These carbon and its compounds class 10 question answer provide clear and step by step answers to all questions. They help students understand the structure, properties, and reactions of carbon compounds in a simple way. These NCERT Solutions for Class 10 also make it easier for students to understand important concepts and prepare effectively for exams.
NCERT Solutions for Class 10 Science Chapter 4 Carbon and Its Compounds
Ever wondered how one element builds everything from fuels to life itself? Let us find the answer through carbon and its compounds! This chapter provides the foundational concepts for the entire field of organic chemistry. Carbon is considered a unique element in the periodic table owing to its property of tetravalency and catenation. These properties also help to explain the ability of carbon to create varied compounds ranging from simpler ones such as methane, to complex ones such as glucose and DNA.
This Story also Contains
- NCERT Solutions for Class 10 Science Chapter 4: Download PDF
- NCERT Solutions for Class 10 Science Chapter 4 (In-text Questions)
- NCERT Solutions for Class 10 Science Chapter 4 (Exercise Questions)
- Practice Questions of NCERT Class 10 Science Chapter 4
- Approaches To Solve Problems of Chapter 4 Class 10 Science
- Topics and Sub-Topics of NCERT Class 10 Science Chapter 4
- NCERT Class 10 Science Chapter 4: Important Compounds, Reactions and E-book
- What Students Learn from NCERT Solutions for Class 10 Science Chapter 4
- NCERT Solutions for Class 10 Science Chapter-wise Links

The important topics related to carbon, its position in the periodic table, behavior of various carbon compounds are well discussed in carbon and its compounds ncert solutions. The NCERT solutions for class 10 science will explain these topics through a series of solved questions. These solutions are one of the important sources for board exams and competitive exams. The practice questions are also added to improve your problem solving skills. We have also added some points to help you build a correct approach to attempt the questions. Students can access these NCERT solutions from our website.
NCERT Solutions for Class 10 Science Chapter 4: Download PDF
Students can download the class 10 science chapter 4 carbon and its compounds solutions pdf for free. These solutions of NCERT help students understand key concepts, reactions, and formulas, making exam preparation easier and more effective.
NCERT Solutions for Class 10 Science Chapter 4 (In-text Questions)
Topic:4.1 Bonding in carbon - The covalent bond, Page no 61
Question 1. What would be the electron dot structure of carbon dioxide which has the formula $\mathrm{CO}_2$ ?
Answer:
The electron dot structure of carbon dioxide, which has the formula $\mathrm{CO}_2$ is:
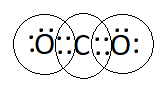
Answer:
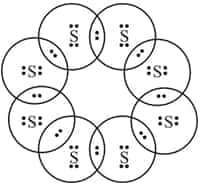
The electron dot structure of a molecule of $S_8$ is:
Topic 4.2: Versatile nature of carbon Page no-68
Question 1. How many structural isomers can you draw for pentane?
Answer:
There are three structural isomers of pentane:
|
n-pentane |
|
|
iso-pentane |
|
|
neo-pentane |
|
Question 2. What are the two properties of carbon which lead to the huge number of carbon compounds we see around us?
Answer:
The two properties of carbon that lead to the formation of a large number of carbon compounds are:
(1) Catenation: Carbon has the unique ability to form bonds with other atoms of carbon, which gives rise to large molecules. The carbon-carbon bond is very strong and hence stable.
(2) Tetravalency: Since carbon has a valency of four, it is capable of bonding with four other atoms. These bonds that carbon forms with other elements are very strong, making these compounds exceptionally stable.
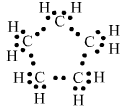
Question 3. What will be the formula and electron dot structure of cyclopentane?
Answer:
The formula of cyclopentane is $C_5 H_{10}$.
And, the electron dot structure of cyclopentane is :
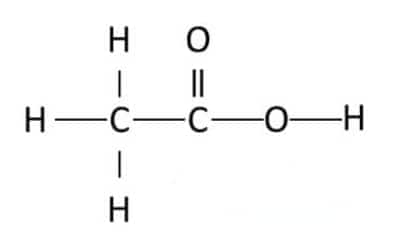
Question 4. Draw the structures for the following compounds.
(i) Ethanoic acid
Answer:
The structure of ethanoic acid is:
Question 4. Draw the structures for the following compounds.
Are structural isomers possible for bromopentane?
Answer:
The structure of bromopentane( $\mathrm{C}_5 \mathrm{H}_{11} \mathrm{Br}$ ) is:
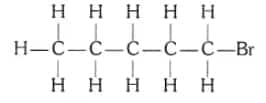
Yes, structural isomers for bromopentane are possible.
Also, Br at different carbons for iso-pentane and neo-pentane.
Question 4. Draw the structures for the following compounds.
Answer:
The structure of Butanone ( $\mathrm{C}_2 \mathrm{H}_5 \mathrm{COCH}_3$ ) is:
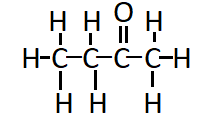
Question 4.
Draw the structures for the following compounds.
Answer :
The structure of hexanal ( $\mathrm{C}_5 \mathrm{H}_{11} \mathrm{CHO}$ ) is:
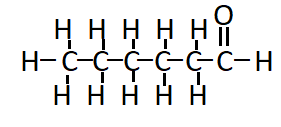
Question 5(i) How would you name the following compound?
$
\mathrm{CH}_3-\mathrm{CH}_2-\mathrm{Br}
$
Answer :
The compound ( $\mathrm{CH}_3-\mathrm{CH}_2-\mathrm{Br}$ ) has 2 carbon atoms. Hence, the parent hydrocarbon is ethane.
A Bromo group is attached to one of the carbon atoms. Thus, the nomenclature of the compound is Bromoethane.
Question 5(ii) How would you name the following compounds?
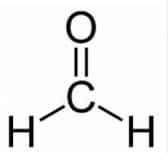
Answer:
The compound ( $\mathrm{H}_2 \mathrm{C}=0$ ) contains 1 carbon atom. Hence, the parent hydrocarbon is methane. It contains the functional group aldehyde.
Thus, the nomenclature of the compound is: Methanal.
Question 5(iii) How would you name the following compound?
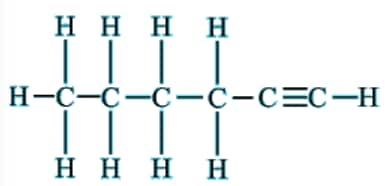
Answer:
The compound has 6 carbons in the chain. Hence, the parent hydrocarbon is hexane. It contains a triple bond and hence the suffix -yne- is used. Thus, the nomenclature of the compound is: Hexyne
Topic 4.3 Chemical Properties of Carbon Compounds Page no. 71
Question 1. Why is the conversion of ethanol to ethanoic acid an oxidation reaction?
Answer:
The conversion of ethanol to ethanoic acid is :
$\mathrm{CH}_3-\mathrm{CH}_2 \mathrm{OH} \xrightarrow[\text { Or acidified } \mathrm{K}_4 \mathrm{Cr}_3 \mathrm{O}, \text { Heat }]{\text { Alkatine } \mathrm{KMnO}_4} \mathrm{CH}_3 \mathrm{COOH}$
Since there is an addition of oxygen in the reaction, this is an oxidation reaction.
Answer:
A mixture of oxygen and ethyne is burnt for welding. But a mixture of ethyne and air is not used. This is because ethyne undergoes incomplete combustion in air, giving a sooty flame.
In oxygen, ethyne gives a clean flame with a temperature as high as 3300 K due to complete combustion.
Topic 4.4: Some Important Carbon Compounds - Ethanol and Ethanoic Acid Page no 74
Question 1. How would you distinguish experimentally between an alcohol and a carboxylic acid?
Answer:
We can distinguish between alcohol and carboxylic acid by reacting them with carbonates/hydrogen carbonates. The acid reacts with hydrogen carbonate to liberate carbon dioxide, which turns lime water milky.
$
\mathrm{CH}_3 \mathrm{COOH}+\mathrm{NaHCO}_3 \rightarrow \mathrm{CH}_3 \mathrm{COONa}+\mathrm{CO}_2+\mathrm{H}_2 \mathrm{O}
$
Whereas alcohol shows no evolution of gas when reacted with carbonates.
$
\mathrm{C}_2 \mathrm{H}_5 \mathrm{OH}+\mathrm{NaHCO}_3 \rightarrow \text { no reaction }
$
Question 2. What are oxidizing agents?
Answer:
The oxidation reaction is the reaction in which oxygen is added or hydrogen is removed.
The substance that adds oxygen to others is known as an oxidizing agent.
Example: Alkaline Potassium permanganate
Topic 4.5: Soaps and Detergents Page no 76
Question 1. Would you be able to check if the water is hard by using a detergent?
Answer:
No, we will not be able to check if the water is hard sing detergents. This is because detergents give rich lather with both hard and soft water without hard-singing scum.
Answer:
Soap molecules form spherical structures known as micelles. The oily dirt is trapped inside these micelles, which remain suspended as a colloid. Hence, to remove these micelles containing the dirt, the clothes are agitated.
NCERT Solutions for Class 10 Science Chapter 4 (Exercise Questions)
These class 10 science chapter 4 carbon and its compounds solutions offer detailed answers to all ecercise problems. They help students practise important reactions, chemical formulas, and properties of carbon compounds. These solutions also strengthen conceptual understanding and improve problem-solving skills for exams.
Question 1. Ethane, with the molecular formula $\mathrm{C}_2 \mathrm{H}_6$ has
(a) 6 covalent bonds.
(b) 7 covalent bonds.
(c) 8 covalent bonds.
(d) 9 covalent bonds.
Answer:
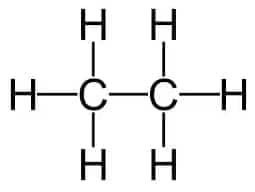
Ethane, with the molecular formula $\mathrm{C}_2 \mathrm{H}_6$ has 7 covalent bonds.
(b) It has $1(C-C)$ and $6(C-H)$ bonds.
Hence, the correct answer is option (b)
Question 2. Butanone is a four-carbon compound with the functional group
(a) carboxylic acid.
(b) aldehyde.
(c) ketone.
(d) alcohol.
Answer:
Butanone is a four-carbon compound with the functional group ketone. (c)
Butan- one: one is the suffix used for the functional group ketone.
Hence, the correct answer is option (c)
Question 3. While cooking, if the bottom of the vessel is getting blackened on the outside, it means that
(a) the food is not cooked completely.
(b) the fuel is not burning completely.
(c) the fuel is wet.
(d) the fuel is burning completely.
Answer:
While cooking, if the bottom of the vessel is getting blackened on the outside, it means that the fuel is not burning completely.
Hence, the correct answer is option (b)
Question 4. Explain the nature of the covalent bond using the bond formation in.
Answer:
Carbon has four electrons in its outermost shell and needs to gain or lose four electrons to attain a noble gas configuration. Carbon overcomes this problem by sharing its valence electrons with other atoms of carbon or with atoms of other elements.
Of the four electrons, three are shared with hydrogen atoms and one with a chlorine atom. Thus, it has three (C-H) and one (C-Cl) covalent bonds.
These bonds formed by the sharing of electrons are known as covalent bonds. Carbon, hydrogen, and chlorine attain the nearest noble gas configuration of Ne, He and Ar, respectively.
Question 5(a) Draw the electron dot structures for
(a) Ethanoic acid.
Answer:
The electron dot structure of ethanoic acid ( $\mathrm{CH}_3 \mathrm{COOH}$ ) is:
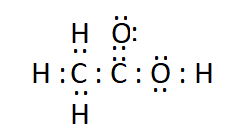
Question 5(b). Draw the electron dot structures for
(b). $\mathrm{H}_2 \mathrm{~S}$ .
Answer:
The electron dot structure of hydrogen sulphide ( $\mathrm{H}_2 \mathrm{~S}$ ) is:
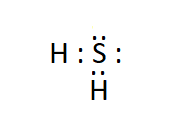
Question 5(c). Draw the electron dot structures for
(c). Propanone
Answer:
The electron dot structure of propanone ( $\mathrm{CH}_3 \mathrm{COCH}_3$ ) is:
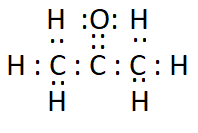
Question 5(d). Draw the electron dot structures for
(d). $F_2$
Answer:
The electron dot structure of $F_2$ is:
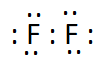
Question 6. What is a homologous series? Explain with an example.
Answer:
A series of carbon compounds having different numbers of carbon atoms but having the same functional group substituting the hydrogen atom is known as a homologous series.
For example, methane, ethane, propane, butane, etc., constitute the alkane homologous series. The general formula for alkanes is $\mathrm{C}_n \mathrm{H}_{2 n+2}$. Methane $\mathrm{CH}_4$; Ethane $\mathrm{C}_2 \mathrm{H}_6$; Propane $\mathrm{C}_3 \mathrm{H}_8$ and so on.
Question 7. How can ethanol and ethanoic acid be differentiated on the basis of their physical and chemical properties?
Answer:
Physical Properties
|
Ethanol |
Ethanoic acid |
|
It has a pleasant smell. |
It has a pungent smell of vinegar. |
|
It has a low boiling point: 351 K. |
It has a comparatively high boiling point: 391 K. |
Chemical Properties
|
Ethanol |
Ethanoic acid |
|
It has no action on litmus paper. |
It turns blue litmus to red. |
|
no reaction with $\mathrm{NaHCO}_3$ |
Liberates $\mathrm{CO}_2$ when reacted with $\mathrm{NaHCO}_3$ |
Answer:
A soap is a sodium or potassium salt of long-chain fatty acids. It has one polar hydrophilic end and one non-polar hydrophobic end. These molecules have a unique orientation inside water in the form of clusters of molecules in which hydrophobic ends are in the interior of the cluster and ionic ends on the surface of the cluster, thus keeping the hydrocarbon portion out of water. This formation is known as a Micelle. Soap in the form of a micelle is able to clean, as the oily dirt is collected in the centre of the micelle.
No, micelle formation does not take place in ethanol because the alkyl chain of soap becomes soluble in alcohol.
Question 9. Why are carbon and its compounds used as fuels for most applications?
Answer:
Carbon compounds have high calorific values. Therefore, carbon and its compounds are used as fuels for most applications. They give a lot of heat and light when burnt in air. Saturated hydrocarbons like methane burn with a clean flame without any smoke and are thus environmentally friendly.
Question 10. Explain the formation of scum when hard water is treated with soap.
Answer:
Hard water contains calcium and magnesium salts. When soap is added to hard water, it reacts with the calcium and magnesium ions of hard water to form an insoluble substance called scum. To overcome this problem, detergents are used.
Question 11. What change will you observe if you test soap with litmus paper (red and blue)?
Answer:
Soap is basic and hence will turn red litmus blue. However, it will not affect blue litmus paper.
Question 12. What is hydrogenation? What is its industrial application?
Answer:
The conversion of an unsaturated hydrocarbon to a saturated hydrocarbon by the addition of hydrogen in the presence of a nickel catalyst is called hydrogenation. This reaction is commonly used in the hydrogenation of vegetable oil into vegetable ghee.
Question 13. Which of the following hydrocarbons undergo addition reactions:
Answer:
Unsaturated hydrocarbons like alkenes and alkynes will undergo addition reactions. Therefore, $C_3 H_6$ (alkene having $-C=C-$ ) and $C_2 H_2$ (alkyne having $-\mathrm{C} \equiv \mathrm{C}-$ ) will give addition reactions.
Question 14. Give a test that can be used to differentiate between saturated and unsaturated hydrocarbons.
Answer:
Butter is a saturated compound and oil is an unsaturated compound.
Test: When oil is added to a test tube containing alkaline potassium permanganate solution $\mathrm{KMnO}_4$, the pink colour of the solution disappears. Therefore, cooking oil causes decolourisation of the solution. This does not happen in the case of butter.
Question 15. Explain the mechanism of the cleaning action of soaps.
Answer:
A soap is a sodium or potassium long-chain fatty acid. It has one polar hydrophilic end and one non-polar hydrophobic end. When soap is at the surface of the water, the hydrophobic tail of soap, being insoluble in water, protrudes out of the water with the ionic end in water.
When there is no more space for soap molecules on the surface, these molecules create a unique orientation inside water in the form of clusters in which hydrophobic ends are in the interior of the cluster and ionic ends on the surface of the cluster, thus keeping the hydrocarbon portion out of water. This formation is known as a Micelle.
Soap in the form of a micelle can clean, as the oily dirt is collected in the center of the micelle. The ionic ends in the micelles remain attached to water. When the dirty clothes are agitated in a soap solution, the oily dirt particles entrapped by soap micelles get dispersed in water and the clothes get cleaned.
Practice Questions of NCERT Class 10 Science Chapter 4
Below the carbon and its compounds ncert solutions that can help you score well in the exams. They provide clear explanations and step by step answers, making it easier to understand complex reactions and concepts.
Question 1. Which of the following is an allotrope of carbon?
(1) Diamond
(2) Graphite
(3) Lonsdaleite
(4) All of them
Answer:
All of them are correct.
Allotropes of carbon:
a) Diamond
b) Graphite
c) Lonsdaleite
Hence, the answer is option (4).
Question 2. Which of the following is an aromatic organic compound?
(1) Non-benzenoid
(2) Benzenoid
(3) Both 1 and 2
(4) None of these
Answer:
Chemical compounds classified as aromatics have conjugated planar ring structures with delocalized pi-electron clouds in place of individual double and single bonds that alternate.
Benzenoid and Non-benzenoid are both aromatic organic compounds.
Hence, the answer is option (3).
Question 3. Given below are the structures of some hydrocarbons. Select the two structures that are related to each other from the given options:
1) (i) and (iv)
2) (ii) and (iv)
3) (ii) and (iii)
4) (i) and (iii)
Answer:
Let us refer to the structures given in the question, We get that
(i) is an alkene.
(ii) is an alkane.
(iii) is an alkane.
(iv) is a chlorinated alkene.
Now, compare the structures given:
(i) and (iv) both are alkenes but are not isomers of each other because (iv) has a chlorine substituent.
(ii) and (iii) have the same molecular formula C4H10 but different structures so these are structural isomers. Hence, these structural forms are related to each other.
Hence, the correct answer is option (3).
Question 4. Alkenes and alkynes both are saturated compounds.
(1) True
(2) False
Answer:
No, it is false.
Alkenes and alkynes both are unsaturated compounds, not saturated compounds.
Hence, the given statement is (False).
Question 5. Organic compounds were initially extracted from the natural substance.
(1) True
(2) False
Answer:
Yes, it is true.
Organic compounds were initially extracted from the natural substance.
Hence, the given statement is (True).
Question 6: Which of the following statements explains why carbon forms a large number of compounds?
(1) Carbon has a large atomic size.
(2) Carbon can form only single bonds.
(3) Carbon shows catenation and tetravalency.
(4) Carbon reacts only with oxygen and hydrogen.
Answer: Tetravalency allows carbon to form four covalent bonds. Catenation allows carbon atoms to bond with one another, forming long chains and rings. These two properties enable carbon to form millions of compounds.
Hence, the correct answer is option (3).
Question 7: Which of the following represents the correct IUPAC name of the compound $\mathrm{CH}_3-\mathrm{CH}_2-\mathrm{CH}_2-\mathrm{OH}^{\prime}$?
(1) Methanol
(2) Ethanol
(3) Propanol
(4) Butanol
Answer: The compound has 3 carbon atoms, so the root name is prop. The functional group is –OH (alcohol), so the suffix is –ol. Hence, the correct name is propanol.
Hence, the correct answer is option (3).
Approaches To Solve Problems of Chapter 4 Class 10 Science
To solve carbon and its compounds class 10 question answers effectively, students have to focus on understanding the fundamental concepts along with practicing the questions given in examples and exercises. You can also refer to notes of this chapter for deep learning of the concepts.
1. Before solving questions, first make sure that you have a good understanding of the topics like covalent bonding in carbon, nature of carbon, properties like catenation, tetravalency, homologous series, various functional groups like alcohol, carboxylic acid, aldehydes, and ketones, etc.
2. Learn to classify types of questions and solve them accordingly. Practice a series of questions like conceptual questions, objective-type questions, nomenclature of compounds and numericals from our systematically prepared carbon and Its compounds ncert solutions.
3. Reaction-based questions are likely to come in boards, so students must practice these questions again and again like questions based on combustion reaction, oxidation and reduction reaction questions, addition and substitution questions, esterification, etc.
4. Identify keywords in questions and relate to the concepts learned and answer accordingly. Answer in a stepwise manner.
5. Learn to draw the structures of compounds like electron dot structure, open and branched chain structure etc. Solve all the questions of the class 10 science chapter 4 carbon and its compounds solutions as they are asked frequently in exams. Students should utilize the sources and content provided online to ensure a complete understanding of the subject.
Topics and Sub-Topics of NCERT Class 10 Science Chapter 4
Given below are topics that are covered in the carbon and its compounds ncert solutions. These topics are explained in a simple and structured way in NCERT textbook:
4.1 Bonding in Carbon – The Covalent Bond
4.2 Versatile Nature of Carbon
- 4.2.1 Saturated and Unsaturated Carbon Compounds
- 4.2.2 Chains, Branches, and Rings
- 4.2.3 Carbon - Will you be my Friend?
- 4.2.4 Homologous Series
- 4.2.5 Nomenclature of Carbon Compounds
4.3 Chemical Properties Of Carbon Compounds
- 4.3.1 Combustion
- 4.3.2 Oxidation
- 4.3.3 Addition Reaction
- 4.3.4 Substitution Reaction
4.4 Some Important Carbon Compounds – Ethanol And Ethanoic Acid
- 4.4.1 Properties of Ethanol
- 4.4.2 Properties of Ethanoic Acid
4.5 Soaps And Detergents
NCERT Class 10 Science Chapter 4: Important Compounds, Reactions and E-book
Here are some highlights of formulas used in class 10 science carbon and its compounds question answer that you need to remember.
Allotropes of Carbon
Diamond: In diamond, every carbon atom is bonded to four other carbon atoms, giving rise to a rigid three-dimensional structure.
Graphite: In graphite, every carbon atom is bonded to three other carbon atoms in the same plane, giving rise to a hexagonal array.
C-60 Buckminsterfullerene: In C-60 Buckminsterfullerene, carbon atoms are arranged in the shape of a football.
Saturated Carbon Compounds
Carbon compounds that involve single bonds are called saturated carbon compounds.
Unsaturated Carbon Compounds
Carbon compounds that involve double or triple bonds are called unsaturated carbon compounds
Functional Group in Carbon Compounds
|
Hetero Atom |
Class of Compounds |
Formula |
|
Cl/Br |
Halo-(chloro, bromo)alkane |
-Cl, -Br |
|
Oxygen |
Alcohol |
-OH |
|
Aldehyde |
-CHO | |
|
Ketone |
-CO | |
|
Carboxylic acid |
-COOH |
Nomenclature of organic compounds
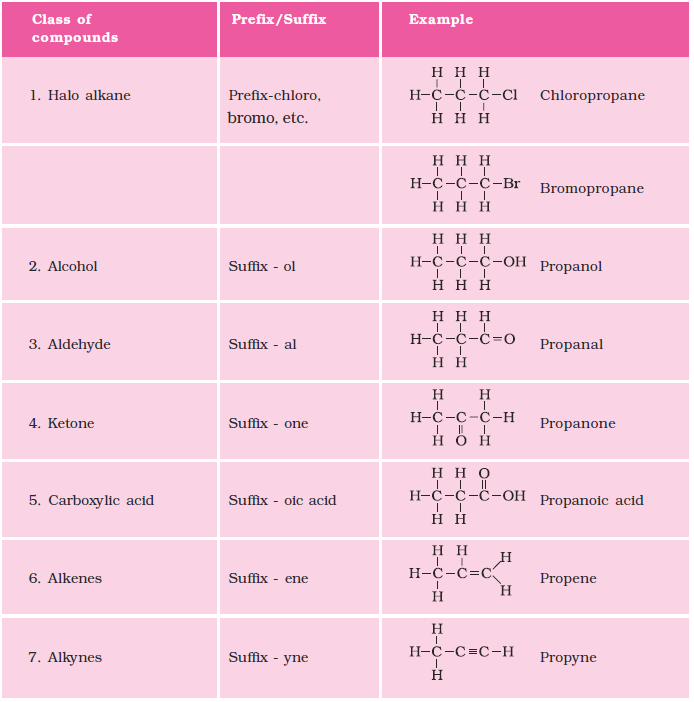
CHEMICAL PROPERTIES OF CARBON COMPOUNDS
Carbon compounds shows wide range of chemical properties due to the versatile bonding nature of carbon. These properties determine how carbon compounds react with other substances and form new products which is an important concept in class 10 science carbon and its compounds question answer.
Combustion
Carbon reacts with oxygen, giving out carbon dioxide, heat and light.
$\mathrm{CH}_4+\mathrm{O}_2 \rightarrow \mathrm{CO}_2+\mathrm{H}_2 \mathrm{O}+$ heat and light
Oxidation
When oxygen is added to the compound, the reaction is called an oxidation reaction. It is done in the presence of an oxidizing agent.
$
\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{OH} \xrightarrow[\text { OrAcilffied } \mathrm{K}_2 \mathrm{C}_2 \mathrm{O}_4]{\text { Alkar }} \mathrm{CH}_3 \mathrm{COOH}
$
Addition Reaction
When hydrogen is added to an unsaturated compound, in the presence of a catalyst such as palladium or nickel giving rise to saturated hydrocarbons, the reaction is called an addition reaction.
Substitution Reaction
When an atom or group of atoms are replaced by another atom, the reaction is called a substitution reaction.
$\mathrm{CH}_4+\mathrm{Cl}_2 \rightarrow \mathrm{CH}_3 \mathrm{Cl}+\mathrm{HCl}$ (in the presence of sunlight)
Reactions of Ethanol
(i) Reaction with sodium –
$2 \mathrm{Na}+2 \mathrm{CH}_3 \mathrm{CH}_2 \mathrm{OH} \rightarrow 2 \mathrm{CH}_3 \mathrm{CH}_2 \mathrm{O}-\mathrm{Na}+($ Sodium ethoxide $)+\mathrm{H}_2$
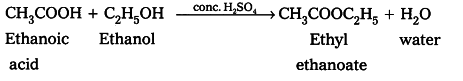
Esterification reaction:
Reaction of ethanoic acid with a base:
$\mathrm{NaOH}+\mathrm{CH}_3 \mathrm{COOH} \rightarrow \mathrm{CH}_3 \mathrm{COONa}+\mathrm{H}_2 \mathrm{O}$
Reaction of ethanoic acid with carbonates and hydrogencarbonates:
$2 \mathrm{CH}_3 \mathrm{COOH}+\mathrm{Na}_2 \mathrm{CO}_3 \rightarrow 2 \mathrm{CH}_3 \mathrm{COONa}+\mathrm{H}_2 \mathrm{O}+\mathrm{CO}_2$
$\mathrm{CH}_3 \mathrm{COOH}+\mathrm{NaHCO}_3 \rightarrow \mathrm{CH}_3 \mathrm{COONa}+\mathrm{H}_2 \mathrm{O}+\mathrm{CO}_2$
Practice the NCERT class 10 science chapter 4 exercise solutions based on the above topics.
To download the E-Book click on the link given below:
What Students Learn from NCERT Solutions for Class 10 Science Chapter 4
These class 10 science chapter 4 carbon and its compounds solutions NCERT help students easily understand concepts. They explain important concepts and reactions in a simple way. These solutions also help students practice questions and prepare well for exams.
- These solutions help students understand the structure, and properties of carbon and its compounds.
-
Students can learn about different types of chemical reactions including addition, substitution, and oxidation reactions using these class 10 science chapter 4 carbon and its compounds question answer.
-
Various organic compounds like hydrocarbons, alcohols, and acids are discussed in these solutions.
-
Using these NCERT Solutions for Class 10 Science Chapter 4 Carbon and Its Compounds students learn how functional groups in carbon compounds affect their chemical reactions and uses.
NCERT Solutions for Class 10 Science Chapter-wise Links
Along with NCERT Solutions for Class 10 Science Chapter 4 Carbon and its Compounds, students can also follow class 10 science chapter-wise solutions. Follow them to score well in the exams.
Frequently Asked Questions (FAQs)
Isomers are compounds that have the same molecular formula but different structural arrangements of atoms. In carbon compounds, isomerism is common due to carbon's ability to form long chains and rings.
Carbon is often referred to as the backbone of organic compounds due to its unique ability to form stable bonds with many elements, including itself. This property allows carbon to create a vast array of complex molecules essential for life, such as carbohydrates, proteins, and nucleic acids.
Saturated hydrocarbons contain only single bonds between carbon atoms e.g., alkanes, meaning they are saturated with hydrogen. Unsaturated hydrocarbons, on the other hand, contain double or triple bonds e.g., alkenes and alkynes, which reduces the number of hydrogen atoms bonded to the carbon framework.
Carbon can form several types of bonds: single bonds, double bonds, and triple bonds. A single bond involves the sharing of one pair of electrons between two carbon atoms, while double and triple bonds involve the sharing of two and three pairs of electrons, respectively.
Allotropes are different forms of the same element, where the atoms are arranged differently. Carbon has several allotropes, including graphite, diamond, and fullerene.
NCERT Solutions for Class 10 Science are a set of step by step answers to all in-text and exercise questions from the NCERT textbook. They help students understand concepts clearly, learn how to solve numerical and theoretical questions, and prepare effectively for exams.
NCERT Class 10 Science Chapter 4 covers the structure, properties, and reactions of carbon compounds, including hydrocarbons, alcohols, acids, and salts. The chapter also explains functional groups, chemical reactions, and the uses of important organic compounds, helping students understand basic organic chemistry.
Carbon and its compounds is a chapter in Class 10 Science that explains the properties, structure, and reactions of carbon compounds. It covers topics like hydrocarbons, alcohols, acids, and salts, along with functional groups, chemical reactions, and the uses of these compounds in daily life.
Carbon is considered versatile due to its ability to form strong covalent bonds with a variety of elements, particularly hydrogen, oxygen, nitrogen, and other carbon atoms. This versatility allows carbon to create an extensive range of structures, from simple molecules to complex macromolecules.
Functional groups are specific groups of atoms that impart particular chemical properties to carbon compounds. They are crucial because they determine how a compound will react in chemical reactions.
Questions related to CBSE Class 10th
On Question asked by student community
HELLO,
I am attaching the link through which you can download and access the Bangalore Sahodaya Class 10th CBSE question paper of Mathematics ( Basic )
Here is the link :- https://school.careers360.com/download/ebooks/bangalore-sahodaya-class-10-mathematics-basic-question-paper-2025-26
It will help you to practice basic level numerical questions, strengthen fundamentals and prepare confidently for the board
HELLO,
Below i am attaching the direct link of Careers360 through which you can download the Bangalore Sahodaya Class 10th Mathematics Basic Question paper 2025 2026
Here is the link :- https://school.careers360.com/download/ebooks/bangalore-sahodaya-class-10-mathematics-basic-question-paper-2025-26
Hope this will help you!
Hello
You will be able to download the CBSE Class 10th Maths Sample Paper 2025-26 using the link which is given below.
https://school.careers360.com/boards/cbse/cbse-10th-maths-sample-papers-2025-26
I hope this information helps you.
Thank you.
Hello,
Here you can access the last 5 years CBSE Class 10 Board Exam Question Papers from the mentioned link below:
https://school.careers360.com/boards/cbse/cbse-previous-year-question-papers-class-10
Hope it helps.
You can check the Class 11 English half yearly question paper and answer key for 2025 26 on the Careers360 website. These papers help students practice, understand the exam pattern, and check their answers for better preparation.
You can visit this Careers360 link to access the English question paper and
Popular CBSE Class 10th Questions
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters