NCERT Solutions for Class 8 Science Chapter 11 - Keeping Time with the Skies
The NCERT Solutions for Class 8 Science Chapter 11 Keeping Time with the Skies explain how movements in the sky help us to predict time. This chapter teaches the concept of Earth's rotation and revolution, as well as the different phases of the Moon. Keeping Time with the Skies chapter is very important to know how festivals are celebrated based on the lunar phases. The NCERT Solutions explain each topic in an easy and understandable manner without causing any confusion, which allows students to perform well in the exams.
This Story also Contains
- Download Keeping Time with the Skies Class 8 Questions and Answers PDF
- Access Class 8 Science Chapter 11 Keeping Time with the Skies Question Answer
- Approach to Solve Keeping Time with the Skies Class 8 Question Answer
- Important Topics of Chapter 11 NCERT Class 8 Science
- Important Questions from NCERT Class 8 Science Chapter 11
- What will You Learn from Keeping Time with the Skies NCERT Solutions?
- Why Class 8 Science Chapter 11 Keeping Time with the Skies NCERT Solutions are Important?
- NCERT Solutions for Class 8 Science: Chapter-wise

Keeping Time with the Skies Class 8 question answer provides a clear explanation of different types of calendars. All the solutions are prepared by subject experts and follow the latest NCERT guidelines. Well-labeled diagrams and real-life examples are added to make students understand the topics in an easy manner. Keeping Time with the Skies Class 8 questions and answers PDF help students build a strong foundation for understanding celestial movements and time measurement.
Download Keeping Time with the Skies Class 8 Questions and Answers PDF
Given below is the link to the PDF for the chapter Keeping Time with the Skies. Students can use the Keeping Time with the Skies Class 8 questions and answers PDF to complete their homework and do the revision quickly. The NCERT Solutions for Class 8 help build a strong foundation for higher classes and competitive exams.
Access Class 8 Science Chapter 11 Keeping Time with the Skies Question Answer
Provided below are the detailed answers to all the questions provided in the textbook. These solutions are carefully prepared to avoid any difficulty for students. Doing the regular practice of Class 8 Science Chapter 11 Keeping Time with the Skies question answers helps students understand the concepts easily. Solutions also explain the role of artificial satellites in communication, weather prediction, etc.
Question 1: State whether the following statements are True or False.
(i) We can only see that part of the Moon which reflects sunlight towards us.
(ii) The shadow of Earth blocks sunlight from reaching the Moon, causing phases.
(iii) Calendars are based on various astronomical cycles which repeat in a predictable manner.
(iv) The Moon can only be seen at night.
Answer:
(i) We can only see that part of the Moon which reflects sunlight towards us. - True
(ii) The shadow of Earth blocks sunlight from reaching the Moon, causing phases. - False
(iii) Calendars are based on various astronomical cycles that repeat predictably. - True
(iv) The Moon can only be seen at night.- False
Question 2: Amol was born on the 6th of May on a full Moon day. Does his birthday fall on the full Moon day every year? Explain your answer.
Answer: No, Amol’s birthday will not fall on the full moon day every year. This is because the Moon follows a different cycle, which is known as the lunar cycle. And this lunar cycle does not match our calendar months. So, the date of the full moon changes every year.
Question 3: Name two things that are incorrect in the figure given below.
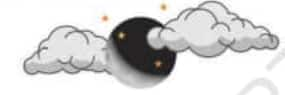
Answer: The two things that are incorrect in the figure are:
Stars are shown near the moon, which is wrong. Stars are only visible at night.
The shadow present on the moon is shown in the wrong direction.
Question 4: Look at the pictures of the Moon in the figure given below, and answer the following questions.

(i) Write the correct panel number corresponding to the phases of the Moon shown in the pictures above.
Picture label (e.g., A, B, C, etc.) | Phase of Moon |
Three days after New Moon | |
Full Moon | |
Three days after the Full Moon | |
A week after the Full Moon | |
Day of New Moon |
(ii) List the picture labels of the phases of the Moon that are never seen from Earth.
Answer:
(i)
Picture label (e.g., A, B, C, etc.) | Phase of Moon |
C | Three days after New Moon |
E | Full Moon |
F | Three days after the Full Moon |
A | A week after the Full Moon |
B | Day of New Moon |
(ii) B is the picture label of the moon phase, which is never seen from the Earth as the moon is not illuminated during that phase.
Question 5: Malini saw the Moon overhead in the sky at sunset.
(i) Draw the phase of the Moon that Malini saw.
(ii) Is the Moon in the waxing or the waning phase?
Answer:
(i) At sunset, the Moon is overhead only during the first quarter (a week after New Moon), when the right half is illuminated. So, we need to draw a half-moon.

(ii) The moon is in the waxing phase because it occurs after New Moon, and the bright part is increasing.
Question 6: Ravi said, “I saw a crescent Moon, and it was rising in the East, when the Sun was setting.” Kaushalya said, “Once I saw the gibbous Moon during the afternoon in the East.” Who out of the two is telling the truth?
Answer: Ravi said he saw a crescent moon rising in the east at sunset, but this is not possible because a crescent moon wouldn't be rising in the east when the sun was setting. On the other hand, Kaushalya said she saw a gibbous moon during the afternoon in the east. This is possible because a waxing gibbous Moon rises in the afternoon and is often visible before sunset.
So, Kaushalya is telling the truth, and Ravi is not.
Question 7: Scientific studies show that the Moon is getting farther away from the Earth and slower in its revolution. Will luni-solar calendars need an intercalary month more often or less often?
Answer: Scientific studies show that the moon is getting farther away from the Earth and slower in its revolution. Because of this, each lunar month will become a little longer.
Luni-solar calendars are different kinds of calendars that use the moon’s phases for counting days and months. The 12 lunar months add up to 354 days and thus fall short by around 11 days compared to the solar year. Thus, every 2–3 years, the difference becomes close to a full month. But since the lunar month becomes longer, the difference between the lunar year and the solar year becomes smaller. So, an intercalary month is needed less often..
Question 8: A total of 37 full Moons happen during 3 years in a solar calendar. Show that at least two of the 37 full moons must happen during the same month of the solar calendar.
Answer: In three years, if we calculate the total months, they would be 36. But as it is mentioned in the question, the total number of full moons in 3 years is 37, then it means one month will have 2 full moons. This occurs because the number of full moons is more than the number of months.
Question 9: On a particular night, Vaishali saw the Moon in the sky from sunset to sunrise. What phase of the Moon would she have noticed?
Answer: If Vaishali saw the moon in the sky from sunset to sunrise, then it is the full moon phase. It is because the full moon rises at sunset, then remains visible all night, and sets as sunrise.
Question 10: If we stopped having leap years, in approximately how many years would the Indian Independence Day happen in winter?
Answer: If we stop having leap years, the calendar will slowly shift. After around 500-600 years, 15th August, which is Independence Day, will fall in winter. This happens because the calendar will not match the Earth’s revolution around the sun.
Question 11: What is the purpose of launching artificial satellites?
Answer: The main purpose of launching artificial satellites is they help us in many ways, like communication, navigation, weather monitoring, disaster management, and scientific research as well.
Question 12: On which periodic phenomenon are the following measures of time based: (i) day, (ii) month, (iii) year?
Answer:
(i) Day - Based on the rotation of the Earth on its axis
(ii) Month - Based on the revolution of the Moon around the Earth
(iii) Year - Based on the revolution of the Earth around the Sun
Also, check the NCERT Books and the NCERT Syllabus here
Approach to Solve Keeping Time with the Skies Class 8 Question Answer
Students can follow the steps given below to answer the questions effectively. Keeping Time with Skies questions and answers provides information about the sun, the moon, and the Earth. These answers allow students to improve their thinking skills and help them relate science to everyday life.
Students can start by understanding how the sun, the moon, and the Earth help us measure time.
They can learn about different phases of the Moon and how its form changes regularly. Students can take help from the Class 8 Science Chapter 11 Keeping Time with the Skies question answer.
Students are advised to prepare flow charts and learn the phases with the diagrams. This makes it easy to remember the phases during exams.
Learn about different types of calendars, such as solar, lunar, luni-solar, Indian national calendar, etc. They are all included in the Class 8 Science Keeping Time with the Skies question answer.
Solve and practice different forms of questions from the NCERT Solutions for Class 8 Science to get familiar with the question pattern.
Important Topics of Chapter 11 NCERT Class 8 Science
This chapter helps students understand how natural movements in the sky, like phases of the moon, etc., help us to measure time. It also includes concepts of day, months, and years based on the sun, moon, and the Earth. Students can use the NCERT Solutions for Class 8 Science Chapter 11 Keeping Time with the Skies for detailed explanations. Given below are a few important topics of this chapter.
How does the Moon’s appearance change, and why
Phases of the Moon
How calendars came into existence
Different types of calendars - lunar calendars, etc.
Are festivals related to astronomical phenomena?
Solar and Lunar eclipses
Measurement of time using celestial bodies
Artificial satellites
Important Questions from NCERT Class 8 Science Chapter 11
Practicing different types of questions helps students gain a better understanding of the chapter. It allows them to connect the concepts of sun, moon, and earth with real-life observations. Given below is a question from this chapter that makes students understand the difficulty level. To gain clarity, students can refer to the NCERT Solutions for Class 8 Science Chapter 11 Keeping Time with the Skies.
Question 1: Which phenomenon occurs when the Moon comes directly between the Earth and the Sun, blocking sunlight from reaching the Earth?
Options
(a) Lunar eclipse
(b) Solar eclipse
(c) New moon
(d) Full moon
Answer: The correct answer is option (b), Solar Eclipse
Explanation: A solar eclipse happens when the Moon comes directly between the Earth and the Sun. It blocks some or all sunlight from reaching the Earth's surface, which forms a shadow. This can only occur during the new moon phase.
Question 2: During which phase of the Moon is a lunar eclipse possible?
Options:
(a) New moon
(b) First quarter
(c) Full moon
(d) Last quarter
Answer: The correct answer is option (c), Full moon
Explanation: A lunar eclipse occurs when the Earth comes between the Sun and the Moon, and the Earth's shadow falls on the Moon. This can only happen during the full moon phase.
Question 3: What is the main purpose of artificial satellites?
Options:
(a) Observe stars
(b) Communication and research
(c) Replace the Moon
(d) Cause eclipses
Answer: The correct answer is option (b), Communication and research
Explanation: Artificial satellites are man-made objects placed in orbit around the Earth for communication, weather forecasting, navigation, and scientific research.
Question 4: Why do most Indian festivals fall on different dates every year?
Options:
(a) Because the Sun moves randomly
(b) Because the lunar calendar is followed
(c) Because festivals are decided by the government
(d) Because the Moon disappears sometimes
Answer: The correct answer is option (b), because the lunar calendar is followed
Explanation: Most Indian festivals follow the lunar calendar, so their dates change every year according to the Moon’s phases. This is why festivals like Diwali, Holi, and Eid do not fall on the same date every year.
Question 5: When I look at the night sky in the early evening, I see some moving stars. What are they, and is their motion periodic?
Options:
(a) Shooting stars, motion is not periodic
(b) Planets, motion is periodic
(c) Distant stars, motion is not periodic
(d) Comets, motion is random
Answer: The correct answer is option (b), Planets, motion is periodic
Explanation: The moving “stars” seen in the night sky are actually planets. Their motion is periodic as they revolve around the Sun in predictable orbits.
Question 6: What causes the phases of the Moon?
Options:
(a) Earth's shadow falling on the Moon
(b) Moon changing its shape
(c) Changing positions of the Earth, Moon, and Sun
(d) Clouds covering the Moon
Answer: The correct answer is option (c), Changing positions of the Earth, Moon, and Sun
Explanation: The Moon does not have its own light. We see different portions of the illuminated part of the Moon depending on its position around the Earth, which causes different phases like new moon, crescent, and full moon.
Question 7: Why do we always see the same side of the Moon from Earth?
Options:
(a) Because the Moon does not rotate
(b) Because the Moon rotates and revolves at the same rate
(c) Because Earth does not rotate
(d) Because the Sun blocks half the Moon
Answer: The correct answer is option (b), because the Moon rotates and revolves at the same rate
Explanation:
The Moon takes the same amount of time to complete one rotation on its axis and one revolution around Earth. This synchronous motion causes the same side of the Moon always to face Earth.
Question 8: What is a constellation?
Options:
(a) A group of planets
(b) A group of stars forming a pattern in the sky
(c) A single bright star
(d) A type of satellite
Answer: The correct answer is option (b), A group of stars forming a pattern in the sky
Explanation:
A constellation is a group of stars that appear to form a recognizable pattern in the night sky. These patterns have been named after animals, objects, or mythological characters, such as Ursa Major and Orion.
Question 9: Which device is used to observe distant objects in the sky more clearly?
Options:
(a) Microscope
(b) Periscope
(c) Telescope
(d) Kaleidoscope
Answer: The correct answer is option (c), Telescope
Explanation:
A telescope is an instrument used to observe distant celestial objects such as stars, planets, and galaxies. It collects and magnifies light, allowing astronomers to study objects that are too far away to be seen with the naked eye.
What will You Learn from Keeping Time with the Skies NCERT Solutions?
The chapter explains how the movement of celestial objects like the Sun and the Moon helps us measure time. Some of the interesting things that students learn are given below:
They will understand how people observed the sky to measure time, as explained in the Keeping Time with the Skies Class 8 question answer.
Students will also learn about the difference between solar time and standard time.
The chapter explains how days, months, and years are calculated from the movement of the Earth and the Moon.
Students will know about the different phases of the moon with the help of the diagrams.
The importance of calendars and clocks is discussed in the Class 8 Science Keeping Time with the Skies question answer.
Why Class 8 Science Chapter 11 Keeping Time with the Skies NCERT Solutions are Important?
Keeping Time with the Skies chapter explains how humans have used the celestial bodies to measure time. Students going through this chapter will gain knowledge about calendars, the motion of Earth in an easy-to-understand way.
Students will understand the principles of calendars, time measurement, and the motion of Earth, Moon, and other celestial bodies.
Class 8 Science Chapter 11 Keeping Time with the Skies NCERT Solutions explains the importance of day, night, months, and years and how they are related to Earth's rotation and revolution.
Knowing the movement of celestial bodies helps students understand the advanced topics in Physics like gravitation and the solar system.
It also develops observation and skills by making students relate daily life events to celestial movements.
NCERT Solutions for Class 8 Science: Chapter-wise
Given below are the links that provide access to well-structured answers for every chapter in the syllabus. These solutions are prepared by subject experts and follow the latest syllabus. It helps students strengthen concepts, improve problem-solving skills, and prepare effectively for exams.
Frequently Asked Questions (FAQs)
NCERT Class 8 Science Chapter 11 question answer sets provide step-by-step solutions to textbook questions and make it easier to understand the topic thoroughly.
The phases of the Moon are the different shapes of the Moon visible from Earth, which change as the Moon orbits the Earth.
Here are the topics covered in the NCERT Solutions for Class 8 Science Chapter 11 Keeping Time with the Skies:
Phases of the Moon
Different types of calendars
Solar and Lunar eclipses
Measurement of time using celestial bodies
Artificial satellites
The NCERT Solutions for Class 8 Science Chapter 11 Keeping Time with the Skies help understand how celestial movements help us measure time. They explain key ideas like shadows, sundials, and phases of the moon in a simple way.
Day and night are caused by the rotation of the Earth on its axis. When one side of the Earth faces the Sun, it is day there; the opposite side has night.
There are a total of 12 questions in the NCERT Solutions for Class 8 Science Chapter 11 Keeping Time with the Skies.
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
