NCERT Solutions for Class 10 Science Chapter 1 Chemical Reactions and Equations
Do you know how milk turns sour, iron rusts and firecrackers explode? Have you seen the transformation of milk into curd or conversion of water into ice? All these transformations discussed above are due to chemical reactions. You all might be thinking, what is a chemical reaction? What happens when a chemical reaction occurs? Chemical reaction is a process in which one or more substances react to form entirely new substances with different properties and composition. From burning of woods to digestion of food these chemical reactions occur everywhere around us.NCERT Solutions for Class 10 Science Chapter 1 Chemical Reactions and Equations helps students to understand all these interesting facts clearly.
This Story also Contains
- NCERT Solutions for Class 10 Science Chapter 1: Download PDF
- NCERT Solution For Class 10 Science Chapter 1 (Intext Questions)
- NCERT Solutions For Class 10 Science Chapter 1 (Exercise Questions with Answers)
- Practice Questions for Class 10 Science Chapter 1
- Approach to Solve Questions of Class 10 Science Chapter 1
- Topics and Subtopics Covered in Class 10 Chapter 1 NCERT
- NCERT Class 10 Science Chapter 1: Important Reactions And E-book
- What Students Learn from NCERT Solutions for Class 10 Science Chapter 1
- Importance of Class 10 Science Chapter 1: Chemical Reactions and Equations
- NCERT Solutions for Class 10 Science Chapter-Wise
.jpg)
NCERT Solution for Class 10 Science are designed by subject experts in a very clear and comprehensive way. This chapter focuses on writing and balancing chemical equations. They follow the CBSE guidelines and give clear explanations, and also help students understand chemical processes and their types. These NCERT solutions serves as an important resource for mastering chemical reactions and equations and helps you to enhance performance in board exams.
NCERT Solutions for Class 10 Science Chapter 1: Download PDF
To get chemical reactions and equations ncert solutions pdf, click below on the icon given below. In this PDF, you will get detailed solutions to all the questions that are given in the NCERT textbook. NCERT Solutions for Class 10 will help you understand the concepts better and improve your problem-solving skills for exams.
Also Read
NCERT Solution For Class 10 Science Chapter 1 (Intext Questions)
If you are looking for accurate and detailed answers to in text questions from class 10 science chapter 1 chemical reactions and equations solutions. These solutions provide step by step explanations to help students understand key concepts and improve exam performance.
Topic 1.1 Chemical equations: Page no-6
Question 1. Why should a magnesium ribbon be cleaned before burning in the air?
Answer:
Magnesium is a very reactive metal so it reacts with oxygen to form a layer of magnesium oxide on its surface.This layer is a stable oxide, so prevents further reaction of magnesium with oxygen. To remove this layer, a magnesium ribbon is cleaned using sandpaper before burning in air.
Question 2. (i) Write the balanced equation for the following chemical reactions.
Hydrogen + Chlorine → Hydrogen chloride
Answer:
The balanced equation for the following chemical reactions is given:
$\mathrm{H}_2(\mathrm{~g})+\mathrm{Cl}_2(\mathrm{~g}) \rightarrow 2 \mathrm{HCl}(\mathrm{g})$
Question 2. (ii) Write the balanced equation for the following chemical reactions.
Barium chloride + Aluminium sulphate → Barium sulphate + Aluminium chloride
Answer:
The balanced equation for the following chemical reactions is given:
$3 \mathrm{BaCl}_2(s)+\mathrm{Al}_2\left(\mathrm{SO}_4\right)_3(\mathrm{~g}) \rightarrow 3 \mathrm{BaSO}_4(\mathrm{~s})+2 \mathrm{AlCl}_3(\mathrm{~s})$
Question 2. (iii) Write the balanced equation for the following chemical reactions.
Sodium + Water → Sodium hydroxide + Hydrogen
Answer:
The balanced equation for the following chemical reactions is given:
$2 \mathrm{Na}(s)+2 \mathrm{H}_2 \mathrm{O}(l) \rightarrow 2 \mathrm{NaOH}(a q)+\mathrm{H}_2(g)$
Question 3. (i) Write a balanced chemical equation with state symbols for the following reactions.
Solutions of barium chloride and sodium sulphate in water react to give insoluble barium sulphate and the solution of sodium chloride.
Answer:
The reaction solutions of barium chloride and sodium sulphate in water react to give insoluble barium sulphate and the solution of sodium chloride is given by :
$\mathrm{BaCl}_2(a q)+\mathrm{Na}_2 \mathrm{SO}_4(a q) \rightarrow \mathrm{BaSO}_4(s)+2 \mathrm{NaCl}(a q)$
Question 3. (ii) Write a balanced chemical equation with state symbols for the following reactions.
Sodium hydroxide solution (in water) reacts with a hydrochloric acid solution (in water) to produce sodium chloride solution and water.
Answer:
The reaction of sodium hydroxide solution (in water) reacts with a hydrochloric acid solution (in water) to produce sodium chloride solution and water is given by :
$\mathrm{NaOH}(a q)+\mathrm{HCl}(\mathrm{aq}) \rightarrow \mathrm{NaCl}(\mathrm{aq})+\mathrm{H}_2 \mathrm{O}(l)$
Topic 1.2: Types of Chemical Reactions Page no- 10
Question 1. (i) A solution of a substance is used for whitewashing.
Name the substance ‘X’ and write its formula
Answer:
The substance ‘X’ is calcium oxide and its formula is CaO.
Question 1. (ii) A solution of a substance is used for whitewashing.
Write the reaction of the substance named in (i) above with water
Answer:
The reaction of calcium oxide with water is given by :
$\mathrm{CaO}(s)+\mathrm{H}_2 \mathrm{O}(\mathrm{l}) \rightarrow \mathrm{Ca}(\mathrm{OH})_2$
Answer :
Water contains one part oxygen and two parts hydrogen. During electrolysis, oxygen and hydrogen are produced in a 1:2 ratio. In electrolysis, oxygen goes in one test tube and hydrogen in a second test tube, so the amount of gas collected in the second test tube is double of the first one.
Topic 1.3: Have you observed the effects of an oxidation reaction in everyday life? Page no-13
Question 1. Why does the colour of copper sulphate solution change when an iron nail is dipped in it?
Answer:
The colour of copper sulphate solution changes when an iron nail is dipped in it because iron displaces copper from copper sulphate forming iron sulphate, which is green.
$\mathrm{CuSO}_4(\mathrm{aq})+\mathrm{Fe}(s) \rightarrow \mathrm{FeSO}_4(\mathrm{aq})+\mathrm{Cu}(s)$
The colour changed from blue to green.
Question 2. Give an example of a double displacement reaction other than the one given in Activity.
Answer:
An example of a double displacement reaction other than the one given in Activity is :
$\mathrm{Na}_2 \mathrm{CO}_3(\mathrm{aq})+\mathrm{CaCl}_2(\mathrm{aq}) \rightarrow \mathrm{CaCO}_3(s)+2 \mathrm{NaCl}(a q)$
Question 3. (i) Identify the substances that are oxidized and the substances that are reduced in the following reactions.
$4 \mathrm{Na}(s)+\mathrm{O}_2 \rightarrow 2 \mathrm{Na}_2 \mathrm{O}(s)$
Answer:
$4 \mathrm{Na}(s)+\mathrm{O}_2 \rightarrow 2 \mathrm{Na}_2 \mathrm{O}(s)$
In the above reaction, Na is oxidised and oxygen gets reduced.
Question 3. (ii)Identify the substances that are oxidized and the substances that are reduced in the following reactions.
Answer:
$\mathrm{CuO}(s)+\mathrm{H}_2(g) \rightarrow \mathrm{Cu}(s)+\mathrm{H}_2 \mathrm{O}(l)$
In the above reaction, CuO is reduced to form Cu and hydrogen gets oxidized to water.
NCERT Solutions For Class 10 Science Chapter 1 (Exercise Questions with Answers)
Get comprehensive and well-structured answers to chemical reactions and equations class 10 question answer. These Solutions of NCERT are designed to help students build a strong foundation and score well in their board exams.
Question 1. Which of the statements about the reaction below are incorrect?
$2 \mathrm{PbO}(s)+\mathrm{C}(s) \rightarrow 2 \mathrm{~Pb}(\mathrm{~s})+\mathrm{CO}_2(g)$
(a) Lead is getting reduced.
(b) Carbon dioxide is getting oxidized.
(c) Carbon is getting oxidized.
(d) Lead oxide is getting reduced.
(i) (a) and (b)
(ii) (a) and (c)
(iii) (a), (b) and (c)
(iv) all
Answer:
$2 \mathrm{PbO}(s)+\mathrm{C}(s) \rightarrow 2 \mathrm{~Pb}(s)+\mathrm{CO}_2(g)$
In the above reaction, PbO reduces to Pb and C(carbon) gets oxidized to carbon dioxide.
Hence, statements (a) and (b) are correct.
Thus, option (i) is correct.
Question 2. $\mathrm{Fe}_2 \mathrm{O}_3+2 \mathrm{Al} \rightarrow \mathrm{Al}_2 \mathrm{O}_3+2 \mathrm{Fe}$
The above reaction is an example of a
(a) combination reaction.
(b) double displacement reaction.
(c) decomposition reaction.
(d) displacement reaction
Answer :
$\mathrm{Fe}_2 \mathrm{O}_3+2 \mathrm{Al} \rightarrow \mathrm{Al}_2 \mathrm{O}_3+2 \mathrm{Fe}$
The above reaction is an example of a displacement reaction.
Hence, the correct answer is option (d).
Question 3. What happens when dilute hydrochloric acid is added to iron filings? Tick the correct answer.
(a) Hydrogen gas and iron chloride are produced.
(b) Chlorine gas and iron hydroxide are produced.
(c) No reaction takes place.
(d) Iron salt and water are produced.
Answer:
When dilute hydrochloric acid is added to iron fillings, then hydrogen gas and iron chloride are produced.
The reaction is given as :
$\mathrm{Fe}(s)+2 \mathrm{HCl}(\mathrm{aq}) \rightarrow \mathrm{FeCl}_2(\mathrm{aq})+\mathrm{H}_2(g)$
Thus, option (a) is correct.
Question 4. What is a balanced chemical equation? Why should chemical equations be balanced?
Answer:
The chemical equation which has an equal number of atoms of all elements on both sides of the reaction is known as a balanced chemical equation.
The law of conservation of mass states that mass can neither be created nor be destroyed so chemical equations should be balanced.
Question 5. (a) Translate the following statements into chemical equations and then balance them.
Hydrogen gas combines with nitrogen to form ammonia.
Answer:
Hydrogen gas combines with nitrogen to form ammonia can be written as :
$3 \mathrm{H}_2(g)+\mathrm{N}_2(g) \rightarrow 2 \mathrm{NH}_3$
Question 5. (b) Hydrogen sulphide gas burns in the air to give water and sulfur dioxide.
Answer:
Hydrogen sulphide gas burns in air to give water and sulfur dioxide can be written as :
$2 \mathrm{H}_2 \mathrm{~S}(\mathrm{~g})+3 \mathrm{O}_2(\mathrm{~g}) \rightarrow 2 \mathrm{SO}_2(\mathrm{~g})+2 \mathrm{H}_2 \mathrm{O}(\mathrm{l})$
Question 5. (c) Barium chloride reacts with aluminium sulphate to give aluminium chloride and a precipitate of barium sulphate.
Answer:
Barium chloride reacts with aluminium sulphate to give aluminium chloride and a precipitate of barium sulphate can be written as :
$3 \mathrm{BaCl}_2(\mathrm{aq})+\mathrm{Al}_2\left(\mathrm{SO}_4\right)_3(\mathrm{aq}) \rightarrow 2 \mathrm{AlCl}_3(\mathrm{aq})+3 \mathrm{BaSO}_4(s)$
Question 5. (d) Potassium metal reacts with water to give potassium hydroxide and hydrogen gas.
Answer: Potassium metal reacts with water to give potassium hydroxide and hydrogen gas is given by :
$2 \mathrm{~K}(s)+2 \mathrm{H}_2 \mathrm{O}(l) \rightarrow 2 \mathrm{KOH}(\mathrm{aq})+\mathrm{H}_2$
Question 6. (a) Balance the following chemical equations.
(a) Balance the following chemical equations.
$\mathrm{HNO}_3+\mathrm{Ca}(\mathrm{OH})_2 \rightarrow \mathrm{Ca}\left(\mathrm{NO}_3\right)_2+\mathrm{H}_2 \mathrm{O}$
Answer:
The balanced chemical equation is given as :
$2 \mathrm{HNO}_3+\mathrm{Ca}(\mathrm{OH})_2 \rightarrow \mathrm{Ca}\left(\mathrm{NO}_3\right)_2+2 \mathrm{H}_2 \mathrm{O}$
Question 6. (b)Balance the following chemical equations.
$\mathrm{NaOH}+\mathrm{H}_2 \mathrm{SO}_4 \rightarrow \mathrm{Na}_2 \mathrm{SO}_4+\mathrm{H}_2 \mathrm{O}$
Answer:
The balanced chemical equation is given as :
$2 \mathrm{NaOH}+\mathrm{H}_2 \mathrm{SO}_4 \rightarrow \mathrm{Na}_2 \mathrm{SO}_4+2 \mathrm{H}_2 \mathrm{O}$
Question 6. (c) Balance the following chemical equations.
$\mathrm{NaCl}+\mathrm{AgNO}_3 \rightarrow \mathrm{AgCl}+\mathrm{NaNO}_3$
Answer:
The balanced chemical equation is given as :
$\mathrm{NaCl}+\mathrm{AgNO}_3 \rightarrow \mathrm{AgCl}+\mathrm{NaNO}_3$
Question 6. (d) Balance the following chemical equations.
$\mathrm{BaCl}_2+\mathrm{H}_2 \mathrm{SO}_4 \rightarrow \mathrm{BaSO}_4+\mathrm{HCl}$
Answer:
A balanced chemical equation is given as :
$\mathrm{BaCl}_2+\mathrm{H}_2 \mathrm{SO}_4 \rightarrow \mathrm{BaSO}_4+2 \mathrm{HCl}$
Question 7. (a) Write the balanced chemical equations for the following reactions.
(a) Calcium hydroxide + Carbon dioxide → Calcium carbonate + Water
Answer:
Calcium hydroxide + Carbon dioxide →Calcium carbonate + Water
The balanced chemical equation for the above reaction is given as :
$\mathrm{Ca}(\mathrm{OH})_2+\mathrm{CO}_2 \rightarrow \mathrm{CaCO}_3+\mathrm{H}_2 \mathrm{O}$
Question 7. (b) Zinc + Silver nitrate → Zinc nitrate + Silver
Answer:
Zinc + Silver nitrate → Zinc nitrate + Silver
The balanced chemical equation for the above reaction is given as :
$\mathrm{Zn}+2 \mathrm{AgNO}_3 \rightarrow \mathrm{Zn}\left(\mathrm{NO}_3\right)_2+2 \mathrm{Ag}$
Question 7. (c)Aluminium + Copper chloride → Aluminium chloride + Copper
Answer:
Aluminium + Copper chloride → Aluminium chloride + Copper
The balanced chemical equation for the above reaction is given as :
$2 \mathrm{Al}+3 \mathrm{CuCl}_2 \rightarrow 2 \mathrm{AlCl}_2+3 \mathrm{Cu}$
Question 7. (d) Barium chloride + Potassium sulphate → Barium sulphate + Potassium chloride
Answer:
Barium chloride + Potassium sulphate → Barium sulphate + Potassium chloride
The balanced chemical equation for the above reaction is given as :
$\mathrm{BaCl}_2+\mathrm{K}_2 \mathrm{SO}_4 \rightarrow \mathrm{BaSO}_4+2 \mathrm{KCl}$
Question 8. (a) Write the balanced chemical equation for the following and identify the type of reaction in each case.
(a) Potassium bromide(aq) + Barium iodide(aq) → Potassium iodide(aq) + Barium bromide(s)
Answer:
Potassium bromide(aq) + Barium iodide(aq) → Potassium iodide(aq) + Barium bromide(s)
The balanced chemical equation for the above reaction is given as :
$2 K B r(a q)+B a I_2(a q) \rightarrow 2 K I(a q)+B a B r_2$
It is a double displacement reaction.
Question 8. (b) Zinc carbonate(s) → Zinc oxide(s) + Carbon dioxide(g)
Answer:
Zinc carbonate(s) → Zinc oxide(s) + Carbon dioxide(g)
The balanced chemical equation for the above reaction is given by :
$\mathrm{ZnCO}_3(s) \rightarrow \mathrm{ZnO}(\mathrm{s})+\mathrm{CO}_2(\mathrm{~g})$
It is a decomposition reaction.
Question 8. (c)Hydrogen(g) + Chlorine(g) → Hydrogen chloride(g)
Answer:
Hydrogen(g) + Chlorine(g) → Hydrogen chloride(g)
The balanced chemical equation for the above reaction is given by :
$\mathrm{H}_2(\mathrm{~g})+\mathrm{Cl}_2(\mathrm{~g}) \rightarrow 2 \mathrm{HCl}(\mathrm{g})$
It is a combination reaction.
Question 8. (d)Magnesium(s) + Hydrochloric acid(aq) → Magnesium chloride(aq) + Hydrogen(g)
Answer:
Magnesium(s) + Hydrochloric acid(aq) → Magnesium chloride(aq) + Hydrogen(g)
The balanced chemical equation for the above reaction is given by :
$\mathrm{Mg}(s)+2 \mathrm{HCl}(\mathrm{aq}) \rightarrow \mathrm{MgCl}_2(\mathrm{aq})+\mathrm{H}_2(g)$
It is a displacement reaction.
Question 9. What does one mean by exothermic and endothermic reactions? Give examples.
Answer:
Exothermic reactions: Reactions in which heat is given out along with the products are called exothermic reactions.
Example : $\mathrm{CH}_4(\mathrm{~g})+2 \mathrm{O}_2(\mathrm{~g}) \rightarrow \mathrm{CO}_2(\mathrm{~g})+2 \mathrm{H}_2 \mathrm{O}(\mathrm{g})$
Endothermic reactions: Reactions in which energy is absorbed are known as endothermic reactions.
Example: The process of photosynthesis.
$6 \mathrm{CO}_2(\mathrm{~g})+6 \mathrm{H}_2 \mathrm{O}(\mathrm{g}) \rightarrow \mathrm{C}_6 \mathrm{H}_{12} \mathrm{O}_6(\mathrm{aq})+6 \mathrm{O}_2(\mathrm{~g})$
Question 10. Why is respiration considered an exothermic reaction? Explain.
Answer:
We know that energy is required to support life. We get energy from the food we eat.
The large molecules of food are broken into simpler substances like glucose during digestion.
Glucose and oxygen react to provide energy to the body. This reaction is a combination reaction named respiration. In this whole process, energy is released, so respiration is considered an exothermic reaction.
$\mathrm{C}_6 \mathrm{H}_{12} \mathrm{O}_6(\mathrm{aq})+6 \mathrm{O}_2 \rightarrow 6 \mathrm{CO}_2(\mathrm{~g})+6 \mathrm{H}_2 \mathrm{O}(\mathrm{l})+$ Energy
Question 11. Why are decomposition reactions called the opposite of combination reactions? Write equations for these reactions.
Answer:
In decomposition reactions, we can observe that a single reactant breaks down to give simpler products. This reaction is a source of energy. Whereas, in a combination reaction, two or more substances combine to give a product and energy is released in this reaction.
Hence, decomposition reactions are called the opposite of combination reactions.
Example : decomposition reaction : $2 \mathrm{H}_2 \mathrm{O}(\mathrm{l}) \rightarrow 2 \mathrm{H}_2(\mathrm{~g})+\mathrm{O}_2(\mathrm{~g})$
And the combination reaction : $2 \mathrm{H}_2(g)+\mathrm{O}_2(g) \rightarrow 2 \mathrm{H}_2 \mathrm{O}(\mathrm{l})+$ Energy
Answer:
The decomposition reaction by heat:
$
2 \mathrm{FeSO}_4(s) \rightarrow \mathrm{Fe}_2 \mathrm{O}_3(s)+\mathrm{SO}_2(g)+\mathrm{SO}_3
$
The decomposition reaction by light :
$
2 \mathrm{AgCl}(s) \rightarrow 2 \mathrm{Ag}(s)+\mathrm{Cl}_2(g)
$
The decomposition reaction by electricity :
$
2 \mathrm{Al}_2 \mathrm{O}_3(\mathrm{aq}) \rightarrow 4 \mathrm{Al}(\mathrm{~s})+3 \mathrm{O}_2(\mathrm{~g})
$
Question 13. What is the difference between displacement and double displacement reactions? Write equations for these reactions.
Answer:
When an element displaces another element from its compound, a displacement reaction occurs.
Example : $\mathrm{Fe}(\mathrm{s})+\mathrm{CuSO}_4(a q) \rightarrow \mathrm{FeSO}_4(a q)+\mathrm{Cu}(s)$
Two different atoms or groups of atoms (ions) are exchanged in a double displacement reaction.
Example : $\mathrm{Na}_2 \mathrm{SO}_4(a q)+\mathrm{BaCl}_2(a q) \rightarrow \mathrm{BaSO}_4(s)+2 \mathrm{NaCl}(\mathrm{aq})$
Answer:
In the refining of silver, the recovery of silver from silver nitrate solution involved displacement by copper metal. The reaction involved can be written as :
$2 \mathrm{AgNO}_3(\mathrm{aq})+\mathrm{Cu}(s) \rightarrow \mathrm{Cu}\left(\mathrm{NO}_3\right)_2(\mathrm{aq})+2 \mathrm{Ag}(s)$
Question 15. What do you mean by a precipitation reaction? Explain by giving examples.
Answer:
Any reaction that produces a precipitate is called a precipitation reaction.
Example : $\mathrm{Na}_2 \mathrm{CO}_3(\mathrm{aq})+\mathrm{CaCl}_2(\mathrm{aq}) \rightarrow \mathrm{CaCO}_3(s)+2 \mathrm{NaCl}(\mathrm{aq})$
Here, we have $\mathrm{CaCO}_3$ as precipitate so it is a precipitation reaction.
Question 16. Explain the following in terms of gain or loss of oxygen with two examples each.
(a) Oxidation
Answer:
Oxidation is a reaction in which the gain of oxygen or loss of hydrogen can be observed.
Example : $2 \mathrm{Cu}+\mathrm{O}_2 \rightarrow 2 \mathrm{CuO}$
$
\mathrm{CO}_2+\mathrm{H}_2 \rightarrow \mathrm{CO}+\mathrm{H}_2 \mathrm{O}
$
Question 16. (b) Reduction
Answer:
The reaction in which the loss of oxygen or gain of hydrogen can be observed is known as the reduction reaction.
Example : $\mathrm{CuO}+\mathrm{H}_2 \rightarrow \mathrm{Cu}+\mathrm{H}_2 \mathrm{O}$
$
\mathrm{ZnO}+\mathrm{C} \rightarrow \mathrm{Zn}+\mathrm{CO}
$
Answer:
A shiny brown-colored element is copper (Cu) and on heating, in the air, it becomes black in colour because of the formation of copper oxide(CuO).
$2 \mathrm{Cu}+\mathrm{O}_2 \rightarrow 2 \mathrm{CuO}$
Question 18. Why do we apply paint on iron articles?
Answer:
To prevent iron from rusting, paint is applied to iron articles. After applying paint iron articles are not in contact with moisture and air and hence rusting is prevented.
Question 19. Oil and fat-containing food items are flushed with nitrogen. Why?
Answer:
We know that nitrogen is an inert gas and does not react with oil and fat-containing food. Whereas, other gases like oxygen react with the oil and fat-containing food and make them rancid. Hence, to remove oxygen and prevent food from acidic food items is flushed with nitrogen.
Question 20. Explain the following terms with one example each.
(a) Corrosion
Answer:
Corrosion is a process in which metals deteriorate due to chemical reactions with moisture, air, and chemicals. The rusting of iron is a major example of corrosion. Iron corrodes in the presence of moisture and air.
$4 \mathrm{Fe}+3 \mathrm{O}_2+\mathrm{H}_2 \mathrm{O} \rightarrow 2 \mathrm{Fe}_2 \mathrm{O}_3 \cdot \mathrm{H}_2 \mathrm{O}$
Question 20. (b) Rancidity
Answer:
The process of oxidation of fats and oils, which can be noticed by a change in color, smell, and taste, is known as rancidity.
Example: When butter is kept in an open atmosphere,e its smell and taste change which results in rancidity.
Practice Questions for Class 10 Science Chapter 1
Practising these class 10 science chapter 1 chemical reactions and equations question answer will help you test your understanding of chemical reactions and equations. They are designed to strengthen your conceptual knowledge and improve your exam preparation.
Question 1. State the change that is observed when a China dish containing copper powder is heated over the flame of a burner. Name the phenomenon responsible for the change and write a balanced equation for the chemical reaction that occurs. How is this reaction different from the reaction that occurs when copperware kept in open air slowly loses its shiny brown surface and gains a coat? Write the chemical name of the coating and state its colour.
Answer:
When a China dish containing copper powder is heated, it undergoes oxidation. Copper reacts with oxygen in the air to form copper(II) oxide, which is black in color.
Balanced Equation for the Reaction:
$2 \mathrm{Cu}(\mathrm{s})+\mathrm{O}_2(\mathrm{~g}) \rightarrow 2 \mathrm{CuO}(\mathrm{s})$
The reason behind this is oxidation of copper in the presence of oxygen
On the other hand, when copper wares are kept in the open air, they react with carbon dioxide, oxygen, and moisture, they slowly tarnish and form a greenish coating. This greenish coating is basic copper carbonate.
Chemical Equation for Tarnishing: $2 \mathrm{Cu}(\mathrm{s})+\mathrm{CO}_2(\mathrm{~g})+\mathrm{H}_2 \mathrm{O}(\mathrm{l}) \rightarrow \mathrm{CuCO}_3 \cdot \mathrm{Cu}(\mathrm{OH})_2(\mathrm{~s})$
Question 2. A chemical compound ' X ' is used to bleach washed clothes in laundry as well as to make drinking water free from germs. Identify ' X '. How is this compound represented? Write the method of its preparation along with the chemical equation for the reaction that occurs.
Answer:
The compound 'X' used to bleach clothes and purify drinking water is bleaching powder (also called calcium oxychloride).
Representation
X= Bleaching powder ($\mathrm{CaOCl}_2$)
Preparation Method: Bleaching powder is prepared by passing chlorine gas over dry slaked lime (calcium hydroxide).
Reaction is as follows:
$2 \mathrm{Cl}_2+2 \mathrm{Ca}(\mathrm{OH})_2 \rightarrow \mathrm{CaCl}_2+\mathrm{CaOCl}_2+2 \mathrm{H}_2 \mathrm{O}$
Question 3. Study the following cases :
(i) $\mathrm{CuSO}_4+\mathrm{Mg} \longrightarrow$
(ii) $\mathrm{FeSO}_4+\mathrm{Pb} \longrightarrow$
(iii) $\mathrm{CaSO}_4+\mathrm{Al} \longrightarrow$
(iv) $\mathrm{ZnSO}_4+\mathrm{Ca} \longrightarrow$
The case/cases in which new product(s) will form is/are :
(1) Only (i)
(2) Only (iii)
(3) (i) and (iv)
(4) (i), (ii) and (iv)
Answer:
Let us look at which reactions will give new products. This happens when a more reactive metal replaces a less reactive one in a compound.
(i) $\mathrm{CuSO}_4+\mathrm{Mg} \rightarrow$
$\mathrm{Mg}+\mathrm{CuSO}_4 \rightarrow \mathrm{MgSO}_4+\mathrm{Cu}$
Magnesium is more reactive than copper → reaction happens → new product forms
(ii) $\mathrm{FeSO}_4+\mathrm{Pb} \rightarrow$
Lead is less reactive than iron → no reaction → no new product
(iii) $\mathrm{CaSO}_4+\mathrm{Al} \rightarrow$
Aluminium is less reactive than calcium → no reaction → no new product
(iv) $\mathrm{ZnSO}_4+\mathrm{Ca} \rightarrow$
$\mathrm{Ca}+\mathrm{ZnSO}_4 \rightarrow \mathrm{CaSO}_4+\mathrm{Zn}$
Calcium is more reactive than zinc → reaction happens → new product forms
Hence, the correct answer is option (3).
Question 4: Which of the following methods can be used to prevent the food from getting rancid:
i. Storing the food in the air-tight containers
ii. Storing the food in refrigerator
iii. Keeping food in open
iv. Keeping food in moist atmosphere
(1) (i) and (ii)
(2) (i) and (iii)
(3) (iii) and (iv)
(4) All of the above
Answer:
Rancidity can be prevented by storing food in airtight containers because in airtight containers there is little exposure to oxygen then oxidation of fats and oils present in food is slowed down and thus rancidity is retarded. Rancidity can be prevented by keeping food in a refrigerator because in a refrigerator when the food is kept the oxidation of fats and oils in it is slowed down due to low temperature and thus rancidity is retarded.
Hence, the answer is the option (1).
Question 5: The white precipitates formed In a chemical reaction between sulphuric acid and barium chloride solution is of:
(1) Sulphur
(2) Barium sulphate
(3) Chlorine
(4) Silver chloride
Answer:
White precipitates formed In a chemical reaction between sulphuric acid and barium chloride solution is of Barium sulphate.
Hence, the answer is the option (2).
Question 6: A reddish-brown layer is deposited on an iron object when it is left exposed to moist air. This process is an example of:
(1) Combination reaction
(2) Decomposition reaction
(3) Oxidation reaction
(4) Displacement reaction
Answer:
The correct answer is option (3)
Oxidation reaction
Question 7: Which of the following is a correctly balanced chemical equation?
(1) $\mathrm{H}_2+\mathrm{O}_2 \rightarrow \mathrm{H}_2 \mathrm{O}$
(2) $\mathrm{Fe}+\mathrm{S} \rightarrow \mathrm{FeS}$
(3) $\mathrm{Mg}+\mathrm{O}_2 \rightarrow \mathrm{MgO}$
(4) $\mathrm{Ca}+\mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{Ca}(\mathrm{OH})_2+\mathrm{H}_2$
Answer:
$\mathrm{Ca}+2 \mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{Ca}(\mathrm{OH})_2+\mathrm{H}_2$ is the fully balanced reaction.
Hence, the correct answer is option (4).
Question 8: A sample of a chromium-containing alloy weighing 3.45 g was dissolved in acid, and all the chromium in the sample was oxidized to $\mathrm{CrO}_4^{2-}$. It was then found that 3.0 g of $\mathrm{Na}_2 \mathrm{SO}_3$ was required to reduce the $\mathrm{CrO}_4^{2-}$ to $\mathrm{CrO}_2^{-}$in a basic solution, with $\mathrm{SO}_3^{2-}$ getting oxidized to $\mathrm{SO}_4^{2-}$. Then the % of chromium in the alloy is $\qquad$ .
(Round off your answer to the nearest integer)
Answer:
The reaction involved is
$
2 \mathrm{CrO}_4^{2-4}+3 \mathrm{SO}_3^{2-}+\mathrm{H}_2 \mathrm{O} \rightarrow 2 \mathrm{CrO}_2^{-}+3 \mathrm{SO}_4^{2-}+2 \mathrm{OH}^{-}
$
Also,
$
\begin{aligned}
\mathrm{molCrO}_4^{2-} & =\left(3.0 \mathrm{gNa}_2 \mathrm{SO}_3\right) \times\left(\frac{1 \mathrm{molNa}_2 \mathrm{SO}_3}{126 \mathrm{gNa}_2 \mathrm{SO}_3}\right) \times\left(\frac{1 \mathrm{molSO}_3^{2-}}{1 \mathrm{molNa}_2 \mathrm{SO}_3}\right) \times\left(\frac{2 \mathrm{molCrO}_4^{2-}}{3 \mathrm{molSO}_3^{2-}}\right) \\
& =1.58 \times 10^{-2} \mathrm{molCrO}_4^{2-}
\end{aligned}
$
Since there is one mole of Cr in each mole of $\mathrm{CrO}_4^{2-}$, then the above number of moles $\mathrm{CrO}_4^{2-}$ is also equal to the number of moles of Cr that were present:
$0.0158 \mathrm{molCr} \times 52.00 \mathrm{~g} / \mathrm{mol}=0.825 \mathrm{gCr}$ in the original alloy.
$\%$ of $\mathrm{Cr}=(0.825 \mathrm{gCr} / 3.450 \mathrm{~g}$ sample $) \times 100 \%=23.9 \% \mathrm{Cr}$
Alternately,
gram equiv of $\mathrm{CrO}_4^{2-} \equiv$ gram equiv of $\mathrm{SO}_3^{2-}$
$\Rightarrow\left(\right.$ Moles of Cr or $\left.\mathrm{CrO}_4^{2-}\right) \times 3=\frac{3}{126 / 2}$
$\Rightarrow$ Moles of $\mathrm{Cr}=\frac{1}{63}$
Therefore, $\%$ of Cr in alloy $=\frac{52 / 63}{3.45} \times 100=23.92 \%$
Hence, the answer is (24).
Question 9: Which type of reaction takes place when a solution of potassium iodide solution is added to a solution of lead nitrate?
(1) Displacement reaction
(2) Double displacement reaction
(3) Addition reaction
(4) Decomposition reaction
Answer:
When a solution of potassium iodide solution is added to a solution of lead nitrate, double displacement reaction takes place.
The reaction occurs as follows:
$\mathrm{Pb}\left(\mathrm{NO}_3\right)_2(\mathrm{aq})+2 \mathrm{KI}(\mathrm{aq})-\rightarrow \mathrm{Pbl}_2(\mathrm{~s})+2 \mathrm{KNO}_3(\mathrm{aq})$
Hence, the answer is the option (2)
Approach to Solve Questions of Class 10 Science Chapter 1
Sometimes chemical reactions and equations ncert solutions seem very difficult, but once we understand the basic rules and strategy, it becomes very easy to solve all the questions related to that particular topic. Students can follow the steps given below to solve the questions in this chapter:
1. Before solving questions from Chemical Reactions and Equations, it is important to understand fundamental concepts like reactants, products, chemical changes, and types of chemical reactions for a strong conceptual foundation.
2. Having a clear understanding of the types of Chemical reactions is important. There are different kinds of reactions like:
(i). Combination: $A+B \rightarrow A B$
(ii). Decomposition: $A B \rightarrow A+B$
(iii). Displacement: $A+B C \rightarrow A C+B$
(iv). Double Displacement: $A B+C D \rightarrow A D+C B$
(v). Redox: Identify the species that is oxidised/reduced.
3. Questions from balancing chemical equations are asked frequently in exams to solve them effectively, first convert the word equation into a chemical equation and balance it by ensuring equal atoms of each element on both sides. Students can refer to the chemical reactions and equations ncert solutions to enhance their understanding of basic concepts.
4. To identify whether the change is Physical or Chemical, note down the indicators such as a change in colour, evolution of gas, precipitate formation, or temperature change to identify reactions practically.
5. Practice chemical reactions and equations class 10 question answer again and again, as it will help in mastering Chapter 1. Start with simpler problems, then gradually move to difficult ones. These questions are asked directly in the CBSE and State board exams.
Topics and Subtopics Covered in Class 10 Chapter 1 NCERT
All the topics and subtopics covered in the NCERT Solutions for Class 10 Science Chapter 1 Chemical Reactions and Equations are listed below. These topics are explained in a systematic manner to make it easier for students to grasp the fundamentals and apply them while solving questions.
1.1. Chemical equations
- 1.1.1. Writing a Chemical Equation
- 1.1.2. Balanced Chemical Equations
1.2. Types of Chemical Reactions
- 1.2.1. Combination Reaction
- 1.2.2. Decomposition Reaction
- 1.2.3. Displacement Reaction
- 1.2.4. Double Displacement Reaction
- 1.2.5. Oxidation and Reduction
1.3. Have you observed the effects of oxidation reactions in everyday life?
- 1.3.1. Corrosion
- 1.3.2. Rancidity
NCERT Class 10 Science Chapter 1: Important Reactions And E-book
Important Formulas from class 10 science chemical reactions and equations question answer are listed below. Go through them to make your basic concept more concrete.
TYPES OF CHEMICAL REACTIONS
1. Combination or Addition reaction: $A+B \rightarrow A B$
2. Decomposition Reaction: $A B \rightarrow A+B$
3. Displacement Reaction: $A+B C \rightarrow A C+B$
4. Double Displacement Reaction or Precipitation Reaction:
$\mathrm{AgNO}_3+\mathrm{NaCl} \rightarrow \mathrm{AgCl}(\mathrm{s})+\mathrm{NaNO}_3$
5. Oxidation and Reduction reactions:
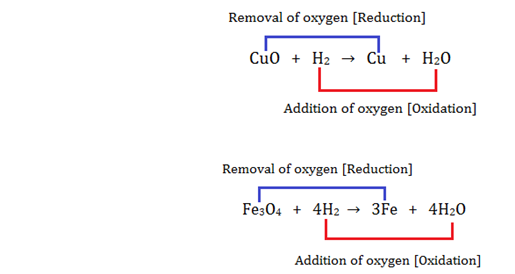
To download the E-book click on the link given below:
What Students Learn from NCERT Solutions for Class 10 Science Chapter 1
These chemical reactions and equations class 10 question answer offers easy to understand explanations and detailed answers to all questions. They help students understand difficult concepts in an easy way. Apart from this, students learn many interesting facts and things, which are given below:
- Students can understand the basic concept of chemical reactions and how these reactions occur.
-
Using these class 10 science chapter 1 chemical reactions and equations solutions students learn how to write and balance chemical equations.
-
Different types of chemical reactions like combination, decomposition, displacement, and double displacement, are explained here in detail.
-
Knowledge of oxidation, reduction, and redox reactions is highlighted in these chemical reactions and equations ncert solutions.
-
The concepts of corrosion and rancidity show how they occur and ways to prevent them.
Importance of Class 10 Science Chapter 1: Chemical Reactions and Equations
The class 10 science chapter 1 chemical reactions and equations question answers help students to understand how substances react and form new products through balanced chemical equations. Given below some points on how these solutions are important:
- Using these solutions students can learn how to write and balance chemical equations.
- The class 10 science chemical reactions and equations question answer explains different types of reactions like combination, decomposition, displacement, and oxidation-reduction.
- They connect concepts to everyday phenomena like rusting of iron, corrosion, and rancidity.
- Concepts like how mass and atoms are conserved during reactions are explained here very well.
NCERT Solutions for Class 10 Science Chapter-Wise
Besides NCERT Solutions for Class 10 Science Chapter 1 Chemical Reactions and Equations students can also follow NCERT Solutions for Class 10 chapter-wise solutions links:
Frequently Asked Questions (FAQs)
NCERT Solutions for Class 10 Science Chapter 1 Chemical Reactions and Equations help by providing clear and step-by-step answers to all textbook questions, making it easier to understand concepts. They strengthen problem-solving skills, improve accuracy in writing and balancing chemical equations, and prepare students effectively for exams.
Chapter 1 introduces the basics of chemical reactions, where substances change to form new substances with different properties. It explains how to write and balance chemical equations, and covers types of reactions like combination, decomposition, displacement, and redox reactions, along with concepts such as corrosion and rancidity.
The main types of chemical reactions include combination reactions, decomposition reactions, displacement reactions, and redox reactions.
Oxidation and reduction are important because they describe the transfer of electrons between substances. This process explains how energy is transferred in chemical reactions.
Catalysts are substances that speed up chemical reactions without being consumed in the process. They work by lowering the activation energy required for the reaction to occur, making it faster.
The solutions help students understand complex concepts easily through clear explanations and answers. They improve problem-solving skills, enhance accuracy in writing and balancing equations.
Redox reactions are characterized by the transfer of electrons between reactants, resulting in changes in oxidation states. They consist of two processes: oxidation and reduction. This distinguishes them from other reactions, where electron transfer may not be a key factor.
A displacement reaction, also known as a replacement reaction, occurs when an element in a compound is replaced by another element.
Balancing chemical equations is crucial because it reflects the law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction. By balancing the equation, we ensure that the number of atoms for each element is the same on both the reactant and product sides, accurately representing the reaction.
This chapter covers several types of chemical reactions, including combination reactions, decomposition reactions, displacement reactions, and redox reactions.
Popular Questions
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
