JEE Main Important Physics formulas
ApplyAs per latest 2024 syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
NCERT Class 12 Chemistry chapter 9 notes discusses the coordination compounds. The chapter coordination compounds Class 12 notes is the continuation of NCERT chapter coordination compounds (Class 12). The NCERT Class 12 chapter 9 notes is a brief outline of the chapter coordination compounds from the NCERT Book. Werner’s theory, coordination entity, ligands, magnetic properties of coordination compound, crystal field theory, colour in coordination compounds, bonding, and stability constant of coordination compounds are the key topics dealt in Coordination compounds Class 12 Notes.
CBSE Class 12 Chemistry chapter 9 notes also cover the basic terms in the chapter. The important topics and examples are also covered in the CBSE class 12 Chemistry chapter 9 notes. All these topics can be downloaded from Class 12 Chemistry chapter 9 notes pdf download.
Also, students can refer,
The postulates from Werner’s theory are listed below:
The central metal or the metal atoms within a coordination compounds will always shows two types of valency. Among these two types, one is known as primary valency and another one is secondary valency.
The primary valency denotes the oxidation state of the atom and the secondary valency will show the coordinate number.
The secondary valences is not variable i.e. constant for every metal atom. Or we can say that the coordination number of any particular atom is fixed.
The metal atom will fulfill both its primary as well as secondary valencies. Generally a negative ion i.e. anion will satisfies the primary valency. And on the other hand, among these two i.e. negative ion or neutral molecules both can satisfy secondary valencies.
A coordination entity comprises a central metal atom or ion bonded with a fixed number of ions or molecules.
For example: [Co(NH3)6]Cl3 is a coordinate compound or we can say coordination entity in which six ammonia molecules are composed of 3 chlorine molecules.
Other examples of coordination entities are as follows:
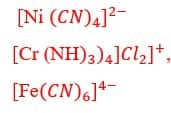
The ions or molecules that are bound to the central atom or ion within the coordination entity are called ligands. They can be simple ions like Cl–, or small molecules like H2O or NH3, they can also be larger molecules like H2NCH2CH2NH2 or N(CH2CH2NH2)3 or even macromolecules, like proteins.
Crystal Field Theory (CFT) is not a basic theory but an electrostatic model. As the theory is based on the electrostatic model of hard spheres and the interaction is done in a purely electrostatic way. The central atom is having a positive charge and ligands have a negative charge and thus this negative charge approaches towards the positive charge and due to ligands point charge degeneracy has been created. This means that all the five “d” orbitals in an isolated gaseous metal atom or ion will have the same energy or we can be told that they are degenerate. However, when this negative field is formed due to ligands in a complex, this will become asymmetrical and then the degeneracy of d orbitals is lifted. It will result in the splitting of the d orbitals. The pattern of splitting will depend on the nature of the crystal field.
The transition metals have a unique ability to form magnets. Metal complexes show paramagnetism due to unpaired electrons as the last electrons will reside in the d orbitals. By considering only monometallic complexes, they have unpaired electrons or an odd number of electrons in which each electron has a magnetic moment associated with spin angular momentum and causes destabilization.
The coordination compound is made up of a ligand and a metal ion, and ligands are responsible for the coloration of the complex compounds. This means that different types of ligands show different colors.
The energy is required to remove the electron from a lower energy state to a higher energy state.
A higher energy state absorbs the color of shorter wavelengths.
We know that the metals of complex compounds are basically from d- orbitals or transition elements which have half-filled or unfilled d orbitals which pull oy the electron from the lower state and forward it to a higher energy level which causes the d-d transition of the metal atom thus radiates the color depending on the type of ligands.
For example, considering the two ligands one is a strong field ligand and the other is a weak field ligand.
The complex [Cr(NH3)6]3+ has strong-field ligands, so it will absorb relatively high-energy photons. This corresponds to the blue-violet light zone, which will give it a yellow color.
The other complex is of weak-field ligands, the [Cr(H2O)6]3+ ion, which absorbs lower-energy photons. This corresponds to the yellow-green portion of the visible spectrum and leads to giving deep violet color.
Discussing other examples based upon the high and low spin is as follows:
The iron(II) complex [Fe(H2O)6]SO4 gives blue-green due to the high-spin complex which absorbs the photons in the red wavelengths.
On the other hand, the complex iron(II) K4[Fe(CN)6] gives pale yellow which absorbs photons in violet wavelengths.
In general, strong-field ligands can cause a large split in the energies of d orbitals, so ligands are generally yellow, orange, or red because they absorb higher-energy violet or blue light.
On the other hand, weak-field ligands are often blue-green, blue, or indigo because they absorb lower-energy yellow, orange, or red light.
400-nm Violet light if absorbed → Green-yellow colour will be observed
430-nm Blue light if absorbed → Orange colour will be observed
450-nm Blue light if absorbed → Yellow colour will be observed
490-nm Blue-green light if absorbed → Red colour will be observed
570-nm Yellow-green light if absorbed → Violet colour will be observed
580-nm Yellow light if absorbed → Dark blue colour will be observed
600-nm Orange light if absorbed → Blue colour will be observed
650-nm Red light if absorbed → Green colour will be observed
Valence bond theory is used as an early understanding of how covalent bonds form.
Resonance and orbital hybridization are the two keys to valence bond theory.
Orbital hybridization occurs when bonding orbitals share the characteristics of several types of orbitals.
Valence bond structures are often used when Lewis structures do not suffice to represent the different forms of coordination molecules that happen as a result of resonance.
resonance: The property of a compound that can be visualized as having two structures and differing only in the distribution of electrons.
orbital hybridization: In chemistry, hybridization is the concept of intermixing atomic orbitals and leads to form new hybrid orbitals suitable for the qualitative description of atomic bonding properties.
The formation of complex compounds can be possible by the help of some steps in which addition of ligands to metal ions can be done and if there are n steps included to form such complexes then it should be in equilibrium state , n represents the coordination number. Every equilibrium has its own equilibrium constant.
The equilibrium constant is also known as formation constant or stepwise stability constant.
It can be used for the measurement of the strength when complexes are formed by the interaction of reagents, and here we see the interaction between ligands and metal ions.
The factors which affects the stability of complex metal are as follows:
Properties of metal ion
Properties of Ligands
Neutral Ligands
The stability constant provides the information that is required to calculate the concentration of the complexes within the solution. There are many areas where stability constant is used in chemistry, biology and medicine.
Significance of NCERT Class 12 Chemistry Chapter 9 Notes
Coordination Compounds notes class 12 will be helpful to revise the topics more conveniently and quickly because the topics are important and placed in one place. Also, this Coordination Compounds notes class 12 is used in preparing the topics offline by just downloading it for future concerns. This is a hustle-free way to remind all the postulates and definitions together by just clicking the link of the Class 12 CBSE Chemistry Syllabus. CBSE class 12 chemistry ch 9 notes will help students to give their best in the examinations. ch 9 chemistry class 12 notes contains Werner’s theory, coordination entity, ligands, nomenclature, isomerism, bonding, magnetic properties of coordination compound, crystal field theory, colour in coordination compounds, bonding, stability of coordination compounds and methods of solving problems related to coordination compounds. Students can refer chemistry class 12 chapter 9 notes pdf for revising the concepts and highlighting the important formulas in the pdf.
The following are the examples of coordination isomers [Cr(NH3)6] [Co(CN)6] and
[Co(NH3)6] [Cr(CN)6] here Co and Cr interchanged from their places.Coordination isomersim is a type of structural isomers in which the composition of metal ions can be determined .
Example : [Pt (NH3)5(Br)] SO4 and
[Co (NH3)5 (SO4)] Br
IUPAC name : Triammine nitrato nickel (III) chloride
The formula of Tetraammineaquachlorocobalt(III) chloride is [Co(NH3)4(H2O)Cl]Cl2
It shows ionization isomerism and linkage isomerism.
a-helix formation -» Intramolecular hydrogen bonding.
NCERT Class 12 Chemistry chapter 9 notes should contain the major terms related to Coordination compounds and Werner’s theory. One can also refer to Class 12 Chemistry chapter 9 notes pdf download or coordination compounds Class 12 notes pdf download.

As per latest 2024 syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters

Get up to 90% scholarship on NEET, JEE & Foundation courses

As per latest 2024 syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters

Enrol in PACE IIT & Medical, Financial District, Hyd for JEE/NEET preparation

Start your JEE preparation with ALLEN

Ace your NEET preparation with ALLEN Online Programs