- Organic Chemistry- Some Basic Principles and Techniques Previous Year Question and Answer
- How to Master Class 11 Chemistry Chapter 8 Organic Chemistry Some Basic Principles and Techniques
- Advantages of Using Class 11 Chemistry Chapter 8 Organic Chemistry: Some Basic Principles and Techniques Notes
- CBSE Class 11 Chemistry Chapter-wise Notes
- NCERT Solutions for Class 11 Chemistry
- Subject Wise NCERT Exemplar Solutions
- Subject Wise NCERT Solutions

Some basic principles and techniques in Organic Chemistry include topics like IUPAC nomenclature, isomerism, reaction mechanisms, and purification techniques. If students are facing difficulty in understanding the concepts, then they can refer to NCERT Class 11 Chemistry notes. These notes cover all the topics as prescribed by the latest CBSE syllabus and selected previous year questions are also added. Students can also access NCERT Class 11 notes for understanding the concepts better.
NCERT Notes for Class 11 Chemistry Chapter 8 Some basic principles and techniques of organic chemistry: Download PDF
Students can download these ncert class 11 chemistry chapter 8 organic chemistry some basic principles and techniques notes pdf for quick revision from the download icon given below. These NCERT notes help students learn the basic principles and techniques of organic chemistry effectively.
NCERT Notes for Class 11 Chemistry Chapter 8
Some basic principles and techniques of organic chemistry build the foundation for understanding complex organic reactions. NCERT Class 11 Chemistry Chapter 8 Notes Organic Chemistry Some Basic Principles and Techniques covers essential concepts like the classification of organic compounds, electron displacement effects, reaction mechanisms, and the basics of IUPAC nomenclature. Whether revising for exams or studying for the first time, these notes are essential. Students can also refer to the NCERT Solutions for additional practice and understanding.
8.1 General Introduction
Organic compounds are vital for sustaining life on earth and include complex molecules like genetic information bearing DNA and proteins that constitute essential compounds of our blood, muscles and skin. Organic compounds appear in materials like clothing, fuels, polymers, dyes and medicines. These are some of the important areas of application of these compounds.
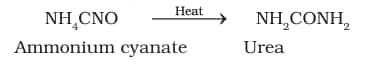
Organic chemistry began around 1780 when chemists diffrenetiates organic and inorganic compounds. Berzilius, a Swedish chemist proposed that a ‘vital force’ was responsible for the formation of organic compounds, but it was disproved in 1828 when Wohler synthesized urea from inorganic ammonium cyanate.
8.2 Tetravalency of Carbon: Shapes of Organic compounds
Carbon forms four covalent bonds, leading to specific geometrical shapes of organic compounds based on its hybridisation. To make your preparation easier, you can also download the organic chemistry some basic principles and techniques class 11 chemistry notes pdf for quick revision and better exam practice.
8.2.1 The Shapes of Carbon Compounds
Molecular structure helps predict organic compound properties, with carbon’s tetravalency and bonding which is explained through its electronic configuration and hybridisation in molecules like methane, ethene, and ethyne.
Hybridisation influences the bond length, bond strength, and electronegativity. The sp hybrid orbital contains more s character and hence it is closer to its nucleus and forms shorter and stronger bonds than the $\mathrm{sp}^3$ hybrid orbital. The $\mathrm{sp}^2$ hybrid orbital is intermediate in s character between sp and $\mathrm{sp}^3$ and, hence, the length and enthalpy of the bonds it forms, are also intermediate between them. These differences influence the physical and chemical properties of molecules.
8.2.2 Some characteristic Features of π Bonds
In a π (pi) bond formation, parallel orientation of the two p orbitals on adjacent atoms is necessary for a proper sideways overlap. Thus, in $\mathrm{H}_2 \mathrm{C}=\mathrm{CH}_2$ molecule all the atoms must be in the same plane. The p orbitals are mutually parallel and both the p orbitals are perpendicular to the plane of the molecule. Rotation of one $\mathrm{CH}_2$ fragment with respect to other interferes with maximum overlap of p orbitals and, therefore, such rotation about carbon-carbon double bond (C=C) is restricted. The electron charge cloud of the π bond is located above and below the plane of bonding atoms. This results in the electrons being easily available to the attacking reagents. In general, π bonds provide the most reactive centres in the molecules containing multiple bonds.
8.3 Structural Representations of Organic Compounds
Organic compounds can be represented in various structural forms to depict the arrangement of atoms and the type of bonding between them.
8.3.1 Complete, Condensed and Bond-line structural Formulas
Complete structural formula
Such a structural formula focuses on the electrons involved in bond formation. A single dash represents a single bond, double dash is used for double bond and a triple dash represents triple bond. Lone-pairs of electrons on heteroatoms (e.g., oxygen, nitrogen, sulphur, halogens etc.) may or may not be shown. Thus, ethane ($\mathrm{C}_2 \mathrm{H}_6$), ethene ($\mathrm{C}_2 \mathrm{H}_4$), ethyne ($\mathrm{C}_2 \mathrm{H}_2$) and methanol ($\mathrm{CH}_3 \mathrm{OH}$) can be represented by the structural formulas as shown below. Such structural representations are called complete structural formulas. Students can understand these concepts with the help of class 11 chemistry chapter 8 organic chemistry some basic principles and techniques notes.


Condensed structural formula
These structural formulas can be further abbreviated by omitting some or all of the dashes representing covalent bonds and by indicating the number of identical groups attached to an atom by a subscript. The resulting expression of the compound is called a condensed structural formula. Thus, ethane, ethene and ethyne can be written as:

Bond-line structural formula
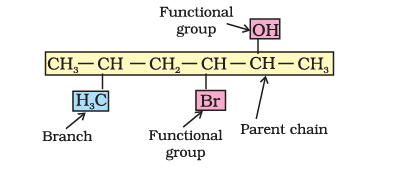
In this bond-line structural representation of organic compounds, carbon and hydrogen atoms are not shown and the lines representing carbon-carbon bonds are drawn in a zig-zag fashion. The only atoms specifically written are oxygen, chlorine, nitrogen etc. The terminals denote methyl (–CH3) groups (unless indicated otherwise by a functional group), while the line junctions denote carbon atoms bonded to an appropriate number of hydrogens required to satisfy the valency of the carbon atoms. For example 3-Methyloctane is represented as follows:

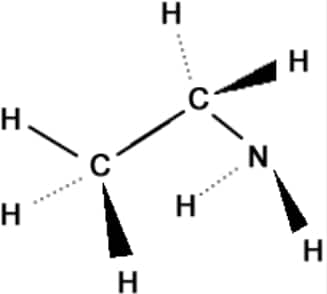
8.3.2 Three-Dimensional Representation of Organic Molecules
To depict their 3D structures, the wedge-dash representation is used. A solid wedge indicates a bond projecting out of the plane towards the viewer, a dashed wedge shows a bond going behind the plane, and a simple line represents a bond lying in the plane of the paper.
Example:
8.4 Classification of Organic Compounds
Organic compounds are classified on the basis of their structure. They are classified as:
i). Acyclic or open chain compounds
ii). Cyclic or closed chain or ring compounds
Acyclic or Open-chain compounds
These are the compounds in which the carbon atoms are linked to each other in such a manner that the molecule is having an open-chain structure. The chain of the carbon atoms may be straight or branched. These compounds are also called as aliphatic compounds. The term aliphatic has been derived from the Greek word aleiphatos meaning fats, since the earliest compounds to be studied were fatty acids or compounds found in fats. Some examples include:


Ethane Acetic acid
Cyclic or closed chain or ring compounds
Cyclic or Closed-chain compounds
These are the compounds in which carbon atoms are linked to each other or to the atoms of other elements in such a manner that the molecule has a closed-chain or cyclic or ring structure. One or more close-chains or rings may be present in the molecule. The compounds with only one ring of atoms in the molecule are known as monocyclic but those with more than one ring of atoms are termed as polycyclic. These are divided into two categories:
(a) Homocyclic compounds: These are the compounds having a ring or rings of carbon atoms only in the molecule. The arbocyclic or hoomocyclic compounds may again be divided into two types, i.e,
- Alicyclic compounds: These are the compounds which contain rings of three or more carbon atoms. These resemble with aliphatic compounds than aromatic compounds in many respects. That is why these are named alicyclic, i.e, aliphatic cyclic. Some examples include,

- Aromatic compounds: These compounds consist of at least one benzene ring, i.e, a six-membered carbocyclic ring having alternate single and double bonds. Generally, these compounds have a fragrant odour and hence, named as aromatic.

The above compounds are also known as benzenoid aromatics as their molecules consist of benzene ring or rings. However, there are aromatic compounds, which have structural units different from benzenoid type and are known as non-benzenoid aromatics.

(b) Heterocyclic compounds: These are cyclic compounds having ring or rings built up of more than one kind of atoms. The most common other atoms besides carbon are O, N and S. Some examples include,

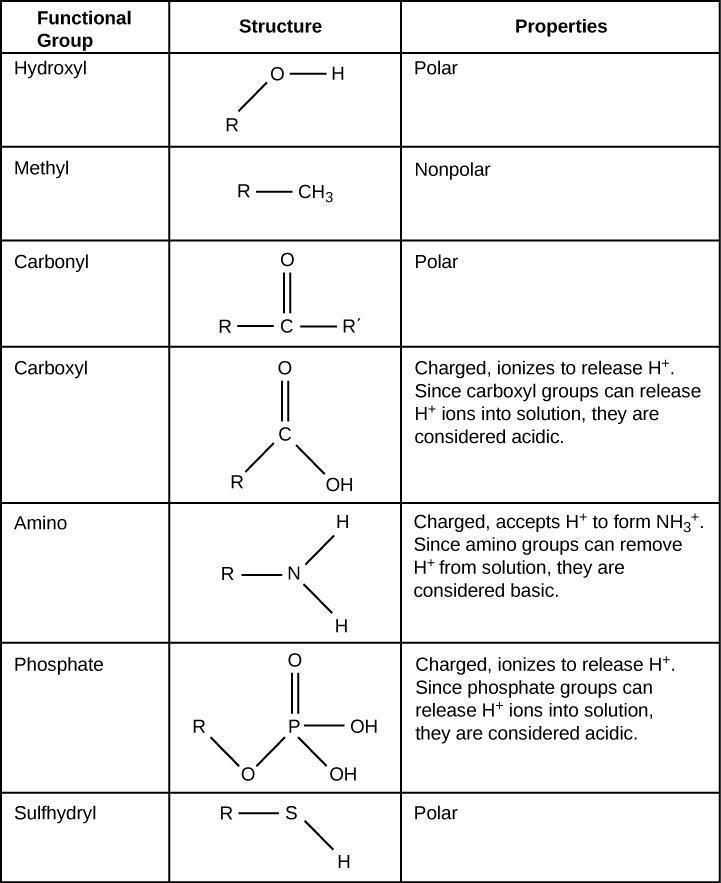
8.4.1 Functional group
There are numerous functional groups available to make compounds, they are groups of atoms when joined specifically to carbon. We can easily determine the chemical behaviour because all of them contain different chemical properties.
8.4.2 Homologous series
The series can be defined as those compounds that have same chemical properties, same functional group attached but only can be differentiated using formula unit-CH2. General formula of such series of compounds are similar.
8.5 Nomenclature of Organic Compounds
Organic chemistry involves millions of compounds, so the IUPAC system was developed to systematically name them.
Before the IUPAC system of nomenclature, however, organic compounds were assigned names based on their origin. For example: urea is found in animal’s urine so the common name is urea. Another example of common naming system is formic acid as this can be obtained from red ants whose scientific name is Formica.
8.5.1 The IUPAC system of nomenclature
The IUPAC name of an organic compound is based on the parent hydrocarbon and Functional group attached to it which is further, modified with prefixes and suffixes. Hydrocarbons, made of carbon and hydrogen, are classified as saturated with single bonds or unsaturated with double and triple bonds.
8.5.2 IUPAC nomenclature of Alkanes
Nomenclature of straight-chain hydrocarbons
The names of such compounds are based on their chain structure, and end with suffix ‘-ane’ and carry a prefix indicating the number of carbon atoms present in the chain (except from CH4 to C4H10, where the prefixes are derived from trivial names). The IUPAC names of some straight-chain saturated hydrocarbons are given in the Table below. The alkanes in this Table differ from each other by merely the number of -CH2 groups in the chain. They are homologues of alkane series. Students can also refer to NCERT solutions for Class 11 Chemistry Chapter 8 for a better understanding of such examples.

Nomenclature of branched-chain alkanes:
The rules for naming branched-chain alkanes are as follows:
-
First of all, the longest carbon chain in the molecule is identified. In the example given below, the longest chain has nine carbons and it is considered as the parent or root chain.

-
The carbon atoms of the parent chain are numbered to identify the parent alkane and to locate the positions of the carbon atoms at which branching takes place due to the substitution of alkyl group in place of hydrogen atoms. The numbering is done in such a way that the branched carbon atoms get the lowest possible numbers. Thus, the numbering in the above example should be from left to right (branching at carbon atoms 2 and 6).
-
The names of alkyl groups attached as a branch are then prefixed to the name of the parent alkane and position of the substituents is indicated by the appropriate numbers. If different alkyl groups are present, they are listed in alphabetical order. Thus, name for the compound shown above is: 6-ethyl-2-methylnonane.
-
If two or more identical substituent groups are present then the numbers are separated by commas. The names of identical substituents are not repeated, instead prefixes such as di (for 2), tri (for 3), tetra (for 4), penta (for 5), hexa (for 6) etc. are used. While writing the name of the substituents in alphabetical order, these prefixes, however, are not considered.
-
If the two substituents are found in equivalent positions, the lower number is given to the one coming first in the alphabetical listing. Thus, the following compound is 3-ethyl-6-methyloctane and not 6-ethyl-3-methyloctane.

8.5.3 Nomenclature of Organic Compounds having Functional Group(s)
Organic compounds having Functional Groups
The longes chain of carbon atoms containing the functional groups is numbered in such a way that the functional group attached to the carbon atom gets the lowest number in the chain.
When there are more functional groups then a priority order is followed as:$-\mathrm{COOH},-\mathrm{SO}_3 \mathrm{H},-\mathrm{COOR}(\mathrm{R}=$ alkyl group $), \mathrm{COCl},-\mathrm{CONH}_2,-\mathrm{CN},-\mathrm{HC}=\mathrm{O},>\mathrm{C}=\mathrm{O},-\mathrm{OH},-\mathrm{NH}_2,>\mathrm{C}=\mathrm{C}<,-\mathrm{C} \equiv \mathrm{C}-$
For example, the IUPAC name of this given compound is written as:

i). The functional group present is an alcohol (OH). Hence the suffix is ‘-ol’.
ii). The longest chain containing -OH has eight carbon atoms. Hence the corresponding saturated hydrocarbon is octane.
iii). The -OH is on carbon atom 3. In addition, a methyl group is attached at 6th carbon.
Hence, the systematic name of this compound is 6-Methyloctan-3-ol.
8.5.4 Nomenclature of substituted Benzene compounds
For IUPAC nomenclature of substituted benzene compounds, the substituent is placed as prefix to the word benzene as shown in the following examples. However, common names of many substituted benzene compounds are also universally used.
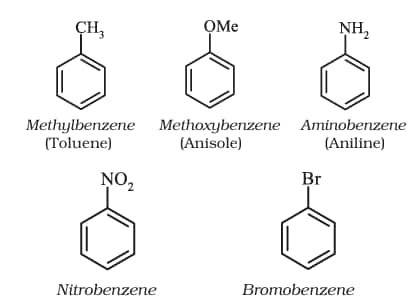
ii). In disubstituted benzene, the ring is numbered to give the substituents the lowest possible positions
For example
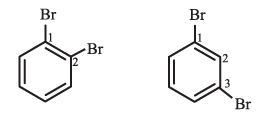
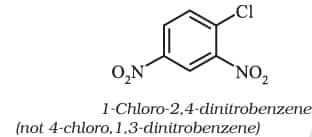
iii). In the trivial system of nomenclature the terms ortho (o), meta (m) and para (p) are used as prefixes to indicate the relative positions 1,2; 1,3 and 1,4 respectively.
8.6 Isomerism
The compounds having the same molecular formula but differ in structure are termed as isomers and the phenomenon are termed as isomerism.
8.6.1 Structural isomerism
Compounds having the same molecular formula but different structures (manners in which atoms are linked) are classified as structural isomers. Some typical examples of different types of structural isomerism are given below:
(i). Chain isomerism: When two or more compounds have similar molecular formula but different carbon skeletons, these are referred to as chain isomers and the phenomenon is termed as chain isomerism. For example, $\mathrm{C}_5 \mathrm{H}_{12}$ represents three compounds as follows:

(ii). Position isomerism: When two or more compounds differ in the position of substituent atom or functional group on the carbon skeleton, they are called position isomers and this phenomenon is termed as position isomerism. For example, the molecular formula $\mathrm{C}_3 \mathrm{H}_8 \mathrm{O}$ represents two alcohols:


Propan-1-ol Propan-2-ol
(iii). Functional group isomerism: Two or more compounds having the same molecular formula but different functional groups are called functional isomers and this phenomenon is termed as functional group isomerism. For example, the molecular formula $\mathrm{C}_3 \mathrm{H}_6 \mathrm{O}$ represents an aldehyde and a ketone as follows:

(iv). Metamerism: It arises due to different alkyl chains on either side of the functional group in the molecule. For example, $\mathrm{C}_4 \mathrm{H}_{10} \mathrm{O}$ represents methoxypropane ($\mathrm{CH}_3 \mathrm{OC}_3 \mathrm{H}_7$) and ethoxyethane ($\mathrm{C}_2 \mathrm{H}_5 \mathrm{OC}_2 \mathrm{H}_5$).
8.6.2 Stereoisomerism
The compounds that have the same constitution and sequence of covalent bonds but differ in relative positions of their atoms or groups in space are called stereoisomers. This special type of isomerism is called as stereoisomerism and can be classified as geometrical and optical isomerism.
8.7 Fundamental Concepts in Organic Reaction Mechanism
The organic molecule or substrate reacts with an appropriate attacking reagent and leads to the formation of one or more intermediate and finally product. A substrate is the reactant that donates carbon to form a new bond, while the other is called the reagent. The step-by-step description of bond changes, electron flow, and reaction rates is known as the reaction mechanism.
8.7.1 Fission of a Covalent Bond
A covalent bond can get cleaved either by:
- Heterolytic cleavage
- Homolytic cleavage
Heterolytic cleavage
In heterolytic cleavage, the bond breaks in such a fashion that the shared pair of electrons remains with one of the fragments. After heterolysis, one atom has a sextet electronic structure and a positive charge and the other, a valence octet with at least one lone pair and a negative charge. Thus, heterolytic cleavage of bromomethane will give $\mathrm{CH}_3{ }^{+}$ and $\mathrm{Br}^{-}$ as shown below.

Homolytic cleavage
In homolytic cleavage, one of the electrons of the shared pair in a covalent bond goes with each of the bonded atoms. Thus, in homolytic cleavage, the movement of a single electron takes place instead of an electron pair. The single electron movement is shown by ‘half-headed’ curved arrow. Such cleavage results in the formation of neutral species (atom or group) which contains an unpaired electron. These species are called free radicals. A homolytic cleavage can be shown as below:

8.7.2 Substrate and Reagent
Ions are generally not formed in the reactions of organic compounds. Molecules as such participate in the reaction. It is convenient to name one reagent as substrate and other as reagent. In general, a molecule whose carbon is involved in new bond formation is called substrate and the other one is called a reagent. When a carbon-carbon bond is formed, the choice of naming the reactants as substrate and reagent is arbitrary and depends on the molecule under observation. Some examples include:
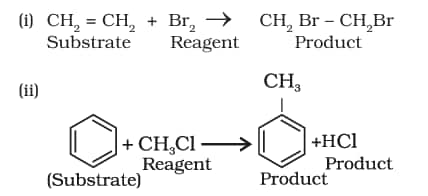
Nucleophiles and Electrophiles
A reagent that brings an electron pair to the reactive site is called a nucleophile (Nu:) i.e., nucleus seeking and the reaction is then called nucleophilic. A reagent that takes away an electron pair from the reactive site is called electrophile (E+) i.e., electron seeking and the reaction is called electrophilic.
During a polar organic reaction, a nucleophile attacks an electrophilic centre of the substrate which is that specific atom or part of the substrate which is electron deficient. Similarly, the electrophiles attack at the nucleophilic centre, which is the electron-rich centre of the substrate. Thus, the electrophiles receive electron pair from the substrate when the two undergo bonding interaction. A curved-arrow notation is used to show the movement of an electron pair from the nucleophile to the electrophile. Some examples of nucleophiles are the negatively charged ions with lone pair of electrons such as hydroxide (HO–), cyanide (NC–) ions and carbanions (R3C:–). Neutral molecules such as $\mathrm{H}_2 \mathrm{O}, \mathrm{R}_3 \mathrm{~N}, \mathrm{R}_2 \mathrm{NH}$, etc., can also act as nucleophiles due to the presence of lone pair of electrons. Examples of electrophiles include carbocations ( +CH3) and neutral molecules having functional groups like carbonyl group (>C=O) or alkyl halides ($\mathrm{R}_3 \mathrm{C}-\mathrm{X}$, where X is a halogen atom). The carbon atom in carbocations has sextet configuration; hence, it is electron-deficient and can receive a pair of electrons from the nucleophiles. In neutral molecules such as alkyl halides, due to the polarity of the C-X bond, a partial positive charge is generated on the carbon atom and hence the carbon atom becomes an electrophilic centre at which a nucleophile can attack.
8.7.3 Electron Movement in Organic Reactions
The movement of electrons in organic reactions can be shown by curved-arrow notation. It shows how changes in bonding occur due to electronic redistribution during the reaction. To show the change in position of a pair of electrons, curved arrow starts from the point from where an electron pair is shifted and it ends at a location to which the pair of electron may move.
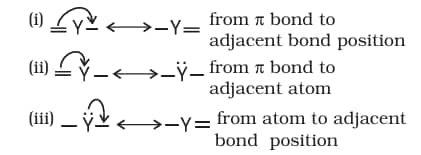
8.7.4 Electron Displacement Effects in Covalent Bonds
The electron displacement in an organic molecule may take place either in the ground state under the influence of an atom or a substituent group or in the presence of an appropriate attacking reagent. The electron displacements due to the influence of an atom or a substituent group present in the molecule cause permanent polarisation of the bond. Inductive effect and resonance effects are examples of this type of electron displacement. Temporary electron displacement effects are seen in a molecule when a reagent approaches to attack it. This type of electron displacement is called the electromeric effect or the polarisability effect. In the following sections, we will learn about these types of electronic displacements.
8.7.5 Inductive effect
When a covalent bond is formed between atoms of different electronegativity, the electron density is more towards the more electronegative atom of the bond. Such a shift of electron density results in a polar covalent bond. Bond polarity leads to various electronic effects in organic compounds. Let us consider chloroethane ($\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{Cl}$) in which the C–Cl bond is a polar covalent bond. It is polarised in such a way that the carbon-1 gains some positive charge (δ+) and the chlorine some negative charge (δ–). The fractional electronic charges on the two atoms in a polar covalent bond are denoted by symbol δ (delta) and the shift of electron density is shown by an arrow that points from δ+ to δ– end of the polar bond.
In turn carbon-1, which has developed a partial positive charge (δ+) draws some electron density towards it from the adjacent C-C bond. Consequently, some positive charge (δδ+) develops on carbon-2 also, where δδ+ symbolises relatively smaller positive charge as compared to that on carbon – 1. In other words, the polar C – Cl bond induces polarity in the adjacent bonds. Such polarisation of σ- bond caused by the polarisation of adjacent σ-bond is referred to as the inductive effect. This effect is passed on to the subsequent bonds also but the effect decreases rapidly as the number of intervening bonds increases and becomes vanishingly small after three bonds. The inductive effect is related to the ability of substituent(s) to either withdraw or donate electron density to the attached carbon atom. Based on this ability, the substitutents can be classified as electron-withdrawing or electron-donating groups relative to hydrogen. Halogens and many other groups such as nitro (-NO2), cyano (-CN), carboxy (-COOH), ester (COOR), aryloxy (-OAr, e.g. – OC6H5), etc. are electron-withdrawing groups. On the other hand, the alkyl groups like methyl (–CH3) and ethyl(–CH2–CH3) are usually considered as electron-donating groups. Download ncert class 11 chemistry chapter 8 organic chemistry some basic principles and techniques notes pdf to get a concise and well-structured summary of concepts, important reactions, and problem-solving techniques for effective revision and exam preparation.
8.7.6 Resonance structure
There are many organic molecules whose behaviour cannot be explained by a single Lewis structure. An example is that of benzene. Its cyclic structure containing alternating C–C single and C=C double bonds shown is inadequate for explaining its characteristic properties.
As per the above representation, benzene should exhibit two different bond lengths, Benzene due to C–C single and C=C double bonds. However, as determined experimentally benzene has a uniform C–C bond distances of 139 pm, a value intermediate between the C–C single(154 pm) and C=C double (134 pm) bonds. Thus, the structure of benzene cannot be represented adequately by the above structure. Further, benzene can be represented equally well by the energetically identical structures I and II.
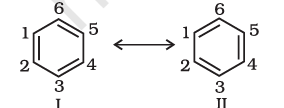
8.7.7 Resonance effect
The resonance effect is defined as ‘the polarity produced in the molecule by the interaction of two π-bonds or between a π-bond and lone pair of electrons present on an adjacent atom’. The effect is transmitted through the chain. There are two types of resonance or mesomeric effects designated as R or M effects.
- Positive Resonance Effect (+R effect): In this effect, the transfer of electrons is away from an atom or substituent group attached to the conjugated system. This electron displacement makes certain positions in the molecule of high electron densities. This effect in aniline is shown as:

- Negative Resonance Effect (- R effect): This effect is observed when the transfer of electrons is towards the atom or substituent group attached to the conjugated system. For example, in nitrobenzene this electron displacement can be depicted as:

The atoms or substituent groups, which represent +R or –R electron displacement effects are as follows :
- +R effect: $-\ddot{\mathrm{X}},-\ddot{\mathrm{O}} \mathrm{H},-\ddot{\mathrm{O} R},-\ddot{\mathrm{O}} \mathrm{COR},-\ddot{\mathrm{H}}_2,-\tilde{\mathrm{N}} \mathrm{HR},-\ddot{\mathrm{N}}_2,-\tilde{\mathrm{N}} \mathrm{HCOR}$
- – R effect: $-\mathrm{COOH},-\mathrm{CHO},>\mathrm{C}=\mathrm{O},-\mathrm{CN},-\mathrm{NO}_2,-\mathrm{SO}_3 \mathrm{H}$
The presence of alternate single and double bonds in an open-chain or cyclic system is termed as a conjugated system. These systems often show abnormal behaviour. The examples are 1,3- butadiene, aniline and nitrobenzene etc. In such systems, the π-electrons are delocalised and the system develops polarity.
8.7.8 Electromeric effect (e effect)
It is a temporary effect. The organic compounds having a multiple bond (a double or triple bond) show this effect in the presence of an attacking reagent only. It is defined as the complete transfer of a shared pair of π-electrons to one of the atoms joined by a multiple bond on the demand of an attacking reagent. The effect is annulled as soon as the attacking reagent is removed from the domain of the reaction. It is represented by E. There are two distinct types of electromeric effect.
(i) Positive Electromeric Effect (+E effect): In this effect the π−electrons of the multiple bond are transferred to that atom to which the reagent gets attached. For example:

(ii) Negative Electromeric Effect (–E effect): In this effect, the π - electrons of the multiple bond are transferred to that atom to which the attacking reagent does not get attached. For example:

NOTE: When inductive and electromeric effects operate in opposite directions, the electromeric effect predominates.
8.7.9 Hyperconjugation
Hyperconjugation is a general stabilising interaction. It involves delocalisation of σ electrons of C—H bond of an alkyl group directly attached to an atom of an unsaturated system or to an atom with an unshared p orbital. The σ electrons of C—H bond of the alkyl group enter into partial conjugation with the attached unsaturated system or with the unshared p orbital. Hyperconjugation is a permanent effect.
To understand the hyperconjugation effect, let us take an example of $\mathrm{CH}_3 \mathrm{CH}_2{ }^{+}$ (ethyl cation) in which the positively charged carbon atom has an empty p orbital. One of the C-H bonds of the methyl group can align in the plane of this empty p orbital and the electrons constituting the C-H bond in plane with this p orbital can then be delocalised into the empty p orbital as shown in the figure given below:

This type of overlap stabilises the carbocation because electron density from the adjacent σ bond helps in dispersing the positive charge.

In general, greater the number of alkyl groups attached to a positively charged carbon atom, the greater is the hyperconjugation interaction and stabilisation of the cation. Thus, we have the following relative stability of carbocations:

Hyperconjugation is also possible in free radicals, alkenes and alkylarenes.
In general, greater the number of hyperconjugative structures, greater is the stability.
8.7.10 Types of Organic reactions and Mechanisms
Organic reactions can be classified into the following categories:
(i). Substitution reactions
(ii). Addition reactions
(iii). Elimination reactions
(iv). Rearrangement reactions
8.8 Methods of Purification of Organic Compounds
Once an organic compound is extracted from a natural source or synthesised in the laboratory, it is essential to purify it. Various methods used for the purification of organic compounds are based on the nature of the compound and the impurity present in it.
Techniques used for purification are:
(i) Sublimation
(ii) Crystallisation
(iii) Distillation
(iv) Differential extraction
(v) Chromatography
8.8.1 Sublimation
It is the transition of a substance directly from the solid phase to the gas phase without passing through the intermediate liquid phase. This method of purification is used for those sublimable substances which are associated with non-volatile impurities. On heating the impure compound, volatile compound gets sublimed and its vapours are collected whereas, the non-volatile impurity remains as such. e.g., camphor, naphthalene, anthracene, benzoic acid, iodine, etc.
8.8.2 Crystallisation
It is the process of formation of solid crystals from solution, melt or by deposition directly from a gas phase. In this process a saturated solution of sparingly soluble compounds is prepared at high temperature and filtered.The clear solution thus obtained is set undisturbed to get cooled so that the pure solid organic substance is seperated out in the form of fine crystals which can be filtered out and dried. For example, Benzoic acid mixed with naphthalene can be seperated using hot water.
Note: Sometimes the process of crystallisation takes a long time. In such cases, crystallisation is initiated by adding a small crystal of some substance. This process is called seeding and the small crystal is known as seed which act as a nuclei for crystallisation.
8.8.3 Distillation
It is a procedure by which two liquids with different boiling points can be separated. Simple distillation can be used effectively to separate liquids that have some major degrees difference in their boiling points. As the liquid being distilled is heated, the vapours that form will be richest in the component of the mixture that boils at the lowest temperature. Purified compounds will boil, and thus turn into vapours, over a relatively small temperature range (2 or 3°C) by carefully watching the temperature in the distillation flask, it is possible to affect a reasonably good separation.
As distillation progresses, the concentration of the lowest boiling component will steadily decrease. Eventually, the temperature within the apparatus will begin to change; a pure compound is no longer being distilled.
Steps to be followed in simple distillation process:
(i) Take a mixture(Acetone and water) in the distillation flask fit it with the thermometer.
(ii) Arrange the apparatus as shown in the given figure.
(iii) Heat the mixture slowly keeping a close watch on thermometer.
(iv) Since the acetone has lower boiling point starts vaporises and condenses in the condenser which is finally collected in the beaker.
Fractional distillation
It is used for the mixture of two liquids which differ in their boiling point by 10-15 K. It is a type of distillation which involves the separation of miscible liquids. The process involves repeated distillations and condensations and the mixture is usually separated into component parts. In this method a fractionating column is used to increase the cooling surface area so that ascending vapour phase become richer in more volatile component and descending liquid phasebecome richer in less volatile component.
The basic principle of this type of distillation is that different liquids boil and evaporate at different temperatures. So when the mixture is heated, the substance with lower boiling point starts to boil first and convert into vapours.
Fractinal distillation is used for seperation of
(i) Acetone (b.pt. 329 K) from methyl alcohol (b.pt. 338 K)
(ii) Crude oil into various useful fractions such as gasoline, kerosene oil, lubricating oil, etc.

Steps to be followed in fractional distillation process:
1. After setting up the apparatus, a mixture of two miscible liquids A and B is taken where A has more volatility than substance B.
2. The solution is added into the distilling flask while the fractionating column is connected at the tip of the flask. Heat is applied which increases the temperature slowly. The mixture then starts to boil and vapours start rising in the flask.
3. The vapours are from the volatile component A. The vapours then start moving through the fractionating column into the condenser where it is cooled down to form a liquid which is collected in the receiver.
Throughout the process, vaporization and condensation take place repeatedly until the two mixtures are separated completely.
Distillation under reduced pressure or vacuum distillation is a type of distillation process that is used for the purification of high boiling liquids and liquids which decompose at or below their normal boiling point.
If the outer pressure is reduced by suction pump or vacuum pump, the liquid boils at lower temperature without decomposition. e.g., glycerol (b.pt. 563 K) can be distilled at 453 K under 12 mm Hg pressure without decomposition.
Raw juice in sugar factories is generally concentrated by vacuum distillation.

Steam distillation process is used for the seperation and purification of organic compounds (solid or liquid) which are volatile in steam, immiscible with water, possess a high vapour pressure of about 10-15 mm Hg at 373 K and contain non-volatile impurities.

In this process steam is passed through the organic mixture to be distilled so that distilling mixture consists of steam and volatile organic compound, which follows that
Atmospheric pressure = vapour pressure of organic substance + vapour pressure of steam.
From the above relation it an be interpretated that organic compounds distil below its normal boiling point without decomposition. For example,
(1) Aniline can be distilled at 371.5 K against its normal boiling point 457 K.
(2) o-Nitrophenol can be seperated from p-Nitrophenol, since o-Nitrophenol is volatile in steam.
8.8.4 Differential extraction
It also means extraction with solvent. This method is based on the fact that organic substances are more soluble in organic solvents than in water. The organic substance is extracted from it's aqueous solution adopting the following process:
(1) The aqueous solution containing organic substance is shaken with a suitable organic solvent which dissolves the substance but is immiscible with water. Two layers are formed- organic layer and an aqueous layer.

8.8.5 Chromatography
(2) The solvent layer containing the organic substance (organic layer) is seperated using a seperating funnel. The impurities remain in the aqueous layer.
(3) The organic solvent is removed by distillation to obtain the organic substance.
The technique for the separation, purification, and testing of compounds. In this process, we apply the mixture to be separated on a stationary phase (solid or liquid) and a pure solvent such as water or any gas is allowed to move slowly over the stationary phase, carrying the components separately as per their solubility in the pure solvent.
There are four main types of chromatography:
1. Adsorption chromatography
2. Column chromatography
3. Thin layer chromatography
4. Partition chromatography
Adsorption chromatography
In the process of adsorption chromatography, different compounds are adsorbed on the adsorbent to different degrees based on the absorptivity of the component. Here also, a mobile phase is made to move over a stationary phase, thus carrying the components with higher absorptivity to a lower distance than that with lower absorptivity.
Column chromatography
It is the technique used to separate the components of a mixture using a column of suitable adsorbent packed in a glass tube, as shown in the figure below. The mixture is placed on the top of the column, and an appropriate eluant is made to flow down the column slowly. Depending upon the degree of adsorption of the components on the wall adsorbent column, the separation of the components takes place. The component with the highest absorptivity is retained at the top, while the other flow down to different heights accordingly.

Thin layer chromatography
In this process the mixture of substances is separated into its components with the help of a glass plate coated with a very thin layer of adsorbent, such as silica gel and alumina, as shown in the figure below.
The plate used for this process is known as chrome plate. The solution of the mixture to be separated is applied as a small spot at a distance of 2 cm above one end of the plate. The plate is then placed in a closed jar containing a fluid termed as an eluant, which then rises up the plate carrying different components of the mixture to different heights.

Partition chromatography
In this process, a continuous differential partitioning of components of a mixture into a stationary phase and mobile phase takes place.Paper chromatography is a type of partition chromatography. A special quality paper known as chromatography paper is used. In this process, chromatography paper is used as a stationary phase which is suspended in a mixture of solvents that act as a mobile phase. Here, we put a spot at the base of the chromatographic paper with the mixture to be separated and as the solvent rises up this paper, the components are carried to different degrees depending upon their retention on the paper. The components are thus separated at different heights.

8.9 Qalitative analysis of Organic Compounds
The elements present in organic compounds are carbon and hydrogen. In addition to these, they may also contain oxygen, nitrogen, sulphur, halogens and phosphorus.
8.9.1 Detection of Carbon and Hydrogen
Carbon and hydrogen are detected by heating the compound with copper(II) oxide. Carbon present in the compound is oxidised to carbon dioxide (tested with lime-water, which develops turbidity) and hydrogen to water (tested with anhydrous copper sulphate, which turns blue).
$\mathrm{C}+2 \mathrm{CuO} \xrightarrow{\Delta} 2 \mathrm{Cu}+\mathrm{CO}_2$
$2 \mathrm{H}+\mathrm{CuO} \xrightarrow{\Delta} \mathrm{Cu}+\mathrm{H}_2 \mathrm{O}$
$\mathrm{CO}_2+\mathrm{Ca}(\mathrm{OH})_2 \longrightarrow \mathrm{CaCO}_3 \downarrow+\mathrm{H}_2 \mathrm{O}$
$5 \mathrm{H}_2 \mathrm{O}+\mathrm{CuSO}_4 \longrightarrow \mathrm{CuSO}_4 \cdot 5 \mathrm{H}_2 \mathrm{O}$
White Blue
8.9.2 Detection of Other Elements
Nitrogen, sulphur, halogens and phosphorus present in an organic compound are detected by “lassaigne’s test”. The elements present in the compound are converted from covalent form into the ionic form by fusing the compound with sodium metal. Following reactions take place:
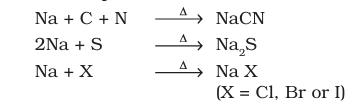
(A). Test for Nitrogen
The sodium fusion extract is boiled with iron(II) sulphate and then acidified with concentrated sulphuric acid. The formation of Prussian blue colour confirms the presence of nitrogen. Sodium cyanide first reacts with iron(II) sulphate and forms sodium hexacyanidoferrate(II). On heating with concentrated sulphuric acid some iron(II) ions are oxidised to iron(III) ions which react with sodium hexacyanidoferrate(II) to produce iron(III) hexacyanidoferrate(II) (ferriferrocyanide) which is Prussian blue in colour.
$6 \mathrm{CN}+\mathrm{Fe}^{2+} \rightarrow\left[\mathrm{Fe}(\mathrm{CN})_6\right]^{4-}$
$3\left[\mathrm{Fe}(\mathrm{CN})_6\right]^{4-}+4 \mathrm{Fe}^{3+} \xrightarrow{\mathrm{xH}_2 \mathrm{O}} \mathrm{Fe}_4\left[\mathrm{Fe}(\mathrm{CN})_6\right]_3 \cdot \times \mathrm{H}_2 \mathrm{O}$
Prussian blue
(B). Test for Sulphur
The sodium fusion extract is acidified with acetic acid and lead acetate is added to it. A black precipitate of lead sulphide indicates the presence of sulphur.
$\mathrm{S}^{2-}+\mathrm{Pb}^{2+} \quad \longrightarrow \mathrm{PbS}$
On treating sodium fusion extract with sodium nitroprusside, appearance of a violet colour further indicates the presence of sulphur.
$\mathrm{S}^{2-}+\left[\mathrm{Fe}(\mathrm{CN})_5 \mathrm{NO}\right]^{2-} \longrightarrow\left[\mathrm{Fe}(\mathrm{CN})_5 \mathrm{NOS}\right]^{4-}$
In case, nitrogen and sulphur both are present in an organic compound, sodium thiocyanate is formed. It gives blood red colour and no Prussian blue since there are no free cyanide ions.
$\mathrm{Na}+\mathrm{C}+\mathrm{N}+\mathrm{S} \longrightarrow \mathrm{NaSCN}$
$\mathrm{Fe}^{3+}+\mathrm{SCN}^{-} \longrightarrow[\mathrm{Fe}(\mathrm{SCN})]^{2+}$
If sodium fusion is carried out with excess of sodium, the thiocyanate decomposes to yield cyanide and sulphide. These ions give their usual tests.
$\mathrm{NaSCN}+2 \mathrm{Na} \longrightarrow \mathrm{NaCN}+\mathrm{Na}_2 \mathrm{~S}$
(C) Test for Halogens
The sodium fusion extract is acidified with nitric acid and then treated with silver nitrate. A white precipitate, soluble in ammonium hydroxide shows the presence of chlorine, a yellowish precipitate, sparingly soluble in ammonium hydroxide shows the presence of bromine and a yellow precipitate, insoluble in ammonium hydroxide shows the presence of iodine.
$\mathrm{X}^{-}+\mathrm{Ag}^{+} \longrightarrow \mathrm{AgX}$
If nitrogen or sulphur is also present in the compound, the sodium fusion extract is first boiled with concentrated nitric acid to decompose cyanide or sulphide of sodium formed during Lassaigne’s test. These ions would otherwise interfere with silver nitrate test for halogens.
(D) Test for Phosphorus
The compound is heated with an oxidising agent (sodium peroxide). The phosphorus present in the compound is oxidised to phosphate. The solution is boiled with nitric acid and then treated with ammonium molybdate. A yellow colouration or precipitate indicates the presence of phosphorus.
$\mathrm{Na}_3 \mathrm{PO}_4+3 \mathrm{HNO}_3 \longrightarrow \mathrm{H}_3 \mathrm{PO}_4+3 \mathrm{NaNO}_3$
$\mathrm{H}_3 \mathrm{PO}_4+12\left(\mathrm{NH}_4\right)_2 \mathrm{MoO}_4+21 \mathrm{HNO}_3 \longrightarrow\left(\mathrm{NH}_4\right)_3 \mathrm{PO}_4 \cdot 12 \mathrm{MoO}_3+21 \mathrm{NH}_4 \mathrm{NO}_3+12 \mathrm{H}_2 \mathrm{O}$
8.10 Quantitative Analysis
Quantitative analysis of compounds helps chemists in the determination of mass per cent of elements present in a compound.
8.10.1 Carbon and Hydrogen
Both carbon and hydrogen are estimated in one experiment. A known mass of an organic compound is burnt in the presence of excess of oxygen and copper(II) oxide. Carbon and hydrogen in the compound are oxidised to carbon dioxide and water respectively.
$\mathrm{C}_{\mathrm{x}} \mathrm{H}_{\mathrm{y}}+(\mathrm{x}+\mathrm{y} / 4) \mathrm{O}_2 \longrightarrow \mathrm{xCO}_2+(\mathrm{y} / 2) \mathrm{H}_2 \mathrm{O}$
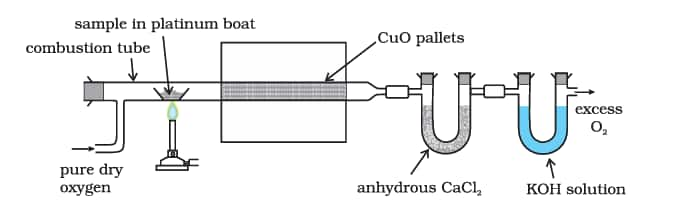
8.10.2 Nitrogen
There are two methods for estimation of nitrogen:
(i) Dumas method and
(ii) Kjeldahl’s method
(i). Duma's Method
The nitrogen containing organic compound, when heated with copper oxide in an atmosphere of carbon dioxide, yields free nitrogen in addition to carbon dioxide and water.
$\mathrm{C}_{\mathrm{x}} \mathrm{H}_{\mathrm{y}} \mathrm{N}_{\mathrm{z}}+(2 \mathrm{x}+\mathrm{y} / 2) \mathrm{CuO} \longrightarrow \mathrm{xCO}_2+\mathrm{y} / 2 \mathrm{H}_2 \mathrm{O}+\mathrm{z} / 2 \mathrm{~N}_2+(2 \mathrm{x}+\mathrm{y} / 2) \mathrm{Cu}$
Traces of nitrogen oxides formed, if any, are reduced to nitrogen by passing the gaseous mixture over a heated copper gauze. The mixture of gases so produced is collected over an aqueous solution of potassium hydroxide which absorbs carbon dioxide. Nitrogen is collected in the upper part of the graduated tube as shown in the figure.
Let the mass of organic compound = mg
Volume of nitrogen collected = V1 mL
Room temperature = T1K
Volume of nitrogen at $\mathrm{STP}=\frac{p_1 V_1 \times 273}{760 \times T_1}$
Where p1 and V1 are the pressure and volume of nitrogen, p1 is different from the atmospheric pressure at which nitrogen gas is collected. The value of p1 is obtained by the relation;
p1 = Atmospheric pressure – Aqueous tension
22400 mL N2 at STP weighs 28g.
$V \mathrm{~mL} \mathrm{~N}_2$ at STP weighs $=\frac{28 \times V}{22400} \mathrm{~g}$
Percentage of nitrogen $=\frac{28 \times V \times 100}{22400 \times m}$

(ii). Kjeldahl's Method
The compound containing nitrogen is heated with concentrated sulphuric acid. Nitrogen in the compound gets converted to ammonium sulphate. The resulting acid mixture is then heated with excess of sodium hydroxide. The liberated ammonia gas is absorbed in an excess of standard solution of sulphuric acid. The amount of ammonia produced is determined by estimating the amount of sulphuric acid consumed in the reaction. It is done by estimating unreacted sulphuric acid left after the absorption of ammonia by titrating it with standard alkali solution. The difference between the initial amount of acid taken and that left after the reaction gives the amount of acid reacted with ammonia.
Organic compound $+\mathrm{H}_2 \mathrm{SO}_4 \longrightarrow\left(\mathrm{NH}_4\right)_2 \mathrm{SO}_4 \xrightarrow{2 \mathrm{NaOH}} \mathrm{Na}_2 \mathrm{SO}_4+2 \mathrm{NH}_3+2 \mathrm{H}_2 \mathrm{O}$

Kjeldahl method is not applicable to compounds containing nitrogen in nitro and azo groups and nitrogen present in the ring (e.g. pyridine) as nitrogen of these compounds does not change to ammonium sulphate under these conditions.
8.10.3 Halogens
A known mass of an organic compound is heated with fuming nitric acid in the presence of silver nitrate contained in a hard glass tube known as Carius tube, in a furnace. Carbon and hydrogen present in the compound are oxidised to carbon dioxide and water. The halogen present forms the corresponding silver halide (AgX). It is filtered, washed, dried and weighed.

Let the mass of organic compound taken = m g
Mass of AgX formed = m1 g
1 mol of AgX contains 1 mol of XMass of halogen in $\mathrm{m}_1 \mathrm{~g}$ of $\mathrm{AgX}=\frac{\text { atomic mass of } \mathrm{X} \times m_1 \mathrm{~g}}{\text { molecular mass of } \mathrm{AgX}}$
Percentage of halogen $=\frac{\text { atomic mass of } \mathrm{X} \times m_1 \times 100}{\text { molecular mass of } \mathrm{AgX} \times m}$
8.10.4 Sulphur
A known mass of an organic compound is heated in a Carius tube with sodium peroxide or fuming nitric acid. Sulphur present in the compound is oxidised to sulphuric acid. It is precipitated as barium sulphate by adding excess of barium chloride solution in water. The precipitate is filtered, washed, dried and weighed. The percentage of sulphur can be calculated from the mass of barium sulphate.
Let the mass of organic compound taken = m g
and the mass of barium sulphate formed = m1 g
1 mol of $\mathrm{BaSO}_4$ = 233 g $\mathrm{BaSO}_4$ = 32 g sulphur
$\mathrm{m}_1 \mathrm{gBaSO}_4$ contains $\frac{32 \times m_1}{233} \mathrm{~g}$ sulphur
Percentage of sulphur $=\frac{32 \times m_1 \times 100}{233 \times m}$
8.10.5 Phosphorus
A known mass of an organic compound is heated with fuming nitric acid whereupon phosphorus present in the compound is oxidised to phosphoric acid. It is precipitated as ammonium phosphomolybdate, $\left(\mathrm{NH}_4\right)_3$, $\mathrm{PO}_4 \cdot 12 \mathrm{MoO}_3$,by adding ammonia and ammonium molybdate. Alternatively, phosphoric acid may be precipitated as $\mathrm{MgNH}_4 \mathrm{PO}_4$ by adding magnesia mixture which on ignition yields $\mathrm{Mg}_2 \mathrm{P}_2 \mathrm{O}_7$.
8.10.6 Oxygen
The percentage of oxygen in an organic compound is usually found by difference between the total percentage composition (100) and the sum of the percentages of all other elements. However, oxygen can also be estimated directly as follows:
A definite mass of an organic compound is decomposed by heating in a stream of nitrogen gas. The mixture of gaseous products containing oxygen is passed over red-hot coke when all the oxygen is converted to carbon monoxide. This mixture is passed through warm iodine pentoxide (I2O5) when carbon monoxide is oxidised to carbon dioxide producing iodine.
Compound $\xrightarrow{\text { heat }} \mathrm{O}_2+$ other gaseous products
$2 \mathrm{C}+\mathrm{O}_2 \xrightarrow{1373 \mathrm{~K}} 2 \mathrm{CO} \times 5 \quad \mathrm{~A}$
$\mathrm{I}_2 \mathrm{O}_5+5 \mathrm{CO} \longrightarrow \mathrm{I}_2+5 \mathrm{CO}_2 \times 2 \quad \mathrm{~B}$
On making the amount of CO produced in equation (A) equal to the amount of CO used in equation (B) by multiplying the equations (A) and (B) by 5 and 2 respectively; we find that each mole of oxygen liberated from the compound will produce two moles of carbon dioxide. Thus 88 g carbon dioxide is obtained if 32 g oxygen is liberated.
Organic Chemistry- Some Basic Principles and Techniques Previous Year Question and Answer
Previous year questions and answers will help students understand important exam questions and frequently asked concepts. These questions with solutions are useful for Chemistry exam preparation and quick revision. The concepts used to solve these questions are explained in organic chemistry some basic principles and techniques class 11 chemistry notes:
Question 1: Hyperconjugation involves delocalisation of ______.
(i) electrons of carbon-hydrogen $\sigma$ bond of an alkyl group directly attached to an atom of unsaturated system.
(ii) electrons of carbon-hydrogen $\sigma$ bond of alkyl group directly attached to the positively charged carbon atom.
(iii) $\pi$-electrons of carbon-carbon bond
(iv) lone pair of electrons
(1). (i) and (ii)
(2). (iii) and (iv)
(3). (ii) and (iii)
(4). None of above
Answer:
The answer is the option (i) and (ii)
Explanation: Hyperconjugation means the delocalization of electrons of the C-H bond of an alkyl group directly attached to an atom of an unsaturated system or to an atom with an unshared p orbital. Electrons of the C-H bond of the alkyl group enter into the partial conjugation with the attached unsaturated system or with the unshared p orbital. Thus, Hyperconjugation is a permanent effect.
Hence, the answer is the option (1).
Question 2: Which of the following pairs are position isomers?
(1) I and II
(2) II and III
(3) II and IV
(4) III and IV
Answer:
The answer is the option (2) II and III
When two or more compounds have functional groups or substituent atoms attached at different positions on the carbon skeleton, they are termed as position isomers, and this phenomenon is termed as position isomerism.
Hence, the answer is the option (2).
Question 3: Which of the following compounds contain all the carbon atoms in the same hybridisation state?
$\begin{aligned} & \text { (i) } \mathrm{H}-\mathrm{C} \equiv \mathrm{C}-\mathrm{C} \equiv \mathrm{C}-\mathrm{H} \\ & \text { (ii) } \mathrm{CH}_3-\mathrm{C} \equiv \mathrm{C}-\mathrm{CH}_3 \\ & \text { (iii) } \mathrm{CH}_2=\mathrm{C}=\mathrm{CH}_2 \\ & \text { (iv) } \mathrm{CH}_2=\mathrm{CH}-\mathrm{CH}=\mathrm{CH}_2\end{aligned}$
(1) (i) and (ii)
(2) (i) and (iv)
(3) (ii) and (iv)
(4) (iii) and (iv)
Answer:
(i) H–C≡C–C≡C–H
All carbons are involved in triple bonds or are sp-hybridised.
All carbon atoms are sp-hybridised
(iv) CH2=CH–CH=CH2
A conjugated diene system.
All four carbon atoms are $\mathrm{sp}^2$ hybridised.
Hence, the answer is the option (2).
Question 4: Mixture of 1 g each of chlorobenzene, aniline and benzoic acid is dissolved in 50 mL ethyl acetate and placed in a separating funnel, 5 M NaOH ( 30 mL ) was added in the same funnel. The funnel was shaken vigorously and then kept aside. The ethyl acetate layer in the funnel contains :
(1) benzoic acid
(2) benzoic acid and aniline
(3) benzoic acid and chlorobenzene
(4) chlorobenzene and aniline
Answer:
NaOH and benzoic acid will react to form salt. Aniline and chlorobenzene, being organic compounds, will remain in the ethyl acetate layer as they will not react with NaOH.
Hence, the correct answer is option (4).
Question 5:
Which of the following is the correct IUPAC name of given organic compound (X) ?

(1) 2-Bromo-2-methylbut-2-ene
(2) 3-Bromo-3-methylprop-2-ene
(3) 1-Bromo-2-methylbut-2-ene
(4) 4-Bromo-3-methylbut-2-ene
Answer:
The naming will we done from the carbon where the functional groups get the lowest number. So here, first carbon will be the one having bromo group, followed by alkene carbon

1-Bromo-2-methyl but-2-ene
Hence, the correct answer is option (3).
How to Master Class 11 Chemistry Chapter 8 Organic Chemistry Some Basic Principles and Techniques
This chapter helps students to understand the fundamentals of organic chemistry, focusing on structure, bonding, nomenclature, and purification methods. These organic chemistry some basic principles and techniques class 11 chemistry chapter 8 CBSE notes help to understand the basic concepts from your NCERT textbook. Given below some points on how to master this chapter.
- Firstly students must understand the IUPAC nomenclature rules for naming organic compounds.
- Then learn about electron displacement effects like inductive effect, resonance, hyperconjugation, and electromeric effect.
- Students must understand the basics of reaction intermediates such as carbocations, carbanions, and free radicals.
- Questions related to reaction mechanisms are often asked in exams. Students can refer to NCERT Class 11 Chemistry Chapter 8 Notes Organic Chemistry Some Basic Principles and Techniques.
- Then read about purification techniques like distillation, crystallisation, chromatography, and sublimation.
- At last students can solve previous year questions from this chapter.
Advantages of Using Class 11 Chemistry Chapter 8 Organic Chemistry: Some Basic Principles and Techniques Notes
NCERT Class 11 Chemistry Chapter 8 Notes Organic Chemistry Some Basic Principles and Techniques covers all important concepts from the NCERT book in a simple and organised manner. The advantages of using these notes are given below:
- These notes cover topics like classification of organic compounds, nomenclature, and types of reactions, electronic displacements, purification and characterisation of organic compounds.
- Students can understand the reaction mechanisms and the basics of organic chemistry using these ncert class 11 chemistry chapter 8 organic chemistry some basic principles and techniques notes.
- These notes are prepared by subject experts in a very clear and comprehensive manner to help students understand the difficult concepts easily and perform well in exams.
CBSE Class 11 Chemistry Chapter-wise Notes
Apart from the class 11 chemistry chapter 8 organic chemistry some basic principles and techniques notes, students can also go through the NCERT notes of other Class 11 chapters listed below:
| NCERT notes for class 11 Chemistry Chapter 1 Some Basic Concepts of Chemistry |
|
NCERT notes for class 11 Chemistry Chapter 2 Structure of Atom |
|
NCERT notes for class 11 Chemistry Chapter 4 Chemical Bonding and Molecular Structure |
|
NCERT notes for class 11 Chemistry Chapter 7 Redox Reactions |
|
NCERT notes for class 11 Chemistry Chapter 8 Organic Chemistry - Some Basic Principles and Techniques |
NCERT Solutions for Class 11 Chemistry
Along with organic chemistry some basic principles and techniques class 11 chemistry chapter 8 CBSE notes, follow the links below to get chapter-wise solutions of NCERT and make your learning better.
Subject Wise NCERT Exemplar Solutions
Students can refer to the links given below for NCERT Exemplar Solutions:
Subject Wise NCERT Solutions
Students can refer to the links given below for NCERT Solutions:
| NCERT Solutions for Class 11 Mathematics |
| NCERT Solutions for Class 11 Chemistry |
| NCERT Solutions for Class 11 Physics |
| NCERT Solutions for Class 11 Biology |
Frequently Asked Questions (FAQs)
A reagent is any substance used to cause a chemical reaction or to detect other substances in organic chemistry. Reagents are vital because they are the chemicals that facilitate reactions, help in the synthesis of new compounds, or act as catalysts.
Basic techniques for the purification of organic compounds include distillation, crystallization, sublimation, and filtration. Distillation separates components based on differences in boiling points; crystallization involves forming solid crystals from a solution to remove impurities; sublimation is the transition of a substance from solid to gas and back, purifying volatile substances; and filtration is used to separate solids from liquids.
The concept of homology in organic chemistry refers to a series of compounds that differ from each other by a specific unit. This concept helps in understanding the relationship between different compounds and predicting properties of similar compounds within a homologous series.
Functional groups are specific groups of atoms within molecules that determine the chemical properties and reactivity of those molecules. They act as the "active sites" in organic reactions. Common functional groups include hydroxyl, carboxyl, amino, and halogens.
In Class 11 Chemistry Chapter 8, nucleophiles are electron-rich species that donate a pair of electrons to form a covalent bond, while electrophiles are electron-deficient species that accept electrons, carbonyl carbons. Understanding nucleophiles and electrophiles is key to grasping organic reaction mechanisms.
The organic chemistry some basic principles and techniques notes class 11 chemistry provide a simplified overview of key concepts such as IUPAC nomenclature, structural representation, electron displacement effects, reaction intermediates, and purification methods. These notes help students quickly revise important topics and build a strong base in organic chemistry.
The organic chemistry some basic principles and techniques class 11 chemistry chapter 8 CBSE notes covers the fundamentals of organic chemistry, including the IUPAC system of nomenclature for naming organic compounds, basic reaction mechanisms, electron displacement effects, and methods for purification of organic substances. It provides the foundational knowledge required for understanding more complex organic reactions in higher classes.
Common techniques used for separation and purification in organic chemistry include distillation, chromatography and recrystallization.
Isomerism refers to the phenomenon where two or more compounds have the same molecular formula but different structures or arrangements of atoms. Isomers can be broadly classified into structural isomers, which differ in the connectivity of their atoms, and stereoisomers, which differ in the spatial orientation of their atoms.
A homologous series is the group of organic compounds that have the same functional group and the same general formula.
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters


